Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A drug is broadly defined as any chemical agent that affects living protoplasm. About one-third of women in the UK take drugs at least once during pregnancy, but only 6% take a drug during the first trimester. In the puerperium, the use of drugs increases substantially with no difference in the pattern of prescribing between mothers who breastfeed and those who bottle-feed.
Possible effects of drugs in pregnancy include:
Teratogenicity (e.g. thalidomide)
Long-term latency (e.g. diethylstilbestrol (DES) – increased risk of vaginal adenocarcinoma after puberty, or abnormalities in testicular function and semen production)
Impaired intellectual or social development (e.g. phenobarbital or sodium valproate).
Prodrugs are pharmacologically inactive derivatives of active drugs. They are designed to maximise the amount of active drug that reaches its site of action through manipulation of the physicochemical, biopharmaceutical or pharmacokinetic properties of the drug. Prodrugs are converted into the active drug within the body through enzymatic or non-enzymatic reactions.
Distribution volume is a hypothetical concept that is defined as the volume that a drug would occupy if the concentration throughout the body were equal to that in plasma. The distribution volume depends on factors like lipid solubility and protein binding.
Clearance is the volume of plasma cleared of the drug in unit time. It determines what dose of drug is necessary to maintain a certain plasma concentration but does not indicate how rapidly the drug disappears when treatment is stopped. Patients with abnormal renal or liver function can have increased clearance times.
A receptor is any cellular molecule to which a drug binds to initiate its effects. Receptors can be proteins (hormones, growth factors and neurotransmitters) or nucleic acids (cancer chemotherapeutic agents). An agonist binds to a physiological receptor and often mimics the regulatory effects of endogenous signalling compounds. An antagonist binds to receptors without regulatory effects and blocks the endogenous agonist. Drugs that stabilise the receptor in its inactive form are called inverse antagonists. Receptors of relevance to clinical practice are summarised in Table 12.1 .
| Receptor | Subtype | Main Actions of Natural Agonist | Drug Agonist | Drug Antagonist |
|---|---|---|---|---|
| Adrenoceptor | α 1 | Vasoconstriction | Prazosin | |
| α 2 | Hypotension, sedation | Clonidine | ||
| β 1 | ↑ Heart rate |
|
|
|
| β 2 |
|
|
||
| Cholinergic | Muscarinic |
|
|
|
| Nicotinic | Contraction of striated muscle |
|
||
| Histamine | H 1 |
|
|
|
| H 2 |
|
|
||
| Dopamine | CNS neurotransmitter | Bromocriptine |
|
|
| Opioid | CNS neurotransmitter | Morphine, pethidine, etc. | Naloxone |
pKa is the pH at which half the drug is in its ionised form.
Henderson–Hasselbalch equation is used to calculate the ratio of ionised to non-ionised drug at each pH.
Absorption is the rate at which a drug leaves its site of administration and the extent to which this occurs.
Bioavailability is the term used to indicate the fractional extent to which a dose of drug reaches its site of action or a biological fluid from which the drug has access to its site of action.
Half-life (t ½ ) is the time taken for the plasma concentration, or the amount of the drug in the body, to be reduced by 50%. The half-life of a drug depends on its rate of clearance and volume of distribution. Highly lipophilic drugs may have an increased clearance but prolonged half-life.
Steady-state concentration is reached when drug elimination is equal to availability with repeated equal doses. It takes repeated dosing for about five half-lives to achieve steady state.
