Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital anomalies of the duodenum often present with obstructive symptoms and consist of intrinsic obstructing lesions, such as duodenal atresia and stenosis, or extrinsic lesions that affect the duodenum, such as midgut volvulus or Ladd bands, annular pancreas, preduodenal portal vein, and duplications cysts; intrinsic and extrinsic lesions may coexist in the same patient.
Duodenal atresia and stenosis represent a spectrum ranging from complete to partial obstruction, and corresponding presentation that ranges from prenatal life to late childhood or even adulthood. Duodenal atresia represents approximately one-half of all intestinal atresias, with a reported incidence of approximately 1 : 7,000 live births. The classification of duodenal atresia by Gray and Skandalakis is most often cited and is similar to that of more distal atresias: type I atresia (approximately 90% of cases) consists of a membrane that may obstruct completely or partially; in type II atresia, the segments are connected by a fibrous cord; in type III atresia, there is a gap separation with a mesenteric defect.
The lumen of the duodenum is obliterated during the 4th to 6th weeks of gestation due to rapid cell division, and it normally recanalizes by the 12th week; duodenal atresia is believed to result from failure of this recanalization process. This differs from atresia of the small and large bowel, which is believed to result from a vascular accident later in gestation. The recanalization error theory was first suggested in 1900 but is very difficult to confirm. Recent studies suggest that disruptions in fibroblast growth factor receptor 2 (FGFR2) function and retinoic acid signaling pathways may play a role in the development of duodenal and possibly other intestinal atresias.
In approximately half of patients, duodenal atresia does not occur in isolation but rather as part of a syndrome or association, such as Down or esophageal atresia. Polyhydramnios, resulting from the proximal bowel obstruction, is seen in 30% to 50% of patients and leads to premature birth in half. Thus the clinical presentation is often modified by associated anomalies and by prematurity.
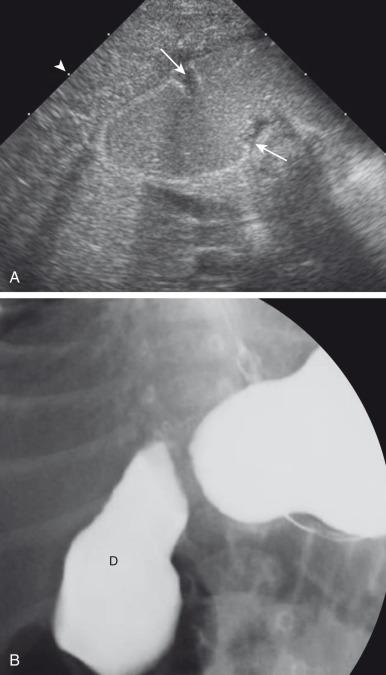
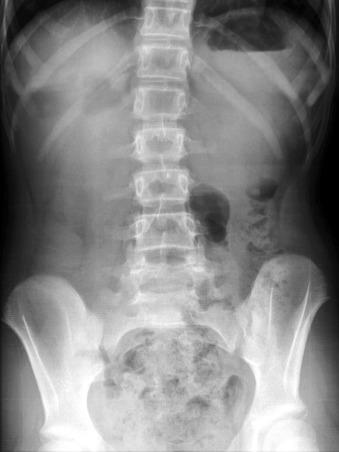
Postnatally, infants with atresia typically present with vomiting within the first 24 hours of life; however, one-third of infants presenting in the neonatal period will have severe stenosis. The infants who have not been diagnosed prenatally will present with bilious vomiting in greater than two-thirds of the cases, in which the obstruction is sited distal to the ampulla of Vater. However, the obstruction occurs proximal to the ampulla of Vater in as many as 23% of patients, and these will present with nonbilious vomiting. Thus the presentation of infants with duodenal stenosis can overlap that of infants with the much more common diagnosis of pyloric stenosis and may be initially encountered at ultrasound ( Fig. 102.1 ). Because the duodenal obstruction is proximal, abdominal distension is not a typical finding, although epigastric fullness may be present secondary to the dilated stomach and duodenum.
Patients with less severe forms of duodenal stenosis may not come to medical attention until later in life, depending on the degree of obstruction. In such patients, presentation may be relatively nonspecific, such as failure to thrive, or rarely pancreatitis, secondary to reflux into the pancreatic duct, proximal to the site of obstruction. Late presentation secondary to obstruction may be precipitated by ingestion of a foreign body that fails to pass and may exacerbate the degree of obstruction, leading to abdominal pain and/or vomiting ( e-Fig. 102.2 ). Annular pancreas and malrotation are seen in approximately one-third of cases.
