Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Liver transplantation (LT) has evolved from a high-risk procedure with significant morbidity and mortality to a standard treatment for patients with liver failure. Patients who undergo successful liver replacement have 1-year and 5-year survival rates that exceed 85% and 70%, respectively (see Chapter 105, Chapter 106, Chapter 107, Chapter 108, Chapter 109, Chapter 110 ). Despite this dramatic improvement in outcome, a significant percentage of patients experience life-threatening complications that can result in the need for reoperation.
Recent changes in the treatment of liver disease have begun to shift the demographics of transplant recipients. Hepatitis C cirrhosis has historically been the main indication for LT in Europe and North America, but with effective antiviral therapy, nonalcoholic steatohepatitis (NASH) is quickly becoming the most common indication. The rapid increase in NASH resulting in decompensated liver failure is expected to surpass the number of effectively treated viral hepatitis with an anticipated increase in end-stage liver disease (ESLD)–related deaths by 178% by 2030. The obesity epidemic has had a 2-fold effect impacting LT complications. The first is increasing the demand for livers, and the second is increasing the prevalence of fatty liver disease in potential organ donors. Fatty liver disease has resulted in an increased number of discarded liver from brain-dead donors, while increasing the number of transplanted liver grafts with a higher degree of macrosteatosis. Although the survival rates with macrosteatosis greater than 30% are comparable to donors with less fatty liver disease, the postoperative complication rates of postreperfusion syndrome (PRS), early allograft dysfunction (EAD), and postoperative dialysis and the need for a second operation are markedly increased. Furthermore, the LT recipients are going to have a higher frequency of obesity, in which a body mass index (BMI) greater than 30 is associated with an increased rate of postoperative cardiopulmonary complications and longer intensive care unit (ICU) stays. Although expertise in the procedure grows, surgeons will have to perform LT in patients who previously were considered poor candidates for surgery and will have to use more marginal donor livers.
Surgical techniques to increase the donor pool include reduced-size grafts, split-liver grafts. With these liver parenchymal–splitting operations has come a new series of complications unique to these procedures. , The incidence of hepatic artery thrombosis (HAT), bile leaks, and stricture is at least two times higher in patients who receive living-donor grafts compared with those who receive cadaveric grafts. There is a learning curve for reducing these complications as well. Another major complication after LDLT is the small-for-size syndrome (SFSS), characterized by hyperbilirubinemia, coagulopathy, and encephalopathy observed shortly after transplant. , The terminology of SFSS is becoming a historic term as early allograft dysfunction (EAD) has become a more appropriate term to describe graft dysfunction after transplant because graft size is not the only factor for slow return of hepatic function in LDLT and cadaveric donors. Despite a higher morbidity rate in recipients of living-donor grafts, patient and graft survival are similar or superior to those observed with deceased donors.
This chapter reviews common early and late complications encountered during and after LT. Because complications after LT represent a continuum, most can occur at any time after surgery.
The process of organ transplantation begins with identification of a suitable organ for a particular recipient. When an organ is identified, procurement teams are dispatched for recovery of various organs. In general, the liver procurement team dictates the conduct of dissection below the diaphragm, and the cardiac team works in concert with the thoracic team, if lungs and heart also are to be recovered. Because the infradiaphragmatic dissection takes considerably longer than the thoracic dissection, careful planning is required to minimize cold-ischemia time for the heart and lungs, which is more time sensitive. The heart procurement team stays in contact with the recipient heart team to minimize total ischemic time.
Under ideal conditions, the conduct of the operation is orderly and well controlled; however, a patient who progresses to brain death rapidly can show cardiac instability and can be difficult to manage. This situation can result in an expedited operation in which perfusion cannulae are hastily placed, and organ procurement rapidly follows. During such events, identification of appropriate anatomy is difficult, and technical errors are more likely to occur.