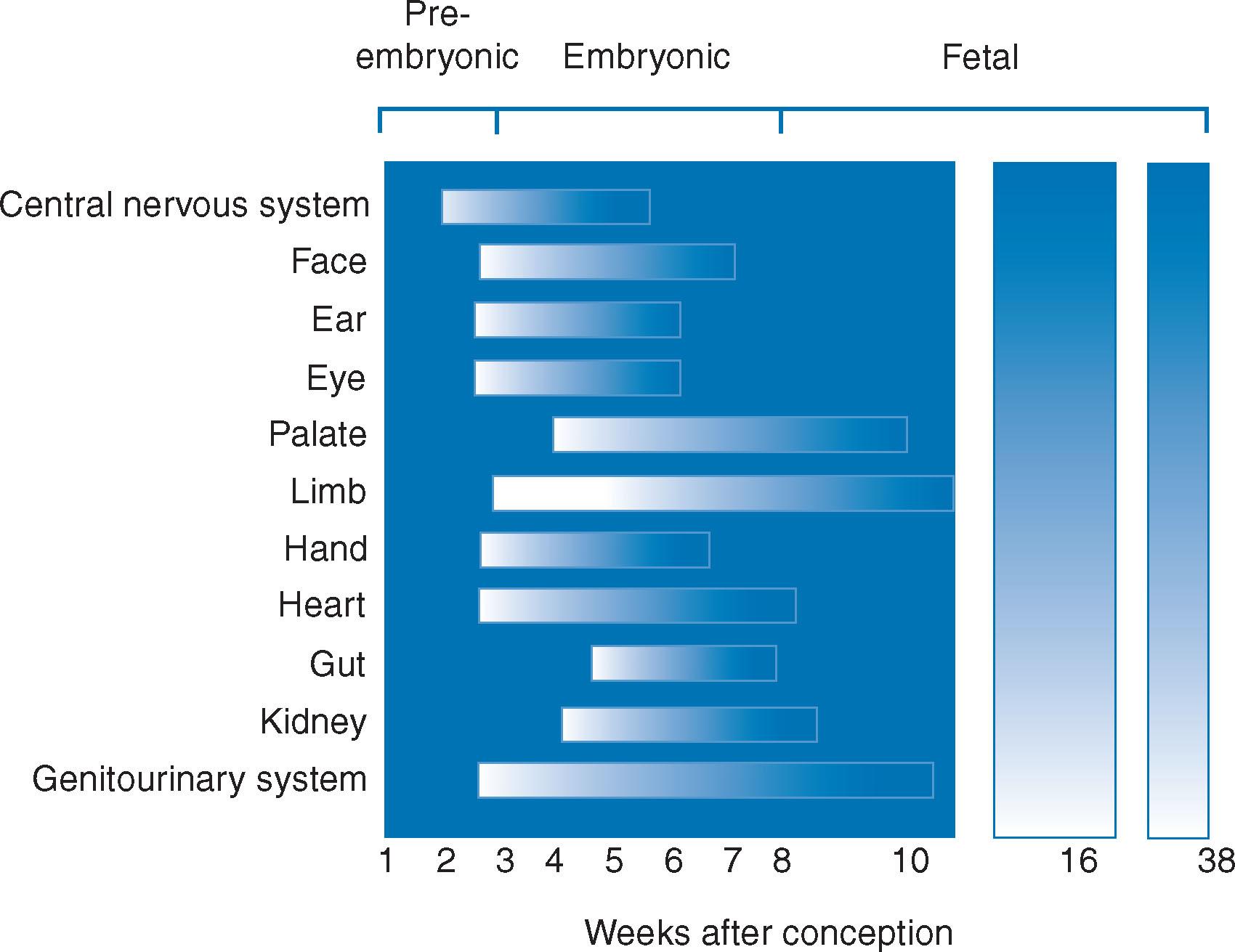
This is defined as structural or functional (e.g. renal failure) dysgenesis of the fetal organs. Typical manifestations of teratogenesis include congenital malformations with varying severity, fetal growth restriction (FGR), carcinogenesis and fetal demise. Lack of understanding of the mechanisms of teratogenicity makes it difficult to predict on pharmacological grounds that a particular drug will produce congenital malformations. The period of highest sensitivity to teratogens is early organogenesis. Later in fetal development, exposure to a teratogen is far less likely to be the cause of a structural defect, but can cause serious functional abnormalities, notably of the neuro behavioural type.