Associated anomalies are common in patients with duodenal atresia/stenosis. Approximately 25% to 40% of infants with duodenal atresia/stenosis have Down syndrome; conversely, approximately 4% of infants with Down syndrome have duodenal obstruction. Hirschsprung disease has been reported in approximately 1% to 3% of patients with duodenal atresia and Down syndrome. Approximately 5% of infants with esophageal atresia, with or without fistula, will also have duodenal atresia. Heterotaxy with left isomerism/polysplenia has been reported in patients with duodenal diaphragm/intraluminal diverticulum. Duodenal atresia can also be part of several familial syndromes with autosomal inheritance, such as Feingold syndrome (autosomal dominant) and Fryns syndrome (autosomal recessive).
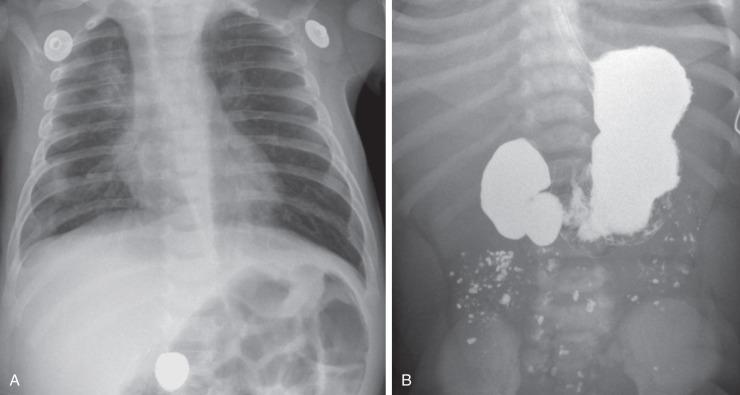
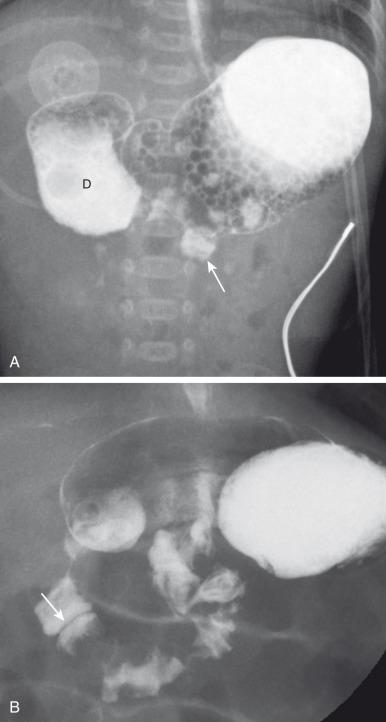
Abdominal radiographs are the starting point in the evaluation of a child with suspected gastrointestinal (GI) obstruction. In the neonate with duodenal atresia, the abdominal radiograph demonstrates the classic “double bubble” representing the dilated stomach and duodenum. Because obstruction has been present in utero, the obstructed proximal duodenum is typically large, approximately one-half to one-third the size of the stomach ( Fig. 102.3A ); at times the pylorus is wide open, and the two bubbles are not distinctly separated ( Fig. 102.3B ) but should still be recognized. This appearance on the abdominal radiograph is diagnostic, and confirmatory contrast examinations are unnecessary. Rarely, air can be seen distally in a patient with complete atresia; this occurs when the atresia is flanked by the branches of an anomalous bifid common bile duct, with separate insertions into the duodenum above and below the point of atresia. Air or contrast may be seen refluxing into the anomalous ducts, which allow the contents of the obstructed proximal duodenum to course into the distal duodenum, bypassing the point of obstruction ( Fig. 102.4 ). In patients with duodenal atresia associated with esophageal atresia with a distal fistula, plain films will demonstrate the double bubble and are diagnostic ( e-Fig. 102.5 ); perforation may occur if these infants need to be mechanically ventilated.