To increase the number of organs available, donation after cardiac death (DCD) has gained popularity since the 1990s. It was estimated that DCD organs could increase the organ donor pool by 10% in 2010. According to United Network for Organ Sharing (UNOS) data, the percent of DCD donors from 2010 to 2019 has increased from 6% to 11% of all donors in the United States, supporting high utilization. In this form of donation, a patient who is deemed hopeless but has not met criteria of “brain death” is allowed to die naturally after removal of supportive measures. After a period of 2 to 5 minutes (dependent on hospital policy) of asystole, organs can be procured for transplantation. Despite the various periods of hypotension and warm ischemia that develop, DCD outcomes have been mixed but generally acceptable. In liver grafts procured from DCD donors, there were concerns for increased biliary strictures and worse long-term graft survival, but a more modern assessment supports comparable outcomes to brain-dead donors with appropriate donor selection. The management of ischemic cholangiopathy will be discussed in a future section. With the use of normothermic regional perfusion , and normothermic machine perfusion, the incidence of this feared complication is believed to have become much less common.
Injuries during liver procurement occur in roughly 20% to 30% of organ donors. , These injuries can be broken down into parenchymal injuries, vascular injuries, and bile duct injuries. The most common major injury during liver procurement is aberrant hepatic artery ligation and division. This injury occurs by failure to recognize a replaced or accessory right or left hepatic artery during hilar dissection or by unintentional division during organ removal. The frequency of these aberrant arterial anatomy is described in a 1000 donor case series by Hiatt et al., in which a replaced/accessory right occurred in roughly 10% of donors, with a similar frequency for left replaced/accessory arteries, and 3% had dual replacement arteries. Despite the lower frequency of the nontraditional vascular anatomy of the liver, hilum–replaced left and right arteries represent two-thirds of all vascular injuries during procurement. Such injuries are serious because segments of the liver are not perfused during recovery and usually require reconstruction on the back table before reimplantation. Injuries to these arteries are associated with a high rate of early HAT (30%) and subsequent need for re-transplantation. The management of HAT is discussed in a later section.
Injuries to the liver parenchyma during graft removal are the most common form of minor liver injury during graft removal. These minor injuries often can be treated with topical hemostatic, cautery, or liver stitch. These injuries can also occur during graft implantation. Parenchymal injuries are associated with long operative times and increased blood loss but do not have an impact on long-term graft function. The more infrequent complication is an injury to the bile duct that can be because of stripping vascular supply or cutting the duct too short. These injuries can be managed by Roux-en-Y biliary reconstruction and typically not associated with graft loss.
Other donor factors that can contribute to morbidity to the organ recipient in relationship to the donor operation include prolonged cold-ischemia time. Grafts that have more than 12 hours of cold ischemia are at an increased risk for graft loss and complications. Additionally, macrosteatosis greater than 30% is also associated with poor early graft function. High BMI donors are used less commonly for liver grafts. Bedside biopsy and procurement biopsy can help improve high BMI donor liver utilization without increased risk to the recipient by confirming the percent of macrosteatosis. It is estimated that macrosteatosis greater than 30% is only prevalent in 21% of high BMI donors and that the majority of these donors can be used for LT. Visceral adipose tissue makes the technical aspects of liver procurement more challenging and time consuming. To minimize dissection-related injuries, some authors suggest the en bloc method of removing abdominal organs with back-table dissection after procurement. Regardless of the technique used, attention to detail and identification of the appropriate anatomy can help avoid graft procurement injury.
Patients with ESLD come to the operating room (OR) with a spectrum of coagulation abnormalities. The adaptive changes in coagulation related to ESLD are a combination of pro and anticoagulants with derangements of fibrinolysis in which individuals are prone to both massive hemorrhage or life-threatening thrombosis. This can be compounded by portal hypertension and adhesions from prior abdominal surgery. The combination of these two conditions results in a significant challenge for the surgeon and anesthesiologist. Patients who have undergone previous operations are at particular risk because prolonged lysis of adhesions may greatly increase the duration of the operation, create additional raw surface areas for bleeding, and lead to derangements in temperature and fluid homeostasis. The net result is worsening coagulopathy, which creates a vicious cycle during the procedure.
Preoperative assessment of the patient’s coagulation status can help guide anesthesia and the surgical team for patients’ risk of massive bleeding in the OR. Attenuating blood loss has been proposed to improve long-term outcomes after LT. Blood loss during surgery has an incremental increase in postoperative complications and decrease in graft survival. In addition, blood utilization in LT is under close scrutiny with the historical reputation of this surgery draining the blood banks. An early report from Stazl in the 1980s estimated that 3% to 9% of all blood products in the Pittsburgh central blood bank were used for LT. A proposed threshold for excessive bleeding in LT is more than 10 units of red blood cells (RBCs). Viscoelastic testing (thrombelastography [TEG] and rotational thrombelastography [ROTEM]) have been used in this setting to predict massive transfusion. A TEG maximum amplitude has been demonstrated to be associated with massive transfusion with a higher performance than INR and MELD. ROTEM has also been demonstrated to outperform conventional assays in predicting nonsurgical bleeding after LT.