The major body structures are formed in the first 12 weeks after conception ( Fig. 12.1 ). Interference in this process causes a teratogenic effect. If a drug is given after this time, it will not produce a major anatomical defect, but possibly a functional one. The overall incidence of major congenital malformations is around 2% to 3% of all births, and of minor malformations, 9%. The part played by drugs is probably small. It has been estimated that 25% of congenital malformations are due to genetic or chromosomal abnormalities, 10% due to environmental causes including drugs and 65% are of unknown aetiology. Even known teratogens do not invariably cause anatomical defects and the mechanism of drug-induced teratogenicity remains unclear. The genetic composition of the fetus, the timing of the insult, maternal age, nutritional condition, disease status and the dose of the drug may play a role. The critical time for drug-induced congenital malformations is usually the period of organogenesis. This occurs approximately 20 to 55 days after conception, that is, 34 to 69 days (7 to 10 weeks) after the first day of the last menstrual period (see Fig. 12.1 ).
Pharmacokinetics is the mathematical description of the rate and extent of uptake, distribution and elimination of drugs in the body. It mainly concerns time. Pharmacok-inetics is important for drugs that are given for more than an isolated dose, and those whose margin of safety is narrow. The pharmacokinetics of a drug depends upon its concentration, structure, degree of ionisation, relative lipid solubility and binding to tissue proteins.
Oral absorption is unpredictable and is dependent on various factors such as gastric emptying time, surface area of absorption, blood flow, lipid solubility and physical state of the drug. Venous drainage from the oral mucosa is to the superior vena cava and hence bypasses first-pass metabolism. Rectal administration causes erratic absorption and irritation of the rectal mucosa but 50% of the dose will bypass the liver. Absorption after subcutaneous or intramuscular injection occurs by simple diffusion.
Distribution occurs in two phases: an initial rapid phase to the liver, kidney and brain followed by a slow phase to the muscles, viscera, skin and fat. The distribution of a drug is determined by its lipid solubility and the pH gradient between the intracellular and extracellular fluids.
Acidic drugs bind to albumin (e.g. salicylates, warfarin, anticonvulsants, non-steroidal anti-inflammatory drugs (NSAIDs))
Basic drugs bind to α 1 -acid glycoprotein (e.g. β-blockers, opioid analgesics, local anaesthetics)
Covalent bonding can occur with reactive drugs (e.g. alkylating agents).