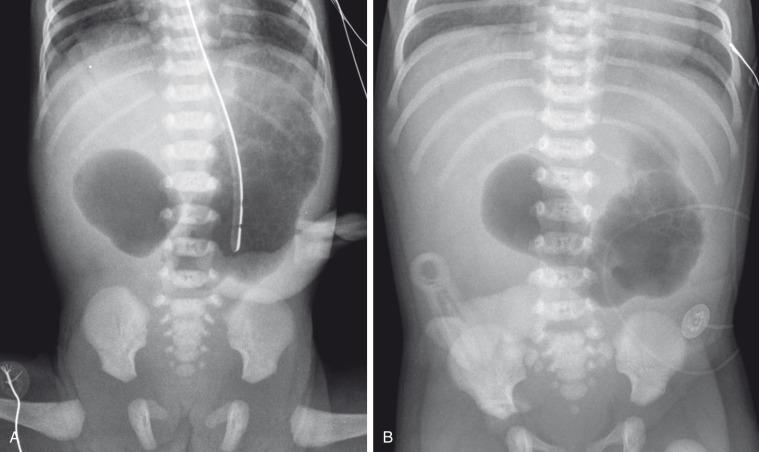
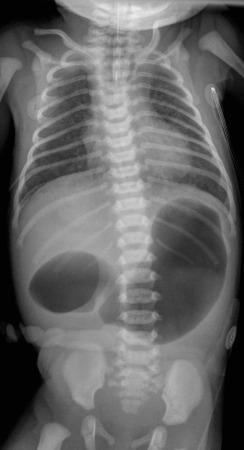
In patients with duodenal stenosis, distension of the stomach and proximal duodenum and decrease in distal gas is commensurate with the degree of obstruction present. However, if the stenosis is mild or the stomach is decompressed via enteric tube, the plain abdominal radiographs may be nonrevealing or even misleading ( e-Fig. 102.6 ).
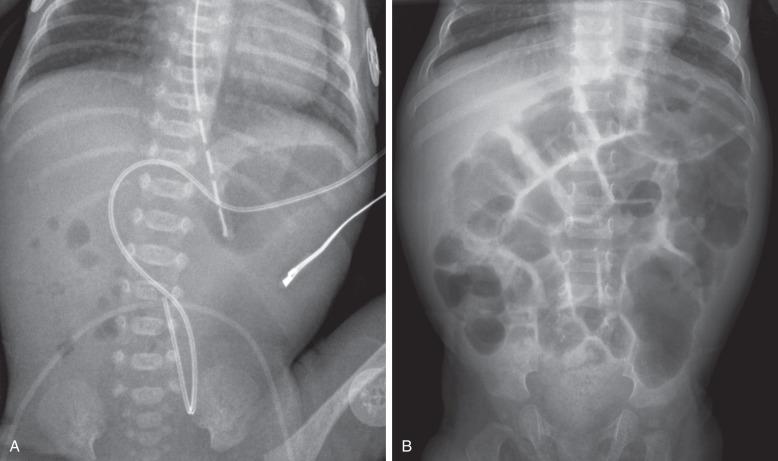
An upper gastrointestinal (UGI) series may be needed to diagnose duodenal stenosis if potentially associated malrotation is a concern or if surgery is to be delayed ( Fig. 102.7 ). Contrast enema has been done in the past to assess the rotation of the bowel, but this examination is not helpful if it is normal or if the cecum is high-riding; cross-sectional imaging may be done in such cases for further evaluation, discussed in greater detail later in this chapter.
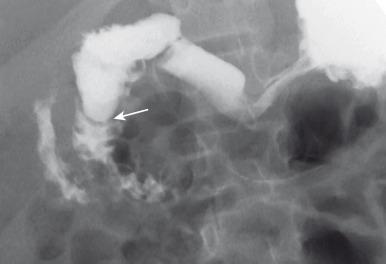
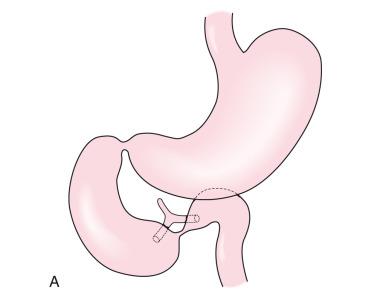
On the UGI examination, patients with duodenal stenosis will demonstrate a partial obstruction ( Fig. 102.8 ); when a web is present, the membrane may be seen as a thin linear filling defect (see Fig. 102.8B ). In older patients, the elongated membrane may have the appearance of an intraluminal diverticulum or “windsock” (see Fig. 102.7 ). As noted earlier, some children with duodenal stenosis may present after ingestion of a foreign body too large to pass through the narrowed canal or orifice (see e-Fig. 102.2 ). In patients with duodenal atresia simulating duodenal stenosis secondary to bypass of the obstruction by anomalous ducts, contrast may be seen within the ducts, as discussed earlier (see Fig. 102.4B ).