Preemptive treatment of coagulopathy before the OR is often not indicated for mild to moderate derangements in coagulation. Hemostatic blood products such as platelets are believed to be rapidly sequestered by the spleen based on historic data. Plasma transfusions can result in exacerbation of venous hypertension and can paradoxically increase bleeding. Although controversial, the early use of prothrombin complex concentrate (PCC) has been associated with reduced blood loss during LT. PCC corrects INR while not requiring the volume of plasma. However, PCC has been associated with life-threatening thrombotic complications and conservative use is advised until the results of ongoing clinical trials are released. Regardless of the institution’s treatment plan for addressing presurgical coagulopathy, high-risk patients warrant careful intraoperative planning, including mobilization of the blood bank and the use of cell saver for autologous blood transfusion.
With evolving measurements of coagulation in LT, it has now been appreciated that there are a cohort of LT recipients who present to surgery with a hypercoagulable profile. Cirrhosis has recently been appreciated to be an independent risk factor for deep vein thrombosis (DVT) in hospitalized patients. NASH is associated with an even higher risk of thrombotic complications. Conventional coagulation measurements do not capture this hypercoagulable state in LT. An elevated preoperative TEG maximum amplitude (clot strength) has been associated with a 5-fold risk of early HAT. This unmeasured hypercoagulability associated with HAT was attributed to the operating surgeon. Although there are no guidelines on managing preoperative hypercoagulability in LT recipients, we have adopted the use of intraoperative heparin bolus after hepatic artery anastomosis in selected recipients (particularly LDLT with PSC of HCC) that have high clot strength (MA >65) on early intraoperative TEG results.
Improvements in surgical techniques and better patient selection have led to the performance of LT without the need for blood transfusion in selected patients. The advent of the transjugular intrahepatic portosystemic shunt (TIPS) can significantly lower portal hypertension preoperatively and may help to reduce blood loss during transplantation; however, a misplaced TIPS in the vena cava or portal vein can be a life-threatening complication during LT. In these situations, TIPS removal is difficult and may lead to massive hemorrhage if vascular control of the native vessels cannot be achieved. Temporary portocaval shunts can also be used to decompress the portal circulation during hepatectomy.
In the presence of severe portal hypertension and underlying coagulopathy, the infusion of fresh frozen plasma, cryoprecipitate, and antifibrinolytic agents can help with hemostasis during LT. These modalities cannot supplant the need for sound surgical technique with adequate control of all surgical bleeding sites. During surgery it is essential to have good communication with the anesthesiology team regarding blood loss assessment of coagulopathy. We have begun to use a scoring system as a tool to communicate with anesthesia for when a patient warrants coagulation assessment for moderate coagulopathy and empiric transfusions for uncontrolled coagulopathy.
An important transition in hemostasis management occurs when the native liver is removed and the new graft undergoes early reperfusion. It has been well appreciated since the origins of LT that recipients develop a hyperfibrinolytic state (excessive clot degradation from elevated levels of tissue plasminogen activator [t-PA]) when the native liver is removed. However, after reperfusion, the majority of patients correct their fibrinolytic state. This is likely because of the rapid clearance of t-PA by the liver, which has a half-life of 5 minutes in humans because of multiple hepatic endothelial receptors. A lack of clearance of t-PA after graft reperfusion can be associated with massive bleeding, which is also exacerbated by metabolic disturbances. In these scenarios, the use of antifibrinolytics may be of value to improve hemostasis control. Bleeding observed after reperfusion may be related to poor initial graft function but also has been related to the release of heparin and heparin-like substances from the graft. Protamine administration is rarely used, and this heparin effect can often be mediated by plasma transfusion. When medical strategies fail to halt reperfusion fibrinolysis, temporary intra-abdominal tamponade with laparotomy packs provides the most conservative management, with temporary vacuum-pack closure and plans for subsequent reexploration. If the graft functions well and the patient responds to core temperature warming and continued resuscitation, packs usually can be removed within 24 to 48 hours. Prophylaxis against intra-abdominal sepsis with parenteral antibiotics is of unproven benefit but may be prudent in the patient with immunosuppression.
