Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The increasing gap between the number of organs available for transplantation and the number of patients listed for transplantation has become a rate-limiting step in reducing both wait times and wait list deaths in patients awaiting transplantation. Before the passage of the first US brain death law in the state of Kansas in 1970, donation after cardiac death (DCD, or donation after circulatory death) was the primary mode of organ donation in the United States. Donor death was determined according to traditional cardiopulmonary criteria—that is, absence of pulse and blood pressures without cardiac activity.
Early organ procurement strategies were relatively crude and variable, which in turn prolonged warm DCD ischemia time (time from donor circulatory arrest to cold perfusion) and resulted in poor outcomes. The impact of the variability of circumstances surrounding donor death, and thus the duration of ischemic time, on DCD graft outcomes did not become apparent until experiences with organs donated after brain death (DBD) increased.
The need for diagnosing brain death was a culmination of critical care physicians’ growing ability to maintain physiologic organ function in patients with little or no hope of neurologic recovery after central nervous system (CNS) insults. The concept was first introduced at a CIBA Foundation meeting in England in 1965 and was subsequently endorsed with formal diagnostic criteria by Harvard Medical School in 1968. , A new debate was sparked over the precise definition and timing of death and the concept of futile care. Acceptance of this medically, philosophically, and legally novel concept of certifying death while maintaining perfusion in a potential donor to guarantee procurement with minimal warm ischemia time and graft damage revolutionized transplantation. Because early experience with DBD organs showed superior outcomes, use of DCD organs declined and was subsequently abandoned.
The success of DBD organs along with the refinements in medical and surgical techniques exponentially increased the number of transplants performed in the United States. The 1984 National Organ Transplant Act led to the formation of the United Network for Organ Sharing (UNOS), a nonprofit entity that provided a basis for standardizing organ procurement organizations (OPOs) throughout the United States, and the Organ Procurement and Transplantation Network (OPTN). Early national OPTN data showed that 10,794 deceased donor transplants were performed in 1988. Six years later, these numbers increased by nearly 50% to 15,210 transplants. Moreover, the number of lung grafts from deceased donors increased annually from 33 to 708. Intestinal transplantation also increased with the introduction of DBD donors. The first intestinal transplant was performed in 1990; by 1994, 96 patients with intestinal failure received intestinal transplants. Concomitant advances in critical care reduced mortality in patients with end-stage organ disease, thereby resulting in increasing wait lists and decreased attrition. This is referred to as the growing “gap” between organ supply and transplantation demand. For example, despite the increased number of transplant centers and use of living donors in 1995, only 33% of registrants waiting for kidney transplant underwent transplantation. However, the rate of transplantation decreased to 10% during 1998 to 2002.
Moreover, the numbers of young and previously healthy DBD donors stagnated because of several statutory changes in areas of gun control, automobile safety (airbags, seatbelts, and lowering of legal blood alcohol limits), and helmet use. This decreased traumatic fatalities and changed the face of DBD organ donors. The demographics of a typical DBD donor transitioned from a young healthy person who was rendered brain dead because of a devastating head trauma to an older person with medical problems who was rendered brain dead because of a neurovascular insult. This transformation eroded some benefit of using a DBD donor and prompted a search for other options.
Because the use of live donor organs has not kept pace with the growing deficit of organ donors, strategies such as regenerative medicine, mechanical devices, and xenotransplantation (use of grafts derived from animal donors) for treating end-organ diseases have been explored. However, these strategies are not ready to replace durable organ replacement. Further, the activity surrounding social and legislative approaches, including increased public awareness, donor registration activities, and interest in presumed consent (requiring individuals to opt out of organ donation to prevent consideration for donation at death), may have peaked because of cultural and philosophical objections. Therefore transplantation is again being performed using organs procured from DCD donors.
In the early 1990s, the Maastricht German transplant group rekindled interest in DCD organs by showing equivalent long-term outcomes in recipients receiving both DCD and DBD renal transplants. , Similar findings from preliminary data have been made regarding DCD liver, lung, , heart, and pancreas transplants. The University of Pittsburgh Medical Center (UPMC) introduced the nation’s first institutional policy to permit and regulate DCD. The need for such a policy arose when several patients/families were asked to participate in donation after previously electing withdrawal of life-sustaining treatment. This request fell outside the current parameters of donation policies and guidelines. The UPMC policy became the first concrete model to use cardiopulmonary criteria to determine death for organ procurement. This policy highlighted a milestone in the evolution of transplantation. Since then, DCD has been adopted by many OPOs and hospitals nationwide. By December 2006 OPTN bylaws required that all OPTN members have a DCD donor protocol in effect. Currently, The Joint Commission now requires that all accredited institutions develop and implement standardized DCD policies.