Treatment of duodenal atresia and stenosis is surgical. When Ladd described surgical correction of duodenal obstruction in 1932, the reported mortality was approximately 40%. In 1990, Kimura et al. described the technique of diamond-shaped anastomosis, which has become standard procedure for open repair, with a mortality of 5% to 10% now largely due only to associated anomalies, particularly those involving cardiac lesions. More recently, laparoscopic duodenoduodenostomy has been introduced, with increasingly good results, and reported improvement in return of bowel function, resulting in reduced length of hospital stay.
Annular pancreas refers to encirclement of the descending portion of the duodenum by pancreatic tissue. The prevalence of annular pancreas is not known, as asymptomatic cases are not always identified; among adults, autopsy series prevalence varies between 1–15 : 100,000 adults, while endoscopic retrograde cholangiopancreatography (ERCP) studies in symptomatic patients indicates a prevalence of 1–4 : 1000. In children, the incidence is estimated at approximately 1–12 : 15,000 births, with a 2 : 1 male:female ratio. Annular pancreas may result in extrinsic duodenal obstruction; however, most cases of duodenal obstruction with annular pancreas are most likely the result of an associated intrinsic duodenal abnormality.
The pancreas arises as a ventral and larger dorsal bud from the duodenum. Normally the ventral bud rotates and fuses with the dorsal bud. When the ventral bud becomes tethered to the duodenum before the onset of the rotation, or if the ventral bud fails to rotate completely before fusion, the result is the annular pancreas. The pancreatic annulus, the portion surrounding the duodenum, frequently has a separate duct entering the duodenum, opposite the ampulla of Vater. Duodenal contents may reflux through this duct into the annulus. Studies in transgenic mice have shown that the annular tissue is derived from the ventral bud; molecular studies have implicated the hedgehog signaling pathway and the transmembrane 4 superfamily 3 gene (TM4SF3) .
Children present with vomiting or feeding intolerance, with a median age of 1 day at diagnosis. Presentation in older children who do not have duodenal obstruction is similar to that in adults, with pancreatitis and jaundice. Associated anomalies are common, and these include malrotation, esophageal atresia, anal atresia, and cardiac defects, particularly in patients with trisomy 21 and Cornelia de Lange syndrome ; annular pancreas has also been reported in patients with heterotaxy.
Plain films will show duodenal dilatation in those patients in whom there is intrinsic and/or extrinsic obstruction. UGI will show narrowing of the descending portion of the duodenum ( Fig. 102.9A ). On ultrasonography, a ring of pancreatic tissue may be seen about the descending portion of the duodenum, although the duodenum might be difficult to follow, and the appearance may resemble a mass at the level of the head of the pancreas ( Fig. 102.9B ). Computed tomography (CT) likewise can show the pancreatic tissue surrounding the duodenum ( Fig. 102.9C and D ), probably more clearly if aided by positive luminal contrast in the duodenum. Magnetic resonance imaging (MRI) also may show the ring of pancreatic tissue ( Fig. 102.9E ), but magnetic resonance cholangiopancreatography (MRCP) will show more definitive findings outlining the annular course of the pancreatic ducts ( Fig. 102.9F ), analogous to the findings seen at ERCP ( e-Fig. 102.10 ).