After reperfusion in grafts that demonstrate good function, de-escalation of hemostatic blood products is warranted. TEG and ROTEM have comparable performance for monitoring of coagulation during LT. Routine use of both TEG and ROTEM have been associated with using less plasma during surgery in randomized clinical trials (RCTs) but are not associated with a reduction in RBC transfusions. Almost all LT recipients will develop inhibition of their fibrinolytic system 2 hours after reperfusion. This fibrinolysis shutdown persists beyond postoperative day 1 in the majority of LT patients. With a lack of capacity to break down endogenous clots, it is essential to not overtransfuse recipients during this time frame. Fibrinolysis shutdown has been associated with decreased graft survival and postoperative thrombotic complications. Platelet transfusions have been associated with decreased survival after transplantation, which has been attributed to the development of postoperative lung injury.
The decision to transfuse hemostatic blood products on LT patients postoperatively should be guided predominantly by clinical judgement with laboratory confirmation of stable hemoglobin levels. Failure to identify coagulopathy will result in a return trip to the OR. There are no definitive thresholds for transfusions. Our group, however, uses a target hemoglobin of 10 to promote platelet margination to optimize hemostasis with thrombocytopenia; platelet transfusion for an absolute platelet count of less than 30,000, or TEG MA 50 in patients with dropping hemoglobin; and plasma transfusion for TEG R time greater than 10 minutes or INR greater than 2 with dropping hemoglobin, and cryoprecipitate for a Clauss fibrinogen less than 100 or TEG angle less than 55 if hemoglobin is dropping. Patients with normal coagulation parameters and dropping hemoglobin should prompt concerns for surgical bleeding. Our group often used the threshold of more than 6 hours of RBC postoperative bleeding as an indicator for the need to return to the OR.
Numerous conditions can interfere with the initial function of the allograft after LT, including donor-related, procurement-related, and recipient-related factors. Donor-related factors that can affect graft function adversely include hemodynamic instability, poor nutritional status, extremes of age, drug toxicity, and steatosis. There is a spectrum of graft dysfunction after LT ranging from early allograft dysfunction (EAD) to the most extreme, primary nonfunction (PNF). Patients with PNF require relisting for transplantation, and the mortality rate can be as high as 50% even after re-transplantation. PNF occurs in 2% to 6% of LTs and because the patients do not retain hepatic function, clinical manifestations are often not subtle, including altered mental status, persistent acidosis, hypoglycemia, coagulopathy, and hemodynamic instability. A major risk factor for PNF is steatosis of the donor liver in addition to female donor, older donor, and long cold and warm ischemia times. Laboratory values concerning for PNF include serum transaminase levels greater than 8000 IU/L within the first 24 to 48 hours in association with diminished bile and urine output. In our practice, postoperative trends in the serum bilirubin and international normalized ratio (INR) have been the most reliable predictors of graft function and outcome. Laboratory studies drawn immediately after surgery serve as the baseline; serum bilirubin and INR should plateau and then trend toward normalization within days with good graft function; any increase in the serum bilirubin and INR portends a worse prognosis and may represent PNF, especially if this occurs rapidly. An elevated INR that neither increases nor decreases may suggest primary graft dysfunction; recovery usually occurs with time, if no additional insults occur, such as infection or rejection.
There are numerous biomarkers and tests that can be performed to assess graft function after transplanatation ; however, there is no definitive test that can differentiate slow graft function that will improve over time from PNF for patients that are extremely sick in the immediate postoperative period. In addition to trends in the bilirubin and INR, a clinical assessment can be helpful in identifying patients with graft dysfunction or nonfunction. Resolution of hepatic encephalopathy, adequate urine production, and absence of metabolic acidosis are reassuring in the early postoperative period. In patients who have received significant quantities of blood products, the development of metabolic alkalosis may be a sensitive indicator of early graft function. Such indicators are based on the ability of the liver graft to process citrate in the administered blood products to bicarbonate ; failure to metabolize citrate to bicarbonate may reflect early allograft dysfunction.
In patients with progressive graft dysfunction or nonfunction, early consideration for relisting with a higher urgency (status 1) may be the only way to salvage the patient and prevent mortality. Similar to patients that experience hypoglycemia with acute liver failure, death usually occurs within hours. In this situation, aggressive metabolic supportive measures are needed to prevent bleeding complications, maintain acid-base status, and prevent permanent brain injury from cerebral edema or a coagulopathic bleed.