Hypoalbuminaemia due to liver disease or nephrotic syndrome results in reduced binding and an increase in the unbound fraction of acidic drugs. An acute-phase response leads to an elevation of α 1 -acid glycoprotein levels and therefore to reduced availability of basic drugs. Fig. 12.2 summarises the different compartments in which drugs can be distributed in the materno-fetal unit.
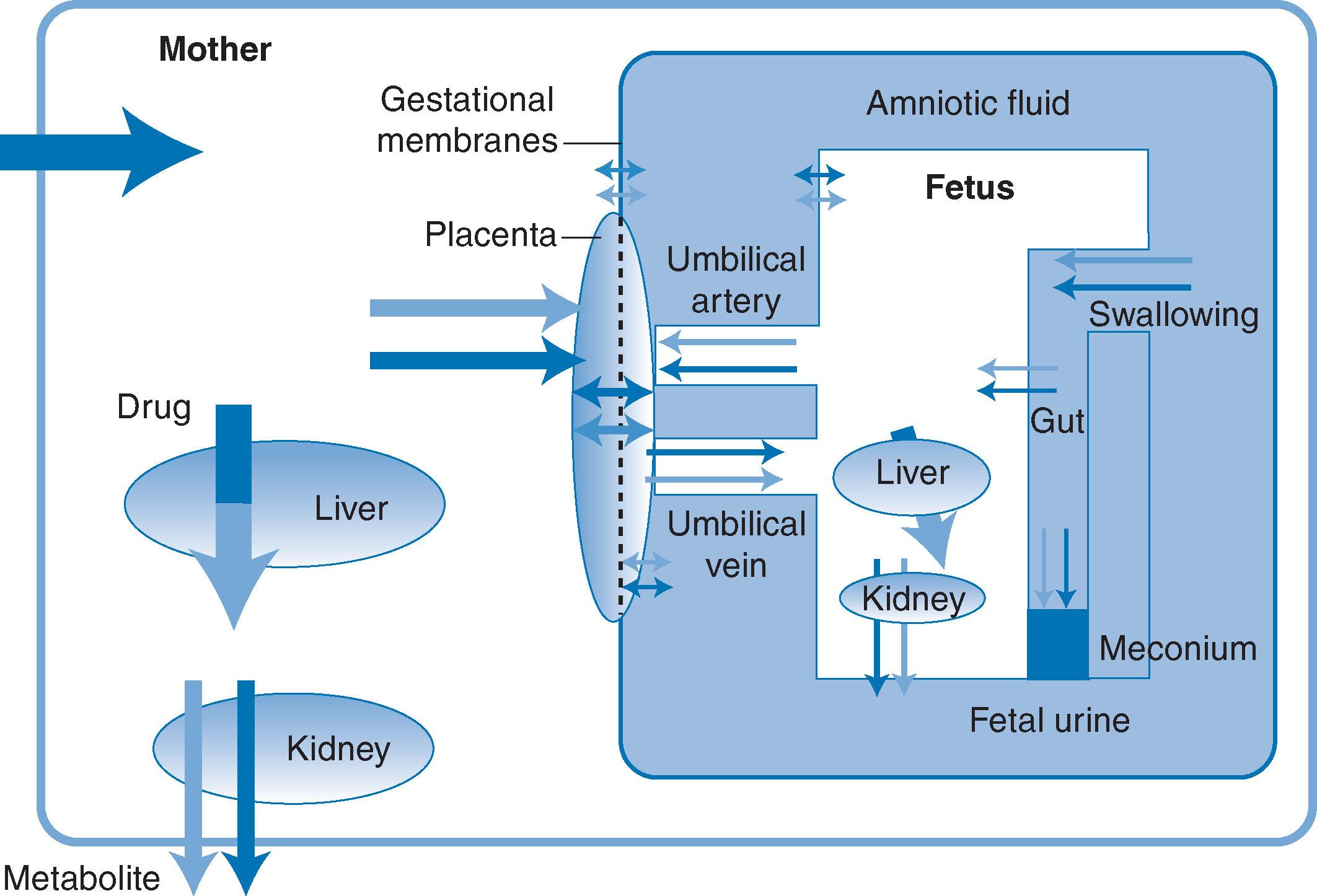
Transcapillary movement : this is transfer of the drug with bulk transfer of water due to hydrostatic or osmotic pressure differences and accounts for the majority of unbound drug transfer.
Paracellular transport : this occurs between cell junctions and is the principal mechanism of excretion of drugs by the kidney.
Passive transport : this is diffusion of the drug through the cell membrane along a concentration gradient by virtue of its lipid solubility.
Active transport : this is characterised by a requirement for energy and involves the movement of a drug against an electrochemical gradient.
Facilitated diffusion : this is a carrier-mediated transport process in which there is no input of energy. Enhanced movement is down an electrochemical gradient.
Drugs that are lipid soluble are less likely to be excreted and polar compounds are likely to be excreted more quickly. The kidneys excrete drugs by filtration, tubular secretion and tubular re-absorption. Changes in renal function affect all three functions and are impaired in the elderly, as adult renal function decreases by 1% per year. Unbound drugs are excreted by filtration. P glycoprotein and multidrug resistance associated protein type 2 secrete ions and conjugated metabolites, respectively, into the tubules. Some of the ways that pregnancy influences pharmacokinetics are summarised in Table 12.2 .
| Maternal Pharmacokinetics | Fetal Pharmacokinetics | Placental Pharmacokinetics |
|---|---|---|
|
|
|
Pharmacodynamics is the study of biochemical and physiological effects of drugs on the body and their mechanism of action. The majority of the drugs pass through cells rather than between them. Broadly speaking, drugs act on four different targets: receptors, enzymes, membrane ion channels and metabolic processes. Drugs commonly act on electrical or chemical signalling pathways and drug action commonly involves a signal transduction pathway, which consists of receptor, cellular target and intermediary molecules.
Drug metabolism will influence the duration and potency of the effect of specific drugs. Drugs are commonly converted to more polar metabolites to facilitate their excretion. This is frequently catalysed by enzymic reactions. While the majority of drug metabolism results in less toxic metabolites, occasionally it can result in the formation of more toxic compounds. A large number of drugs are metabolised by hepatic phase I and II reactions.