After more than a decade of ongoing scrutiny surrounding ethical issues and outcome assessment, several key issues regarding DCD remain controversial in both the lay and medical communities. These include (1) criteria for identifying potential DCD donors, thus avoiding the financial and emotional burden of “failed” DCD; (2) optimization of DCD donor management; and (3) standardization of DCD procurement protocols to ensure a successful multidisciplinary effort with reproducible results. These issues are explored in this chapter after a brief discussion on the definition and current status of DCD.
Although seemingly straightforward, successful use of a DCD donor involves identification and classification of potential donors, appropriate diagnosis of death, and compliance with local policy of mandated wait time between pronouncement of death. The initial step in DCD organ transplantation is the recognition of potential donors with sufficient time to prepare and preserve optimal organ function before procurement. DCD is defined as organ procurement after the determination of death, which is characterized by an irreversible cessation of cardiopulmonary functions. Critical care physicians and OPO staff must be familiar with diagnoses and clinical circumstances that qualify a patient as a potential DCD donor. Candidates are patients in whom withdrawal of futile life-sustaining treatment is being planned. Because optimal preservation of organ function is facilitated by coordinated perimortem care, graft quality can be compromised in situations where a patient’s wishes regarding organ donation are unknown or where DCD is not offered as an option until late. Organ suitability may decline while attempts are being made to educate staff and families. Moreover, a treating physician must ensure that for patients on life support, withdrawal of life support must be independent of the decision to donate organs. At present, an OPO is responsible for coordinating surgical recovery and for preserving and transporting organs and tissues. An OPO should be notified within 1 hour as soon as a patient’s death is imminent from natural causes or withdrawal of life support.
The once-popular practice of managing potential DCD donors by placing vascular and/or intraperitoneal catheters to infuse cold organ preservation solution before the availability of consent for procurement has now largely been abandoned. This practice stimulated contentious debate from both the medical and lay communities. Unlike several European countries, no US state adopted this presumed consent into law.
Management of DCD donors is facilitated by a classification scheme developed by the Maastricht group in 1994 and revised in 2000. An international consensus conference was held in Paris in 2013 to clarify the Maastricht classification. Maastricht categories define potential donors by circumstances under which cardiovascular death occurs. A distinction is made between donors whose cardiopulmonary failure is uncontrolled or emergent (categories 1, 2, and 4) and those whose death according to cardiopulmonary criteria occurs in a controlled manner after withdrawing futile life-sustaining support (category 3). Prior versions of the classification had a category 5, which is medically assisted cardiocirculatory death or euthanasia. This category has been mostly removed, except in Belgium and the Netherlands, where euthanasia is legal. The revised Maastricht classification is outlined in Table 160.1 . Recent initiatives in the northeast United States involve training prehospital personnel to rapidly converse with preconsented victims of unsuccessful resuscitation after cardiopulmonary arrest (category 2) to determine potential DCD donors. Category 3 donors constitute the majority of US and European DCD donors. It is difficult to compare DCD outcomes according to Maastricht categories because few authors use this classification when reporting DCD results. Therefore, for uniformity, the remainder of this chapter focuses on category 3 donors.
| Category | Description | Condition |
|---|---|---|
1
|
Cardiac arrest outside hospital and no resuscitation attempted
|
Uncontrolled |
2
|
Cardiac arrest followed by unsuccessful resus-citation either inside or outside hospital
|
Uncontrolled |
| 3 | Cardiac arrest after planned withdrawal of life support | Controlled |
| 4 | Cardiac arrest in a brain-dead patient awaiting organ procurement | Uncontrolled Controlled |
Category 3 standardization is outlined in Fig. 160.1 (the UNOS Critical Pathway for DCD). Typical patients may have the following characteristics: absence of or hyperactive respiratory drive, lack of adequate respiratory muscle strength, and severe hypoxemia or inadequate circulation in the absence of treatment with inotropic or vasopressor drugs. These patients are usually supported using ventilators or mechanical circulatory assistance such as ventricular-assist devices (VADs) or intraaortic balloon pumps. These patients may have also experienced severe neurologic insults but may not have met brain death criteria. Conscious patients usually develop degenerative neuromuscular diseases or end-stage cardiopulmonary diseases and are often ventilator or VAD dependent. These patients or their families may decide to discontinue life-sustaining support and request their organs to be donated.