PNF, as previously mentioned, is at the extreme of graft dysfunction after transplant. EAD is a broader term that captures LT recipients who do not necessarily require re-transplantation but have less favorable outcomes. EAD occurs in roughly 25% of recipients , and is associated with up to a 7-fold increased risk of early graft loss and 10-fold risk of mortality after transplant. Although clinical gestalt for slow graft function is made after reperfusion of the liver, the time frame for diagnosis of EAD with objective laboratory data is commonly calculated at 7 days after LT. Risk factors for EAD in adults include high levels of macrosteatosis, donor location, donor weight, DCD donor, recipient obesity, recipient HCC, severity of postreperfusion syndrome, warm and cold ischemia time, operative time, and the amount of transfused fresh frozen plasma. Because EAD presents a spectrum of graft dysfunction after surgery, newer definitions of EAD have included a continuous variable termed the model for early allograft function (MEAF), which can grade the severity of dysfunction and ranges from 1 to 10 based on postoperative day 3 laboratory values. Each increase in MEAF score was associated with progressively lower graft survival time. There are numerous other definitions of EAD in the literature that have variances in how well they perform. We currently prefer to use the Othoff et al. definition because of its simplicity and agreement between LDLT and cadaveric liver recipients.
In LDLT, graft size was an initial concern for development for EAD. SFSS can be a significant cause of live-donor graft dysfunction and graft loss, but additional evidence has suggested the importance of pressure and flow as major contributors of the syndrome rather than size alone. , With subsequent work, it has been demonstrated that a graft to body weight ratio of 0.8 can be transplanted successfully. A key prognostic factor for good graft function with smaller-sized livers is achieving a portal systemic gradient less than 15 mm Hg. Most recently, EAD serves as a better predictor for long-term graft function than small graft size. Increasing age of the donor, the BMI of the donor, left liver grafts, and enlarged spleen in the recipients are risk factors for LTLD EAD.
EAD has been proposed as an appealing target to improve graft outcomes, yet specific mechanisms driving this process remain unclear. , There has been one successful RCT targeting EAD. In this study, 65 patients were randomized to steroids or standard of care if they met the EAD definition of a bilirubin greater than 10 on postoperative day 7. The steroid intervention group received methylprednisolone intravenously once daily for 5 days. Both arms of the trial received 250 mg of oral ursodeoxycholic acid three times a day for this duration. At the end of 2 weeks, the treatment arm had lower alkaline phosphatase levels, low bilirubin levels, and shorter hospital length stay. There were not reported increased rates of infections or other steroid-related complications.
Vascular complications are a major source of morbidity and graft loss in LT patients, especially those who receive live-donor grafts. Arterial complications, of which HAT is the most common, account for 64% to 82% of the vascular complications encountered. The overall incidence of HAT is 1.6% to 8% in various adult series, but it can be 15% to 26% in pediatric patients. , The incidence of HAT in LDLT varies widely and is influenced by the type of graft, surgeon experience, and donor anatomy. Risk factors for HAT include donor cytomegalovirus (CMV) positivity in a CMV-negative recipient, prolonged operating time, redo LT, arterial conduit, variate arterial anatomy, and low surgical volume. Preoperative transarterial chemoembolization (TACE) has been associated with complications related to the hepatic artery but not specific to HAT.
HAT is divided into early and late forms. The time cut off for early HAT remains an academic debate and can range from 7 to 100 days. Regardless, UNOS policy defines early HAT as lasting less than 7 days with an AST of greater than 3000 and INR greater than 2.5 or acidosis (arterial PH < 7.3 or lactate > 4) to qualify for status 1A. Early HAT of less than 14 days regardless of other factors qualifies the recipients as a MELD 40. An attempt at revascularization should be conducted in the setting of early HAT because salvage is possible, and the alternative is re-transplantation, which uses another organ and the timing remains of an offer for the organ is indeterminate. Alternatively, late HAT will typically present as a biliary complication or identified incidentally on imaging for an alternative indication. Bilomas are the most common presenting symptom of late HAT and can be salvaged with percutaneous drainage and antibiotics. Late HAT has a lower frequency than early HAT and tends to have a better prognosis with a lower rate of requiring re-transplantation. Asymptomatic individuals can often be overserved and followed for biliary complications. Risk factors for late HAT include CMV infection and variant arterial anatomy.