Phase I metabolism occurs in the endoplasmic reticulum and involves the formation of more polar metabolites of the original compound. These reactions can involve oxidation (catalysed by cytochrome P450 enzymes), hydrolysis, reduction, cyclisation or decyclisation. The polar metabolites may be directly excreted, usually in the urine, or may be converted further by phase II reactions.
Phase II reactions occur in the cytoplasm and commonly involve conjugation with sulphates, glucuronides, glutathione or amino acids and result in the formation of metabolites that are usually less toxic and more easily excreted.
The metabolism of a drug can be affected by enzyme induction, protein binding and the liver extraction ratio. Table 12.3 summarises the common drugs that influence the activity of the liver microsomal enzymes.
| Microsomal Induction (Cytochrome P450) | Microsomal Inhibition |
|---|---|
| Smoking | Oestrogen |
| Anticonvulsants | Ciprofloxacin |
| Progestogen | Fluconazole |
| Rifampicin | Omeprazole |
| Theophylline | Quinidine |
| Ethanol | Erythromycin, sulphonamide |
| Griseofulvin | Grapefruit juice, metronidazole |
Drugs that are likely to precipitate drug interactions are those that are highly protein bound, alter metabolism of other drugs or alter renal or hepatic metabolism. Drugs that are affected by drug interactions are those that have a steep dose–response curve and those that have a low toxic : therapeutic ratio (e.g. aminoglycosides, anticoagulants, anticonvulsants, antihypertensives, cardiac glycosides, cytotoxic drugs, oral contraceptives).
Pharmacokinetic interactions can be related to:
Absorption
Drugs that decrease gastric emptying (e.g. morphine, anticholinergics)
Chelation of calcium, aluminium, magnesium salts by tetracycline
Binding of warfarin and digoxin by cholestyramine
Protein-binding displacement interactions
For example, warfarin and phenytoin are displaced by sulphonamides, salicylates, phenylbutazone and valproate
Metabolism interactions with induction or inhibition of cytochrome P450 or phase I functionalisation reactions (e.g. oral contraceptives decrease anticoagulant effect of warfarin). Table 12.3 summarises drugs that commonly influence microsomal enzymes
Excretion interactions
Probenecid and penicillin at the renal tubules
Quinidine doubles digoxin levels
Diuretics causing lithium retention.
Pharmacodynamic interactions could be antagonism at same site (e.g. pethidine/naloxone), synergism at same site (e.g. verapamil/β-blockers increase arrhythmias) or indirect, for example when alterations in coagulation, fluid and electrolyte balance affect drug action.
Liver disease can lead to impaired drug metabolism. The severity of the liver damage reflects the extent of the reduced metabolism, but clinical liver enzymes are of little value in predicting this. Drugs with high hepatic first-pass metabolism are most severely affected.
The distribution volume for all drugs increases
There is delayed gastric emptying, resulting in slow peak levels of readily absorbed drugs (e.g. paracetamol) and increased bioavailability of slowly absorbed drugs (e.g. digoxin)
Nausea and vomiting in early pregnancy increases the clearance time affecting the dosage of drugs (e.g. anti-epileptics)
Increased body fat increases clearance of lipophilic drugs (e.g. thiopental) even though the plasma half-life is prolonged
Decreased albumin and raised free fatty acids lead to increase in free levels of albumin-bound drugs. Therefore measurement of these drugs may not reflect the actual concentration and saliva monitoring may be needed
Increased alveolar ventilation and cardiac output seen in normal pregnancy may lead to enhanced alveolar and intramuscular drug absorption
Renal blood flow increases and glomerular filtration rate (GFR) increases by 50% leading to enhanced renal clearance of many medications
α 1 -Acid glycoprotein levels do not change, but there is a large transplacental concentration gradient that affects transfer of drugs
Maternal albumin concentrations progressively decrease during pregnancy and fetal albumin concentrations progressively increase. They achieve equivalence at around week 30 of gestation. Albumin-bound drugs may be transferred to the fetus in a higher concentration. The placenta has cytochrome P450 sulphating and acetylating enzymes that can metabolise drugs.
