Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Advanced age is a major risk factor for the development of cardiovascular disease. Why age increases the risk of cardiovascular disease is debatable. The increased risk might arise simply because there is more time to be exposed to risk factors such as hypertension, smoking, and dyslipidemia. In other words, the aging process itself has little impact on the cardiovascular system. However, an emerging view is that the accumulation of cellular and subcellular deficits in the aging heart and blood vessels renders the cardiovascular system susceptible to the effects of cardiovascular diseases. Although increased exposure to risk factors likely contributes to the development of cardiovascular disease in aging, there is considerable evidence that the structure and function of the human heart and vasculature change importantly as a function of the normal aging process. These changes occur in the absence of risk factors other than age and in the absence of overt clinical signs of cardiovascular disease.
Studies in blood vessels from apparently healthy humans have shown that the vasculature changes with age, a process known as remodeling. The centrally located large elastic arteries dilate, something that is evident to the naked eye, and that is well seen in arterial radiographic studies. Structural changes due to remodeling are apparent even in early adulthood and increase with age. Aging-related arterial remodeling is important, because it is thought to provide an ideal setting in which vascular diseases can thrive. Structural changes that occur in the arteries of normotensive aging humans are observed in hypertensive patients at much younger ages.
These readily visible changes arise from microscopic changes in the wall structure of these large elastic arteries. The arterial wall is composed of three different layers, or tunics. The outermost layer, tunica adventitia, is composed of collagen fibers and elastic tissue. The thicker middle layer, the tunica media, is composed of connective tissue, smooth muscle cells, and elastic tissue. The contractile properties of the arterial wall are determined primarily by variations in the composition of the media. The innermost layer of the arterial wall, tunica intima, consists of a connective tissue layer and an inner layer of endothelial cells. Endothelial cells are squamous epithelial cells that play an important role in the regulation of normal vascular function, and endothelial dysfunction contributes to vascular disease. Age-associated changes in these different layers have a profound effect on the structure and function of the vasculature in older adults.
One of the most prominent age-related changes in the structure of the vasculature in humans is dilation of large elastic arteries, which leads to an increase in lumen size. In addition, the walls of large elastic arteries thicken with age. Studies of carotid wall intima plus media (IM) thickness in adult human arteries have shown that IM thickness increases almost threefold by 90 years of age. Increased IM thickness is an important risk factor for atherosclerosis independent of age. Thickening of the arterial wall in aging is due mainly to an increase in the thickness of the intima. Whether thickening of the media occurs in aging is controversial. However, studies have shown that the number of vascular smooth muscle cells in the media declines with age, whereas the remaining cells increase in size. Whether these hypertrophied smooth muscle cells are fully functional or whether this is one way in which aging is deleterious to vascular function is not yet clear. The major structural changes in the vasculature with age are illustrated in Figure 16-1 .
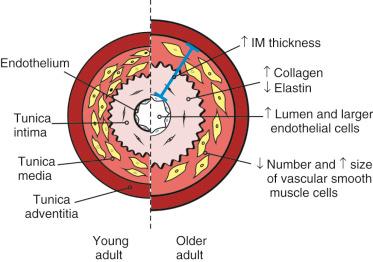
Age-associated thickening of the intima is due, in part, to an increase in infiltrating vascular smooth muscle cells. In addition, the collagen content of the intima and collagen cross-linking increase markedly with age in human arteries. However, the elastin content of the intima declines, and elastin fraying and fragmentation occur. It has been proposed that repeated cycles of distention followed by elastic recoil may promote the loss of elastin and deposition of collagen in aging arteries. These changes in collagen and elastin content are believed to have important effects on the distensibility or stiffness of aging arteries, as discussed in more detail later (see “ Arterial Stiffness in Aging Arteries ”).
In addition to alterations in intimal connective tissues in aging, studies in human arteries have shown that the aging process modifies the structure of endothelial cells themselves. Endothelial cells increase in size with age or hypertrophy. In addition, endothelial cell shape becomes irregular. The permeability of endothelial cells increases with age, and vascular smooth muscle cells may infiltrate the subendothelial space. There also is considerable evidence that the substances released by the endothelium are modified by age. The impact of these changes on vascular function is discussed in more detail in the next section.
Once regarded as an almost inert lining of the blood vessels, the vascular endothelium is now recognized to be a metabolically active tissue involved in the maintenance and regulation of blood flow. In younger adults, the vascular endothelium synthesizes and releases a variety of regulatory substances in response to chemical and mechanical stimuli. For example, endothelial cells release substances such as nitric oxide, prostacyclin, endothelins, interleukins, endothelial growth factors, adhesion molecules, plasminogen inhibitors, and von Willebrand factor. These substances are involved in the regulation of key functions, including vascular tone, angiogenesis, thrombosis, and thrombolysis. There is growing evidence that the aging process may disrupt many of these normal functions of the vascular endothelium.
Endothelial dysfunction is usually measured as a disruption in endothelium-dependent relaxation. Endothelium-dependent relaxation is mediated by nitric oxide, which is released from the endothelium by mechanical stimuli, such as increased blood flow (shear stress), and by chemical stimuli (e.g., acetylcholine, bradykinin, adenosine triphosphate [ATP]). When nitric oxide is released from the endothelium, it causes vascular smooth muscle relaxation by increasing intracellular levels of cyclic guanosine monophosphate (cGMP). The increased cGMP prevents the interaction of the contractile filaments actin and myosin. The increase in vascular stiffness in aging arteries is partly explained by a decrease in the production of nitric oxide by the vascular endothelium. This leads to impairment in blood vessel relaxation as people age.
The mechanism whereby nitric oxide activity is reduced in aging remains controversial. Nitric oxide is synthesized in endothelial cells by a constitutive enzyme called endothelial nitric oxide synthase (eNOS or NOS III). There is evidence that the levels of eNOS are reduced in aging, which could account for the decrease in nitric oxide activity in aging vasculature. Other studies have suggested that factors such as the production of oxygen free radicals in aging endothelial cells may impair nitric oxide production. Further studies will be needed to understand fully the mechanism or mechanisms responsible for endothelial dysfunction in aging vasculature.
There is good evidence that endothelial dysfunction is an important cause of cardiovascular disease, independent of age. Therefore, age-related endothelial dysfunction is likely to make a major contribution to the increased risk of cardiovascular disease in older adults.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here