Doppler ultrasound (US) is the best screening method for HAT and should be used liberally in the first 2 weeks after transplantation with any change in graft function or significant elevation in bilirubin or transaminases. Because collateral blood flow through the gastroduodenal artery can result in a false-negative result, care must be taken to establish arterial flow within the hepatic parenchyma. In cases of suspected HAT revealed by US, confirmation should be made by celiac arteriography ( Fig. 111.1 ), multiphase computed tomography (CT), or operative exploration. Failure to make a rapid diagnosis can result in hepatic necrosis and graft loss.
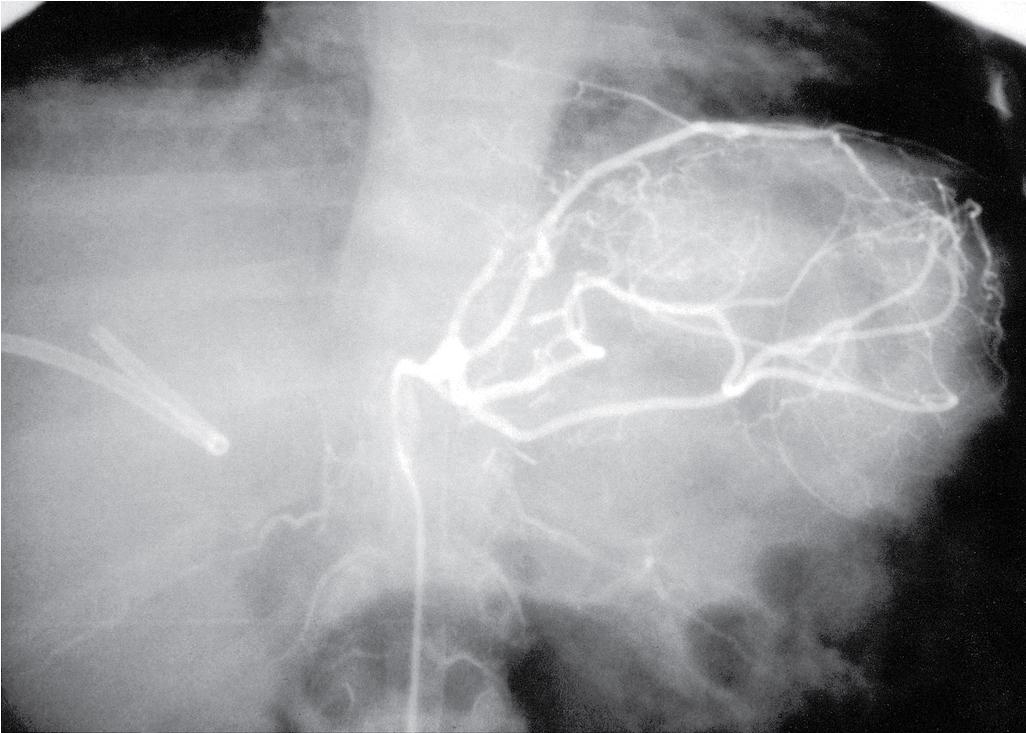
Intimal dissection of the recipient hepatic artery down to the origin of the celiac axis is a common cause of intraoperative HAT. In these cases, immediate reconstruction with a donor iliac allograft is indicated ( Fig. 111.2 ). Under no circumstances should the patient leave the OR without a completely revascularized graft. When a donor iliac allograft is unusable or unavailable, an autogenous saphenous vein graft should be used. Rarely, an artificial conduit made from Dacron or expanded polytetrafluoroethylene can be used.