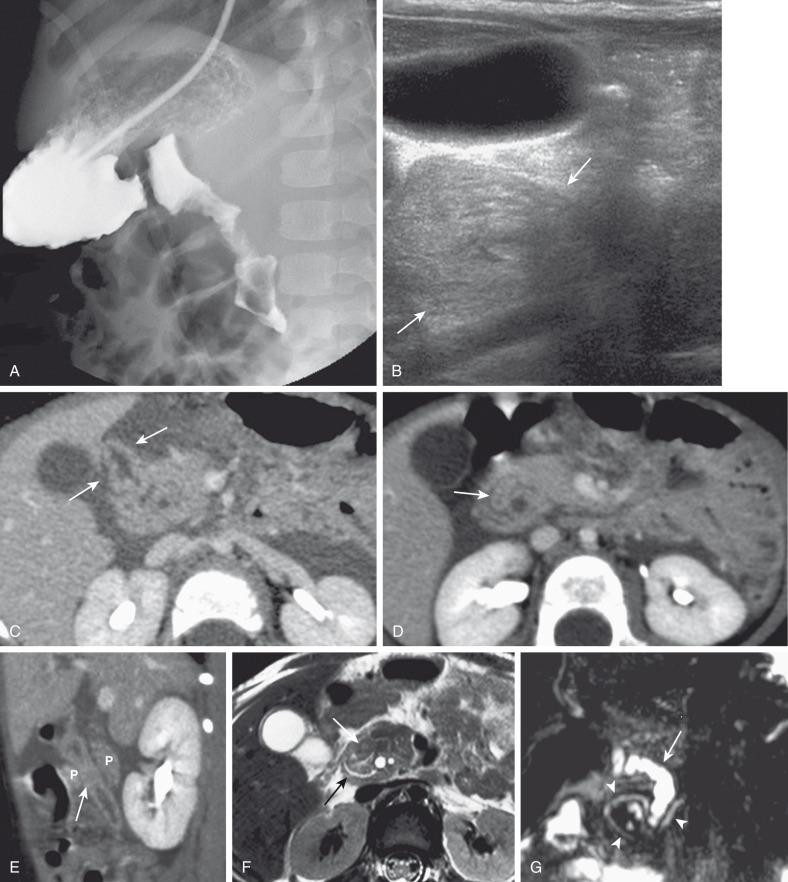
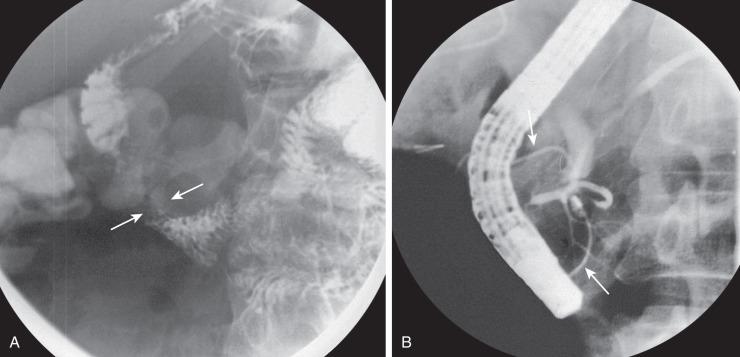
As in the case of intrinsic duodenal obstruction, treatment is surgical bypass; interruption or division of the pancreatic tissue around the duodenum is not indicated, due to the likelihood of postoperative leakage or pancreatitis. More recently, laparoscopic surgery has also been undertaken with good results in experienced hands.
The embryonic midgut, which is supplied by the superior mesenteric artery and extends from the distal descending duodenum to the distal transverse colon, undergoes 270 degrees of counterclockwise rotation (as viewed from the front) through a complex series of steps to achieve its final position, with the duodenojejunal junction in the left upper quadrant and the cecum in the right lower quadrant.
The term malrotation indicates that the normal rotation of the midgut was not completed, and therefore represents a misnomer, as the term “incomplete rotation” would more accurately characterize the resulting spectrum of abnormalities, which affect both the duodenojejunal and the cecal poles of the midgut, singly or in unison. The prevalence of malrotation is difficult to determine, inasmuch as asymptomatic cases may not be recognized; it has been most often quoted as 1 : 500 live births, which seems excessive as that is similar to the incidence of pyloric stenosis (2–5 : 1000). More recent information suggests that malrotation is far less common, approximately 3.9 per 10,000.