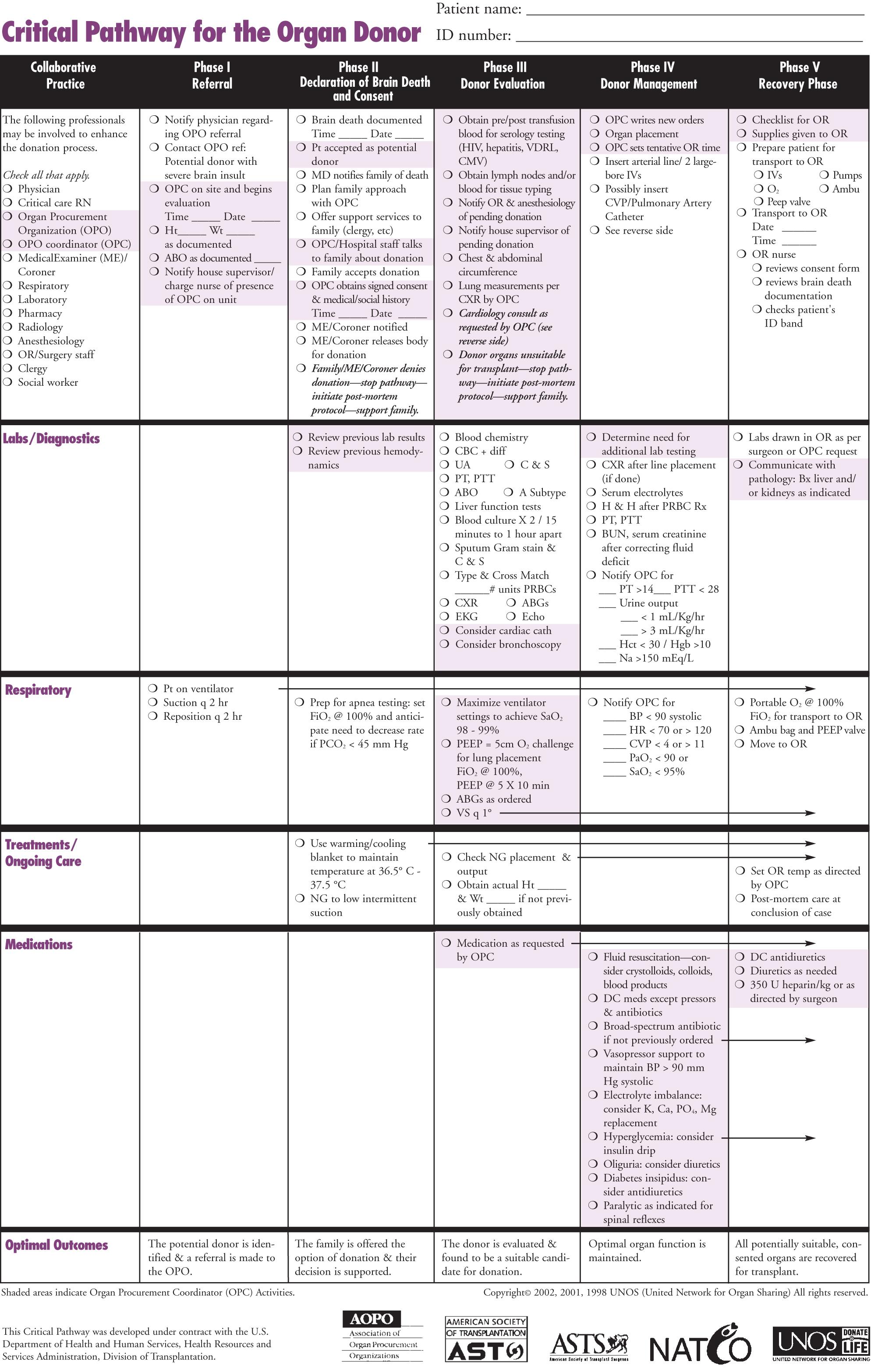
The other category of potential DCD donors includes patients with impending cardiopulmonary death, the timing of which is either predictable based on patient-/family-requested withdrawal of care or unpredictable because of premature cardiac arrest before withdrawal. Given the lack of perfusion in DCD donors, prompt identification of death is needed to minimize organ ischemia, especially if uncontrolled cardiac arrest occurs. Organ procurement from DCD donors under uncontrolled conditions is technically feasible but is not physiologically ideal because of the inherent ischemic insult.