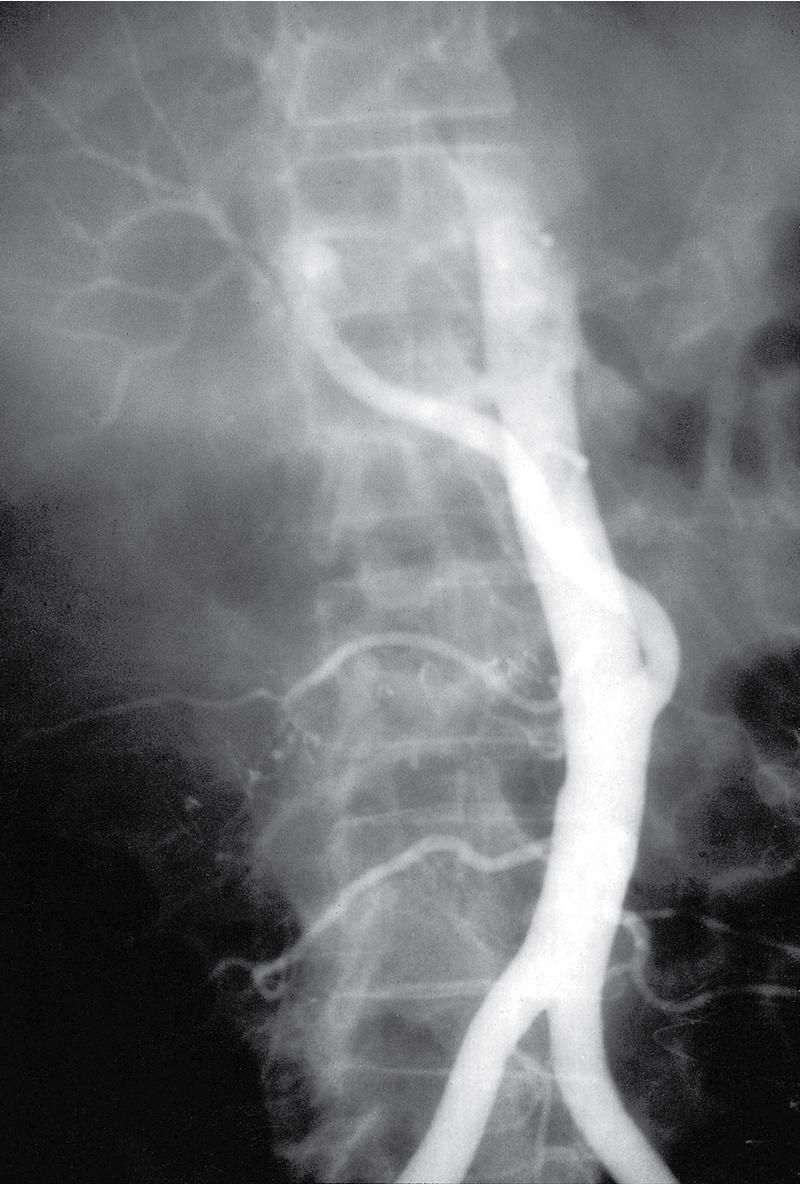
The clinical presentation of HAT observed postoperatively ranges from a completely asymptomatic patient with minimal alterations in liver function to a critically ill patient with fulminant hepatic necrosis. A more common presentation of HAT is with postoperative biliary complications, including leaks and stricture formation. Treatment for HAT in the early postoperative period is the same, whether symptoms are present or not; rapid reestablishment of arterial inflow should be attempted, which generally requires urgent operation with arterial reconstruction by using a donor iliac artery allograft or autogenous graft material. Some authors have attempted thrombolysis using fibrinolytics followed by stent placement, but this is not common practice and durable successful results remain limited. Most often, hepatic artery intimal dissection is not amenable to thrombolytic therapy, and attempts at systemic thrombolysis waste valuable time and resources and worsen outcome. The majority of patients (50%–70%) will ultimately require re-transplantation.
Portal vein thrombosis (PVT) observed before surgery was previously considered an absolute contraindication to orthotopic LT (OLT). Although this problem no longer precludes successful LT, its presence may substantially increase the surgical complexity and perioperative morbidity. Depending on the patient population, PVT can be found in 5% to 25% of LT recipients. The severity of thrombosis ranges greatly between patients. Portal vein inflow is essential for successful LT and imaging with duplex of CT before implant can be an invaluable resource for intraoperative planning. Scoring systems have been created for intraoperative planning. An example is from Yerdel et al. in which grade 1 is less than 50% PVT, 2 is greater than 50% with minimal superior mesenteric vein (SMV) obstruction, 3 is 100% PVT, and 4 is 100% PVT with SMV thrombosis. The authors advocate that intraoperative thrombectomy can be used for grades 1 and 2, a jump graft can be used for grade 3, and grade 4 is a relative contraindication to transplant unless bypass is achievable to a nonmesenteric venous source. Reconstruction of the portal can be accomplished using a donor iliac vein allograft anastomosed between the SMV and liver allograft portal vein. Successful reconstructed inflow for grade 4 has been reported in more than 75% of patients but is prone to stenosis. Living-donor grafts that have relatively short portal vein segments can be difficult to reconstruct, and some institutions consider PVT an absolute contraindication to LDLT.