To understand the etiology of malrotation, or incomplete rotation, the normal process of rotation must be understood. The gut begins as a straight tube, with the duodenojejunal junction and the cecum along a straight line. As the gut begins to grow, it forms a primary loop about the axis of the superior mesenteric artery, with the apex at the omphalomesenteric duct: the proximal portion is the prearterial (duodenojejunal) limb, and the distal portion is the postarterial (ileocecal or cecocolic) limb. Postnatally, the site of the Meckel diverticulum marks the junction of the pre- and postarterial limbs. Initially, the duodenum and cecum rotate 90 degrees counterclockwise, such that the duodenojejunal junction comes to lie in the right upper quadrant, and the cecum comes to lie in the left lower quadrant. At approximately 6 weeks of gestation, continued growth of the intestinal tube results in herniation of the bowel into the umbilical cord and a second counterclockwise turn of 90 degrees at the duodenojejunal junction. By the 10th week of gestation, the bowel begins its return into the abdominal cavity, beginning with the prearterial segment, and the duodenum undergoes its final 90 degrees of rotation and fixation at the ligament of Treitz in the left upper quadrant; the cecum similarly undergoes its final 180 degrees of rotation, to terminate in the right lower quadrant, and undergo fixation to the posterior peritoneum through shortening and resorption of its dorsal mesentery. The bowel is thus suspended from a mesentery, which is attached to the posterior abdominal wall, and which extends from the left upper to the right lower quadrants ( Fig. 102.11 ). The configuration of the duodenal C-loop mirrors the 270 degrees of rotation that it has undergone. The final steps in bowel rotation and fixation involve resorption of the dorsal mesenteries of the ascending and descending colon and elongation of the ascending colon with descent of the cecum, processes that are ongoing until several months of postnatal life.
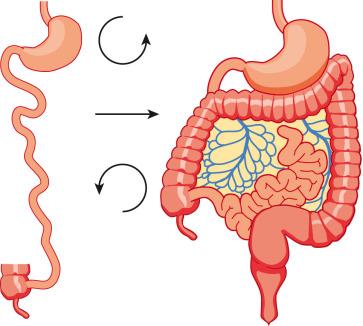
Malrotation arises when the process of normal rotation is arrested, and this can occur at any point along the 270-degree arc of rotation of the duodenojejunal or cecal segments. If it is arrested after the initial 90 degrees of counterclockwise rotation, the duodenojejunal junction and the small bowel will be located on the right side of the abdomen, and the cecum and the rest of the colon will be located in the left side of the abdomen, with the terminal ileum entering the cecum from right to left. Despite the fact that the initial 90 degrees of counterclockwise rotation have occurred in both the duodenojejunal junction and the cecum, this arrangement is nevertheless known as nonrotation , because of the parallel appearance of the small and large bowel and the absence of the duodenal C-loop; this type of malrotation is characterized by a relatively long mesenteric pedicle. Progression of the rotation process beyond the nonrotation stage brings the duodenojejunal junction and the cecum into closer proximity, and arrest along this spectrum typically results in a shorter mesenteric pedicle from which the bowel is suspended ( Fig. 102.12 ), which increases the risk for midgut volvulus. Reversed rotation is a rare form of malrotation, in which the gut undergoes 90 degrees of clockwise rotation, instead of the normal 270 degrees of counterclockwise rotation. Starting from the initial straight tube, this rotation results in the location of the duodenojejunal junction in the left upper quadrant and of the cecum in the right lower quadrant. However, in these cases, the ceco-colic loop returns into the abdomen first, and this results in location of the transverse colon, not the duodenum, between the aorta and the superior mesenteric artery.
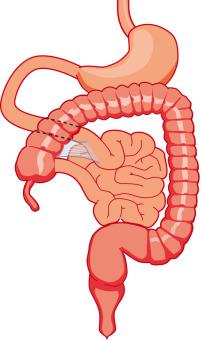
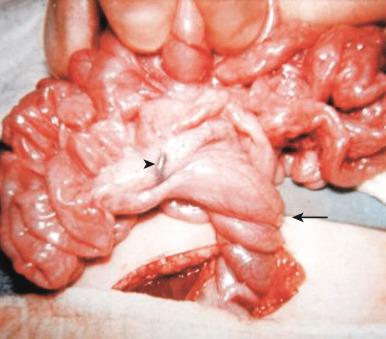
There are also several disorders of fixation that commonly occur in patients with malrotation. Ladd bands are the result of abnormal mesenteric attachments that are formed in patients in whom the rotational process is incomplete. These bands, named after Dr. William E. Ladd, typically extend from the liver edge to the malrotated cecum, crossing over and causing obstruction and kinking of the duodenum ( e-Fig. 102.13 ); however, they can rarely occur more distally, crossing over the jejunum, ileum, or colon.