Various modalities have been proposed to help physicians identify death based on the absence of cardiac sounds, pulse, respiration, and response to stimuli. Confirmatory tests such as intraarterial monitoring or Doppler studies recommended by the Institute of Medicine (IOM) can be used to expedite the confirmation of death; however, these tests are not widely accepted at present. A DCD work group assembled in 2006 indicated that electrocardiographic silence was not required for determining death but was sufficient to show the absence of circulation.
However, there is no agreement on the observation time required to rule out spontaneous unassisted cardiopulmonary resuscitation or autoresuscitation. The DCD work group and the Society of Critical Care Medicine (SCCM) recommend that potential donors be observed for at least 2 minutes but not more than 5 minutes to ensure the absence of spontaneous circulation. These recommendations pertain to the period between the loss of circulation and declaration of death and not between the declaration of death and organ procurement. Fugate and colleagues identified variability among DCD protocols within the United States, particularly for defining the observation of potential donors to rule out autoresuscitation. Although most centers followed the 2- to 5-minute observation period, there was variability in the definition of the period starting either before or after declaration of death, thus implying a total of 10 minutes. A prospective study by Dhanani and colleagues on the timing of determination of death in DCD donors showed that the longest period of arterial blood pressure resumption after declaration of death was 89 seconds. Moreover, only 4 of 41 patients examined showed return of blood pressure after cessation; however, this only lasted between 1 and 172 seconds. Given the variability and limited data on the duration of observation, specific guidelines for the precise minimal duration of irreversible circulation are warranted. At present, local protocols are used to stipulate the requirements of the determination of death and duration of observation time before organ procurement.
An important part of identifying potential DCD donors includes predicting the occurrence of rapid physiologic deterioration and death in less than 30–60 minutes (depending on the organ to be procured) after withdrawing life-sustaining treatment. Failure of a potential donor to progress to cardiac death within the prescribed time disqualifies the donor because of the extent of organ warm ischemia time (WIT). Factors such as age, comorbidities, and preterminal vasopressor requirement can be used as predictors; however, no strict criteria have been universally adopted. Lewis and colleagues from the University of Wisconsin developed a tool that uses clinical parameters to predict the suitability of DCD candidates. This resulted in the development of guidelines to predict the likelihood of circulatory death within 2 hours after withdrawing life support. The authors proposed four readily obtainable clinical criteria: (1) requirement for vasopressors to support blood pressure, (2) absence of primary brain injury, (3) history of mechanical ventilation for ≥6 days, and (4) respiratory rate of less than 20 breaths/minute (in the absence of mechanical ventilation). They noted that presence of two or more of these indicators accurately predicted death within 60 minutes after withdrawing life-supporting treatments, with a sensitivity and specificity of 81% and 78%, respectively. Munshi and colleagues observed that controlled ventilation, oxygenation, vasopressor use, Glasgow Coma Scale/Score, and brainstem reflexes consistently predicted time to death in DCD donors. Other novel predictors, such as physician opinion and simultaneous withdrawal of all support, are promising and warrant additional study. Robust analysis of retrospective DCD data would enable intensivists and OPO staff to more precisely identify potential DCD donors, help minimize the financial impact on hospitals and donors that “fail to progress,” and prevent unnecessary stress and disappointment for families during a psychologically vulnerable time.
Familiarity with relative and absolute contraindications for DCD, some of which overlap those associated with DBD, is important. These include the multiple-operated abdomen, active sepsis, active or recent extracranial primary malignancy, and active infections such as hepatitis B or COVID-19. With regard to virologic status, OPOs are well versed in performing rapid serologic testing to rule out latent viral infections and should be involved as early as feasible to initiate testing.