PVT observed after transplantation is a rare complication that can occur in the immediate postoperative period, usually for technical reasons, such as incomplete thrombectomy or twisting of the anastomosis. Early PVT has a poor prognosis with a mortality rate of 50%, predominantly because of later complications after attempted repair. Risk factors for early PVT include postoperative platelet transfusions, RBC transfusion, and PVT before transplant. PVT observed several months to years after transplantation usually results from intimal hyperplasia with gradual cavernous transformation with collaterals. A high index of suspicion is needed to make the diagnosis. Accumulation of ascites, splenomegaly, or the presence of varices after transplantation should prompt investigation. Early thrombosis is best treated with reoperation, thrombectomy, and systemic anticoagulation. Partial PVT can alternatively be approached with systemic heparinization and followed with serial ultrasounds to evaluate for regression. The treatment of late thrombosis and portal vein stenosis has been reported to be increasingly successful with interventional procedures with angioplasty, stent placement, and anticoagulation. Selective shunting procedures can sometimes be considered in the setting of control variceal hemorrhage with adequate liver function.
Inferior vena cava (IVC) obstruction is a rare complication that occurs in 1% of patients after LT. , Historically, the donor IVC was anastomosed with the suprahepatic and infrahepatic IVC. The piggyback transplantation , with the anastomosis of the donor suprahepatic IVC to the confluence of the recipient middle and left hepatic veins, leaving the recipient IVC in situ, has become increasingly popular. The piggyback technique has been associated with reduced blood loss, shorter warm ischemia times, and lower rates of acute kidney injury after LT. However, there have been reported concerns for an increased rate of outflow obstruction because of anastomotic strictures or kinking at the hepatic veins, which tend to have high frequencies in LTLD pediatric LTs.
Early after transplant, high outflow obstruction can be confused with increased central venous pressure (CVP) because of high volume resuscitation manifesting as DGF, ascites, and peripheral edema. This nonanatomic source of outflow obstruction can be treated with diuretics when tolerated by the patient, targeting CVP below 11. When CVP has been ruled out as a source of venous outflow obstruction, the patients can be evaluated with venography of the vena cava with segmental pressures above and below the anastomosis. A gradient of more than 10 mm Hg in the setting of clinical symptoms of lower extremity edema, ascites, and slow hepatic function can warrant intervention, which often includes angioplasty. Later-onset anastomotic stenosis can be treat with repeat angioplasty and stenting with durable long-term results.
An additional cause of outflow obstruction includes thrombosis. Treatment of IVC thrombosis usually depends on the cause. Direct surgical removal is difficult in a critically ill patient and requires extensive mobilization of the right colon and small bowel mesentery to facilitate exposure. Nonoperative management of IVC thrombosis was successful in our experience and that reported by others. Our experience suggests that a more conservative management algorithm that uses invasive radiologic procedures and systemic anticoagulation can treat this complication satisfactorily. Thrombolytic therapy can also be adjunctive in resolving “fresh” thrombus formation.
In some cases, high-grade venous outflow obstruction cannot be corrected, and TIPS may be used as a bridge to mediate ascites and bleeding complications for patients that are candidates for re-transplantation. However, the use of TIPS post-transplantation is associated with accelerated decline in hepatic function and is not a durable therapy because the patient will progress to need another LT. An additional cause of symptoms that can mimic outflow obstruction includes veno-occlusive disease sinusoidal obstruction syndrome (VOD/SOS). This disease process is believed to be related to acute rejection and drug toxicity, which often occurs months after transplant. VOD/SOS was originally described in bone marrow transplants and has more recently been identified as a rare cause of liver failure after transplantation. VOD/SOS is diagnosed via liver biopsy and demonstrates fibrous obliteration of the small hepatic veins. The most effective treatment in bone marrow patients is Defibronate. Success with LT recipients is not as clear, and the medication that activates the fibrinolytic system poses a risk of bleeding. Alternative strategies include steroids, increasing immunosuppression, and TIPs as a last-line therapy.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here