Virtually all drugs cross the placenta and achieve equal concentrations on either side over repeated administration. Most drugs have a molecular weight below 1000 daltons (Da), and molecules of this size cross the placenta (<600 Da cross easily). Lipid-soluble drugs are readily transferred across the placenta. Diffusion is the most important mode of transfer of drugs through the placenta. Fetal plasma is more acidic and leads to ion trapping of basic drugs.
The adrenal cortex synthesises two classes of steroid: the corticosteroids (glucocorticoids and mineralocorticoids), which have 21 carbon atoms, and the androgens, which have 19. Cortisone is the main glucocorticoid and aldosterone is the main mineralocorticoid. Cortisol is produced at a rate of 10 mg/day.
Corticosteroids act with specific receptor proteins in target tissues to modulate proteins synthesised by various target tissues. Hence, most effects of corticosteroids are not immediate but become apparent after several hours. The receptors are members of the nuclear receptor family. The glucocorticoid receptor is predominantly in the cytoplasm in an inactive form until it binds to glucocorticoids. Steroid binding results in receptor activation and translocation to the nucleus. The activated receptor interacts with specific DNA sequences in the regulatory regions of genes called glucocorticoid response elements (GREs) and these provide specificity to the induction of gene transcription.
Mineralocorticoids act similarly though the exact mechanism is unclear.
Hydrocortisone and numerous congeners including the synthetic analogues are orally effective. They can be administered intravenously to achieve high concentrations. Absorption from the skin is low but, if they are applied to a large area or on an occlusive dressing, the absorption may be sufficient to cause systemic effects. After absorption, >90% of cortisol is reversibly bound to protein. Two plasma proteins account for almost all of the steroid-binding capacity: corticosteroid-binding globulin (CBG) and albumin. A state of physiological hypercortisolism occurs during pregnancy. The elevated circulating oestrogens induce CBG production, and CBG and total plasma cortisol increase several-fold.
Glucocorticoids are administered in multiple formulations for disorders that share an inflammatory or immunological basis. With the exception of patients receiving replacement therapy for adrenal insufficiency, glucocorticoids are neither specific nor curative, but rather are palliative because of their anti-inflammatory and immunosuppressive actions.
Prednisolone is the biologically active form of prednisone. The placenta can oxidise prednisolone to inactive prednisone or even less active cortisone. Only 10% of the maternal prednisolone dose crosses the placenta. Four large epidemiological studies including steroids that readily cross the placenta (betamethasone and dexamethasone) have looked at the use of corticosteroids in first trimester and found an association with non-syndromic orofacial clefts. However, the overall risk is low. The Michigan Medicaid surveillance study looked at 229,101 patients exposed to prednisolone, prednisone and methylprednisolone during the first trimester; the data did not support an association between these agents and congenital defects. There are isolated reports of cataracts in the newborn if prednisolone was used throughout the pregnancy. During lactation, the infant is exposed to minimal amounts of steroid through the breast milk. At higher doses (>20 mg), it is recommended to wait at least 4 hours after a dose before nursing the baby.
Betamethasone administration to women with threatened preterm labour is associated with a decrease in respiratory distress syndrome, periventricular leukomalacia and intraventricular haemorrhage in pre-term infants. It can induce hyperglycaemia and may rarely precipitate myasthenic crisis or hypertensive crisis in the mother. Approximately 80% of the maternal betamethasone dose crosses the placenta. Single courses of betamethasone have no effects on the fetus but multiple courses have been associated with lower birth weights and reduced head circumference at birth. Follow-up studies have not shown any differences in cognitive and psychosocial development when compared with controls.
Hydrocortisone and its inactive precursor, cortisone, appear to present a small risk to the human fetus. Approximately 50% of the maternal dose of hydrocortisone crosses the placenta. These corticosteroids produce dose-related teratogenic and toxic effects in genetically susceptible experimental animals consisting of cleft palate, cataracts, spontaneous abortion, IUGR and polycystic kidney disease. However, there are no data to support these effects in the great majority of human pregnancies, although the small increase in incidence of cleft lip with or without cleft palate is supported by large epidemiological studies.
