Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
DNA repair and the cellular response to DNA damage are critical for maintaining genomic stability.
Defects in DNA repair or the response to DNA damage encountered from endogenous or external sources results in an increased rate of genetic mutations, often leading to the development of cancer.
Inherited mutations in DNA damage response pathway genes often result in cancer susceptibility.
The major active pathways for DNA repair in humans are nucleotide excision repair, base excision repair, mismatch DNA repair, translesional DNA synthesis, and homologous recombination or nonhomologous end joining processes for double-strand break repair.
Inherited defects in nucleotide excision repair lead to the skin cancer–prone syndrome xeroderma pigmentosum, Cockayne syndrome, and trichothiodystrophy.
Inherited defects in base excision repair can result in colorectal cancer susceptibility.
Inherited defects in mismatch repair result in Lynch syndrome (hereditary nonpolyposis colorectal cancer syndrome), leading to increased incidence of gastrointestinal cancers, endometrial cancer, and other malignancies.
Biallelic inherited defects in DNA double-strand break repair and response pathways underlie a number of rare, recessive cancer-prone disorders, including ataxia-telangiectasia, Nijmegen breakage syndrome, Bloom syndrome, Werner syndrome, Rothmund-Thomson syndrome, and Fanconi anemia.
Persons with autosomal dominant Li-Fraumeni syndrome, who are highly prone to cancer because of inherited monoallelic p53 mutations, and with breast-ovarian cancer syndrome, who are highly prone to cancer because of inherited mutations of the BRCA1 and BRCA2 genes, exhibit defects in multiple DNA repair and DNA damage response pathways.
Because most cancer therapeutic agents that are currently used damage DNA, understanding how normal cells and tumor cells respond to and repair DNA damage is an important aspect of understanding cancer therapeutic responses and toxicities of treatment.
The presence of mutations in DNA damage response and repair pathways in tumors presents opportunities for developing therapeutics that target alternative damage response pathways and can be selectively lethal to the mutated tumor cells, an approach called synthetic lethality.
Genomic instability, particularly that conferred by underlying DNA repair defects, can lead to enhanced responses to immune checkpoint inhibitors owing to increased expression of mutant neoantigens.
Cancer is a disease caused by the accumulation over time of changes to the normal DNA sequence resulting in alterations, loss, amplification, or changes in expression of genes important for normal cellular functions and growth properties, including proto-oncogenes and tumor suppressor genes. Nearly all cancers are clonal in origin—that is, they originate from a single progenitor cell rather than a group of cells. The development of cancer in a particular cell type or tissue is caused by a series of specific mutations, each of which could be caused by DNA replication errors or unrepaired endogenous or exogenous DNA damage or could be the result of inherited mutations. For the most common cancers, multiple genetic events occur in many different genes during the process of carcinogenesis, suggesting that an early and perhaps necessary event in the cancer process is an underlying defect in mechanisms to maintain genomic stability. In fact, alterations in the specific genes required for recognizing, processing, and responding to DNA damage may result in an enhanced rate of accumulation of additional mutations, recombinational events, chromosomal abnormalities, and gene amplification. Whether such a “mutator phenotype” is a required event for human tumorigenesis remains a controversial issue, but cellular responses to DNA damage are clearly important determinants of both cancer development and outcomes following cancer treatments.
Cancer cells must be able to tolerate increased amounts of unrepaired DNA damage associated with genomic instability, and therefore inactivate DNA damage–inducible signaling and checkpoint pathways. Therefore, DNA repair and the DNA damage response are essential not only for the basic processes of transcription and replication necessary for cellular survival, but also for maintaining genomic stability and avoiding the development of malignancies. This chapter reviews the major DNA repair mechanisms active in mammalian cells, and our understanding of DNA damage signaling pathways that integrate with other cellular processes regulating transcription, replication, cell division and apoptosis in response to DNA damage. The relevance of these mechanisms to cancer is explored by focusing on several human cancer predisposition syndromes caused by underlying defects in DNA damage processing. Dysregulation of the DNA damage response is also associated with increased sensitivity or resistance of tumors to various classes of therapeutics, and can be further exploited. Finally, new opportunities for cancer treatment will be discussed based on targeting DNA repair or DNA repair deficiencies and genomic instability.
Studies of cancer genetics have defined three general groups of genes involved in the development of human cancers: oncogenes, tumor suppressor genes, and DNA damage repair and response genes. The latter set of genes is particularly important to hereditary cancer susceptibility owing to their direct involvement in maintaining genomic stability. Much of what we know regarding cancer genes in sporadic tumors comes from the study of relatively rare inherited cancer syndromes caused by mutations passed along in the germline DNA of families and predisposing to the development of cancers, often at a very young age and at a high incidence, in affected carriers. Individuals who inherit a germline mutation in genes involved in or required for DNA repair usually are at increased risk for the development of cancer because of the enhanced frequency of mutations and increased genomic instability. Susceptibility to cancer may also be affected by environmental factors and multiple low-penetrance modifier genes.