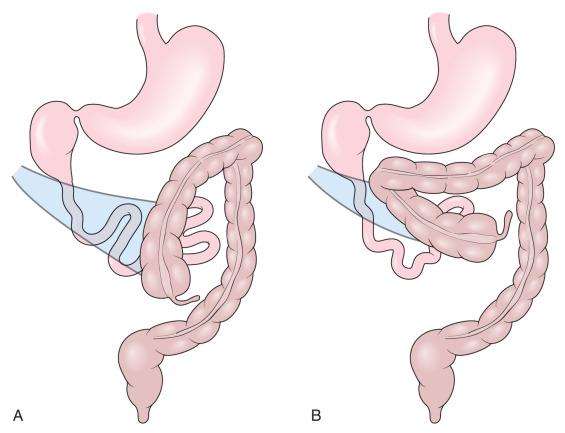
Internal hernias are the result of abnormal or incomplete fixation of the ascending or descending portions of the colon, resulting in a defect through which the bowel can herniate. Herniation into a defect in the ascending colon results in a right paraduodenal or mesocolic hernia, whereas herniation into a descending colonic defect results in a left paraduodenal or mesocolic hernia. In persons with normal bowel rotation, failure of complete fixation of the ascending colon, cecum, or sigmoid colon can result in volvulus of these structures, typically not occurring until late adult life.
The presentation of patients with malrotation depends on whether there is obstruction, whether the obstruction is acute or chronic, and on associated malformations. Presentation may occur in utero, and the neonate may demonstrate short bowel secondary to in utero bowel necrosis and resorption, or with apple-peel type of atresia. In asymptomatic patients, malrotation may be discovered in the course of evaluation for other clinical concerns.
It is important to differentiate the midgut volvulus that occurs in patients with malrotation from the segmental volvulus that can occur in multiple other conditions, which involves only a small segment of small or large bowel, many of which will be discussed later. In midgut volvulus, the duodenum rotates clockwise about the axis of the superior mesenteric artery ( Fig. 102.14 ) and is obstructed at its third portion, distal to the ampulla of Vater. Because approximately 60% to 80% of patients with midgut volvulus present in the first month of life, the typical presentation is that of a previously well neonate who experiences the acute onset of bilious vomiting. The infant may experience crampy abdominal pain, which could be confused with colic. If the obstruction is significant, the abdomen may become initially scaphoid after distal intestinal contents are evacuated; this lack of distension, together with lack of abdominal tenderness during this early stage, may lead to underappreciation of the urgency of the condition. Vascular compromise may lead to intraluminal bleeding and hematochezia, seen in 10% to 15% of patients with volvulus. As ischemia of the midgut continues, the abdomen becomes distended and firm, with physical signs of peritonitis, and the patient will present in shock with cardiovascular collapse.
Diagnosis of malrotation and midgut volvulus is even more challenging in premature infants. Preterm infants are less likely to present with bilious vomiting and instead display nonspecific signs and symptoms, including apnea and gastric retention, culminating in abdominal distension and shock with necrotic bowel at operation.
Partial or intermittent midgut volvulus tends to present more insidiously, with an average duration of symptoms of 28 months or more before the diagnosis. There is usually a history of abdominal pain, which is often vague and intermittent but could be severe, often associated with intermittent vomiting and/or failure to thrive. Impairment of venous and lymphatic flow leads to signs and symptoms of malabsorption. The correct diagnosis is frequently delayed, with interim diagnoses including central or psychogenic vomiting, milk or other food allergies, and various malabsorption syndromes, such as celiac disease.
Patients with duodenal obstruction secondary to Ladd bands may present acutely, with sudden onset of bilious vomiting, or may present with more chronic symptoms such as failure to thrive and intermittent abdominal pain. Patients may also present with more distal obstruction.
Either right or left paraduodenal or mesocolic hernias produce symptoms by entrapment of bowel. Left hernias are more common and herniate through the fossa of Landzert; right hernias extend through the fossa of Waldeyer. These present with abdominal pain and vomiting secondary to obstruction, which can progress from partial to complete obstruction, and to ischemia and bowel necrosis.