It is important to remember that in some women the benefits of corticosteroids can far outweigh the fetal risks when used to treat maternal inflammatory and autoimmune disease, and these agents should not be withheld if the mother’s condition requires their use.
General anaesthetics act by increasing the sensitivity of the γ-aminobutyric acid (GABA) A receptor to GABA thus enhancing inhibitory neurotransmission and depressing nervous system activity. Glycine receptor-mediated activation of chloride channels is responsible for inhibition of neurotransmission in the spinal cord and brain stem. Ketamine, nitrous oxide and xenon act via N -methyl- d -aspartate (NMDA) receptors and cause long-term modulation of synaptic responses.
Intravenous (i.v.) anaesthetics are unique drugs that induce anaesthesia rapidly as they quickly achieve high concentrations in the central nervous system (CNS). Intravenous anaesthetics affect synaptic function by inhibiting excitatory synapses and enhancing inhibitory synapses their pharmacological effects are terminated by redistribution to tissues with low blood flow. Commonly used drugs are thiopental and propofol for induction of anaesthesia. Thiopental is an ultrashort-acting agent that has quick entry into the CNS followed by quick redistribution of the drug. After i.v. administration, it causes unconsciousness with amnesia without analgesia or muscle relaxation. It is used mainly as an induction agent and by infusion during short procedures. It is also used to control convulsions in status epilepticus and eclamptic convulsions not responding to magnesium sulphate.
Inhalational anaesthetics can hyperpolarise neurones and hence reduce both pacemaker neurone and post-synaptic neurone action potentials. Halothane is commonly used. Due to its high lipid solubility and increased clearance from lungs, induction is slow and speed of recovery is also lengthened. Some 80% is excreted unchanged and 20% is metabolised by cytochrome P450 enzymes to trifluoroacetylate, which can bind to several liver proteins. Hypersensitivity to these proteins leads to halothane-induced hepatotoxicity.
A side effect of the drug is uterine smooth muscle relaxation and this can be helpful for manipulation of fetus (version) and for manual removal of placenta. It can also lead to an increased risk of postpartum haemorrhage. It is a triggering agent for malignant hyperthermia.
Nitric oxide (NO) is very insoluble in blood and other tissues. Due to its high insolubility, rapid induction and rapid emergence occurs during anaesthesia. On discontinuation of nitrous oxide it can diffuse from blood to alveoli and decrease the concentration of oxygen in alveoli (diffusional hypoxia). Hence 100% oxygen should be administered during recovery from NO. NO is a weak anaesthetic and analgesic at 20%, and is a sedative. A 50% concentration is frequently used to provide analgesia in labour and outpatient dentistry.
A collaborative perinatal project showed no embryonic or fetal effects of NO. Its use during delivery may lead to neonatal depression and fetal accumulation of nitrous oxide, which increases over time; hence, it is safer to keep the induction to delivery time as short as possible.
These agents are used as an adjunct to anaesthetics to provide muscle relaxation. Based on their mechanism of action they are divided into depolarising (e.g. succinylcholine) and non-depolarising (e.g. pancuronium). The actions of neuromuscular blocking agents are reversed by acetylcholine esterase inhibitors (e.g. neostigmine) and muscarinic receptor antagonists (e.g. glycopyrrolate). The only depolarising agent in use is succinyl choline, which acts by depolarising the membrane by opening sodium channels. A series of repetitive excitation followed by block transmission and neuromuscular paralysis occurs. Competitive antagonists act by decreasing the frequency of channel opening events that result in an action potential. At increasing doses the drug binds to the channels in a non-competitive manner.
Depolarising muscle relaxants (e.g. suxamethonium and succinylcholine) can cause histamine release and hyperkalaemia (and therefore should be avoided in patients with heart disease, trauma and burns). Malignant hyperthermia occurs due to calcium release from the sarcoplasmic reticulum of the skeletal muscle. Clinical features include contracture, rigidity and heat production resulting in hyperthermia-accelerated muscle metabolism and acidosis. Malignant hyperthermia is treated with dantrolene which inhibits calcium release.
Local anaesthetics cause a reversible block in the action potential responsible for nerve conduction. They decrease the permeability of the nerve to sodium and block propagation of electrical impulses. Combination with adrenaline (epinephrine) doubles their duration of action. Excessive administration can cause cerebral irritation and convulsions.