UNOS disseminates US transplant-related data and has reported DCD statistics since 1994. Data are available on the UNOS website ( www.unos.org ) and in UNOS annual reports. The annual number of DCD donors increased steadily from the mid-1990s to the early 21st century ( Table 160.2 ). In all, 42 DCD recoveries were performed in 1993, which represented <1% of the total recoveries in that year. In 2012 DCD recoveries showed a 12-fold increase of 12% of all organ procurements. The rate of DCD donations in the United States continues to increase steadily from 2012, comprising nearly 20% of all donors in 2018.
| Year | # Deceased Donors | # DCD Donors | DCD as a Percentage of Total Donors |
|---|---|---|---|
| 1993 | 4861 | 42 | 0.86 |
| 1995 | 5362 | 64 | 1.20 |
| 1997 | 5478 | 78 | 1.43 |
| 1999 | 5825 | 87 | 1.49 |
| 2000 | 5985 | 104 | 1.74 |
| 2001 | 6080 | 169 | 2.77 |
| 2002 | 6190 | 156 | 2.52 |
| 2003 | 6456 | 268 | 4.15 |
| 2004 | 7150 | 319 | 4.15 |
| 2005 | 7593 | 556 | 7.32 |
| 2006 | 8019 | 538 | 6.71 |
| 2007 | 8086 | 793 | 9.80 |
| 2008 | 7990 | 728 | 9.11 |
| 2009 | 8022 | 803 | 10.01 |
| 2010 | 7943 | 831 | 10.46 |
| 2011 | 8125 | 956 | 11.77 |
| 2012 | 8144 | 993 | 12.19 |
| 2017 | 10,286 | 1883 | 18.31 |
| 2018 | 10,721 | 2130 | 19.87 |
Despite ethical controversies, the real barrier to the widespread acceptance of DCD graft use is the poor outcome observed in early DCD experiences. Suboptimal organ function characterized by primary nonfunction, delayed graft function (DGF), and/or abbreviated graft survival have traditionally threatened the success of DCD organs because of the ischemic insult associated with cardiopulmonary arrest. Although these observations were valid at that time, they accumulated during early experiences with transplantation and were thus inherently confounded by era bias.
On a cellular level, DBD and DCD organs show different injury profiles. DBD is characterized by a surge in serum catecholamines because of brain death, which induces hypotension and subsequent organ hypoperfusion. Animal studies have shown that hemodynamic instability of DBD organs may be further exacerbated by inflammatory cytokines such as interleukin (IL)-1, IL-6, and IL-8 that directly affect renal grafts. In vivo studies evaluating DCD kidney grafts have shown less upregulation of inflammatory markers ; however, these studies showed that prolonged WIT induced alternative pathways of injury, such as those related to hypoxia. Rosenberger and colleagues found that hypoxia-inducible factors were correlated with renal allograft ischemia time. The increased incidence of DGF with DCD renal grafts was associated with a hypoxia-specific injury. The primary lesson from the early DCD era was that the metabolically active renal cortex, biliary epithelium, pulmonary alveoli, and pancreatic islets were sensitive to ischemia, with warm ischemic injury manifesting as acute tubular necrosis, ischemic-type biliary strictures (ITBSs), pulmonary fibrosis, and impaired beta-cell function, respectively. These manifestations were postulated to result in both poor initial graft function and long-term complications. However, contemporary outcomes for each organ have improved.
Recent data have shown equivalent outcomes for DCD vs. DBD grafts. Early experience with DCD kidneys showed that graft survival in recipients of controlled DCD kidney grafts is equivalent or comparable to that of DBD. Long-term outcomes of DCD kidneys also had similar graft outcomes to DBD kidneys. , A recent study by Gill and colleagues observed slightly improved allograft survival from DCD kidneys compared with DBD if warm ischemia time was less than 48 minutes. Similarly, Butler and colleagues did not observe a difference in likelihood of graft loss for DCD vs. DBD allografts. Although DCD kidneys have comparable graft survival rates to DBD kidneys, DCD kidneys have higher rates of DGF. Donor age of >60 years (compared with donors’ age of <40 years) and prolonged cold ischemia time (CIT; >24 vs. <12 hours) have been cited as factors affecting DCD graft function.
Much of the available outcome data on the transplantation of DCD pancreatic grafts are derived from cases of simultaneous pancreas-kidney (SPK) transplants. These data show that graft and patient survival rates in recipients of DCD pancreatic grafts are similar to those in recipients of DBD pancreatic grafts. , Transplantation of DCD pancreas-alone grafts by using the same protocols as those used for DCD SPK grafts has provided favorable results, although there was a higher odds of graft thrombosis compared with DBD organs. Fortunately, these findings were not observed in the setting of heparin administration. Donor selection (i.e., age) had a higher impact on graft outcomes vs. DCD status. More experience is needed to confirm these findings.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here