Malrotation occurs as part of a constellation of anomalies in as many as 30% to 60% of patients, depending on the reporting series. Malrotation can occur in patients with duodenal stenosis and annular pancreas, which have been previously discussed but can also be found in patients with other intestinal atresias. Malrotation can also be found as part of the spectrum of findings in patients with Hirschsprung disease and anorectal malformations, cloacal exstrophy, Eagle-Barrett (prune belly) syndrome, megacystis-microcolon-intestinal hypoperistalsis (Berdon syndrome), Cornelia de Lange, Marfan, and Meckel syndrome. Malrotation is reported to be 25 times more common than in the general population in patients with trisomy 13, 18, and 21. Malrotation is an intrinsic component of entities in which gut rotation cannot occur, such as gastroschisis, omphalocele, and Bochdalek diaphragmatic hernia; it is also extremely common if not invariable in patients with heterotaxy.
Plain abdominal radiographic findings in patients with malrotation without obstruction or volvulus may show an abnormal distribution of stool, with absence of stool pattern in the right lower quadrant. In patients with nonrotation, all colonic stool may be seen confined to the left half of the abdomen ( Fig. 102.15 ). However, although it may occasionally be suspected, malrotation is not a plain film diagnosis.
In patients with volvulus, the radiographs may be completely normal at the onset of the bilious vomiting ( Fig. 102.16A ) and should not lead to a false sense of security. As unabated or increased duodenal obstruction occurs, distal bowel contents are evacuated; this lack of abdominal distension may again lead to a false sense of security or to the suspicion of pyloric stenosis if the stomach is distended and the significance of the bilious vomiting is overlooked ( Fig. 102.16B ). It is important to note that in acute volvulus the duodenum may be distended, but typically duodenal distension is subtle ( Fig. 102.16C ), different from the obvious double bubble of duodenal atresia, because the time span for the development of dilatation is typically much shorter.
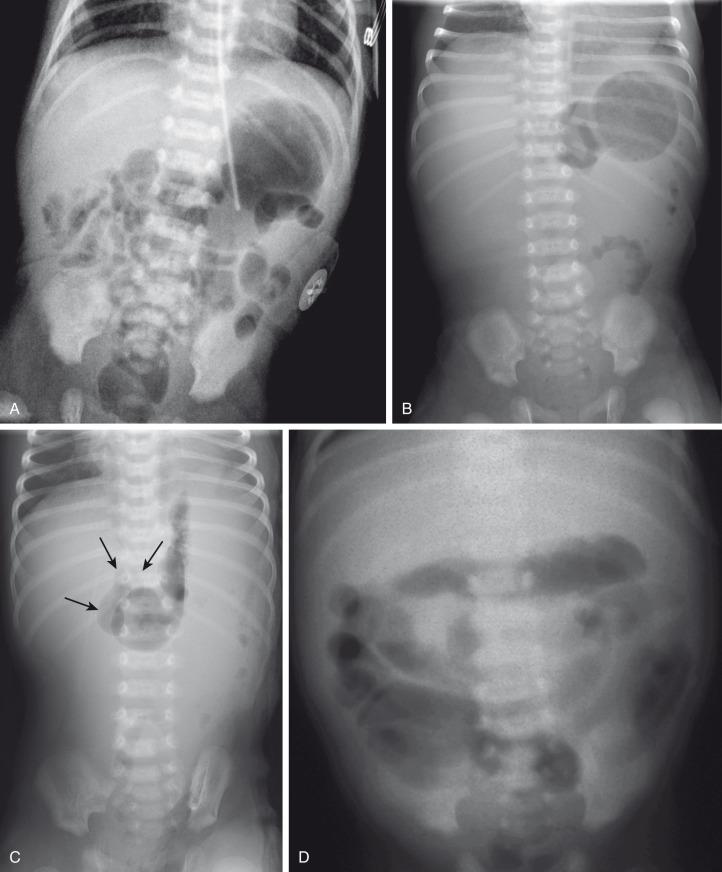
Ominous plain radiographic findings of supervening ischemia in patients with volvulus include abdominal distension, separation of the gas within bowel loops secondary to increased luminal fluid, tubular appearance of bowel loops, and fold thickening. Diffuse fluid and gaseous distension of the bowel suggests gangrenous bowel and a poor prognosis ( Fig. 102.16D ).
In premature infants, bilious vomiting may be absent, and the clinical picture may be nonspecific and suggestive of the more common entities of sepsis and necrotizing enterocolitis ; radiographs obtained at the time of systemic manifestations may show dilated bowel loops and suggest profound ileus and/or necrotizing enterocolitis ( e-Fig. 102.17 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here