Warfarin interferes with cyclic conversion of vitamin K to its active metabolite, which is essential in carboxylation of glutamic acid residues of vitamin K-dependent coagulation factors (II, VII, IX, X). Carboxylation is necessary for binding of these factors to calcium and phospholipids. As protein S levels are also dependent on vitamin K activity, warfarin administration causes a prothrombotic state prior to the onset of an anticoagulant effect. It causes embryopathy in 5% to 10% of pregnancies where there is first-trimester exposure. The clinical features are similar to those of chondromalacia punctata (stippled epiphysis, nasal and limb hypoplasia). The embryopathy is secondary to vitamin K involvement in the post-translational modification of proteins enabling them to bind calcium. The use of warfarin in the second and third trimester is associated with recurrent micro-haemorrhages in the brain leading to optic atrophy, dorsal midline dysplasia and mental retardation. It is avoided after 36 weeks to prevent maternal and neonatal complications related to delivery.
Heparin is the anticoagulant of choice from the fetal perspective as it does not cross the placenta. It is a glycosaminoglycan and acts through interaction with antithrombin III. Antithrombin III inactivates thrombin, factor Xa and factor IXa. Two major side effects that can occur with heparin treatment are heparin-induced thrombocytopenia and osteoporosis. There are two types of thrombocytopenia that occur in association with heparin treatment. Non-immune heparin-associated thrombocytopenia is associated with a mild reduction in platelet count and occurs 2 to 5 days after heparin injection. Immune thrombocytopenia occurs due to IgG antiplatelet antibodies, 3 to 4 weeks after therapy, and increases the risk of thrombus formation.
These are oral anticoagulants that specifically inhibit factors IIa or Xa. They are also known as new oral anticoagulants (NOACs). Factor Xa is a clotting factor in the coagulation pathway that leads to thrombin generation and clot formation. They act by inhibition of prothrombinase complex-bound and clot-associated factor Xa, resulting in a reduction of thrombin in the coagulation cascade. The main advantage of direct oral anticoagulants (DOACs) is the oral administration and that they do not need drug monitoring or dose adjustments. Examples are Rivaroxaban and Apixaban.
All DOACs can cross the placenta and although no specific embryopathy pattern has been established, they are contraindicated in pregnancy and breastfeeding.
The pharmacokinetics of all antiepileptics is altered in pregnancy and therapeutic drug monitoring can be of benefit. Phenytoin, primidone, phenobarbital, carbamazepine and sodium valproate all cross the placenta and are teratogenic. Major abnormalities produced by anticonvulsants are neural tube, orofacial and congenital heart defects. Fetal hydantoin syndrome includes prenatal and postnatal growth restriction, motor or mental deficiency, short nose with broad nasal bridge, microcephaly, hypertelorism, strabismus, low-set or abnormally formed ears, limb and positional deformities. Sodium valproate and carbamazepine mainly cause neural tube defects and spina bifida (always lumbar). Valproate is no longer prescribed to women of childbearing age due to its significant association with neural tube defects and neurodevelopmental delay. Phenobarbital appears to be safer than phenytoin. The risk of teratogenicity rises with the use of more than one drug. The newer anticonvulsants are often prescribed along with other drugs, and it is difficult to ascertain teratogenic risk of these drugs in isolation.
Altered pharmacokinetics in pregnancy may lead to changes in drug levels and for most drugs the concentration of the free drug falls. If a woman is fit free, there is usually no need to measure serial drug levels or adjust the dose for most anticonvulsants. An exception is lamotrigine as levels of this drug invariably fall in pregnancy. In women who have regular seizures, and who are dependent on critical drug levels, it is worth monitoring drug levels and increasing dosages of anticonvulsants should be guided by serum concentrations. Vitamin K is given in the last 4 weeks of pregnancy to prevent haemorrhagic disease of the newborn. Carbamazepine, phenytoin and valproic acid are safe in breastfeeding. Succinimides (e.g. ethosuximide) are commonly used to treat petit mal epilepsy and are thought to have a low or no teratogenic potential.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here