Many converging lines of experimental evidence reveal the complexity of the cellular responses to DNA damage and their role in malignant transformation. A number of interrelated biochemical pathways exist that influence the following actions: (1) the metabolism of potentially mutagenic or carcinogenic agents, (2) the efficiency and manner by which damaged DNA is recognized and repaired, (3) cell cycle progression and the coordination of DNA replication and cell division relative to the repair of lesions, and (4) the decision point determining survival or the active induction of programmed death of cells carrying different types and amounts of DNA damage. Many cellular pathways have evolved that require hundreds of gene products for the repair of DNA damage, and involve excision of damaged DNA bases, joining of broken DNA strands, or replication bypass of DNA lesions ( Table 11.1 ). The central role of DNA damage responses in neoplastic transformation has been highlighted by the discovery that mutations in numerous classes of genes required for DNA repair and the maintenance of genomic integrity result in a predisposition to the development of many malignancies. In fact, a number of rare, inherited disorders have been described that appear to be caused by defects in the repair of DNA lesions ( Table 11.2 ), and many of these are associated with an increased risk of the development of cancers.
| DNA Repair | Pathway Type of DNA Damage | Approximate No. of Genes |
|---|---|---|
| Nucleotide excision repair | Bulky or helix-distorting DNA adducts, for example, ultraviolet photoproducts and carcinogen adducts |
37 |
| Base excision repair | Oxidative DNA damage; spontaneous depurination | 40 |
| Mismatch repair | Mispaired nucleotides 1–15 nucleotide insertion-deletion loops |
26 |
| Homologous recombination | Double-strand DNA breaks, DNA cross-links | 20 |
| Nonhomologous end joining | Double-strand DNA breaks | 10 |
| Syndrome | Gene(s) | Biologic Functions | Clinical Features | Hypersensitivities |
|---|---|---|---|---|
| Xeroderma pigmentosum | XPA-XPG | Nucleotide excision repair | Sunlight hypersensitivity | UVR, chemical carcinogens |
| XPV | Translesional DNA synthesis | Neurologic defects; skin cancers | ||
| Cockayne syndrome | CSA, CSB | Transcription-coupled repair | Growth retardation | UVR, chemical carcinogens |
| XPB, XPD | Mental retardation Reactive oxygen species |
|||
| XPG | Premature aging; sunlight hypersensitivity | |||
| Trichothiodystrophy | XPB, XPD, TTDA | Nucleotide excision repair; transcription | Sulfur-deficient brittle hair; dry, scaly skin; mental and physical retardation; sunlight sensitivity | UVR |
| Hereditary nonpolyposis colorectal cancer (Lynch syndrome) | MLH1, MSH2, MSH6, PMS2 | Mismatch repair | 6-Thioguanine and cisplatin resistance. Colorectal, endometrial, gastric, bile duct cancers | |
| Ataxia-telangiectasia | ATM | DNA damage-responsive kinase | Cerebellar ataxia; telangiectasia; immunodeficiency; lymphomas | Ionizing radiation |
| Ataxia-telangiectasia–like disease | MRE11 | Double-strand break repair | Similar to ataxia-telangiectasia | |
| Nijmegen breakage syndrome | NBS1 | Double-strand break repair | Microcephaly; immunodeficiency; lymphomas; neuroblastoma; rhabdomyosarcoma | Ionizing radiation |
| Bloom syndrome | BLM | DNA helicase; homologous recombination at stalled replication forks? | Sunlight hypersensitivity; growth retardation; leukemias, lymphomas; breast and intestinal cancers | UVR, hydroxyurea |
| Werner syndrome | WRN | DNA helicase; homologous recombination?; translesional synthesis? | Premature aging; atherosclerosis; soft tissue sarcomas; melanoma, thyroid cancer | 4-NQO, camptothecin; hydroxyurea |
| Rothmund-Thomson syndrome | RECQL4 | DNA helicase | Growth deficiency; sunlight sensitivity; osteogenic sarcomas; squamous cell carcinomas | UVR |
| Fanconi anemia | FANCA–G | Interstrand cross-link repair | Growth retardation | Bifunctional alkylating agents |
| BRCA2 | Homologous recombination | Anatomic defects; bone marrow failure; myeloid leukemia; squamous cell cancers | Ionizing radiation | |
| Li-Fraumeni syndrome | p53 | Apoptosis; cell cycle checkpoints; nucleotide excision repair | Breast cancer; brain cancers; adrenocortical carcinoma; leukemia; bone and soft tissue sarcomas | ? |
| Li-Fraumeni–like syndrome | CHEK2 | DNA damage responsive kinase | Similar to Li-Fraumeni syndrome | |
| Breast-ovarian cancer syndrome | BRCA1, BRCA2 | Double-strand break repair | Breast cancer, ovarian cancer | Ionizing radiation, cisplatin, PARP inhibitors |
DNA undergoes many types of spontaneous modifications, and it also can react with physical and chemical agents, some of which are endogenous products of normal cellular metabolism (e.g., reactive oxygen species), whereas others, including ionizing radiation and ultraviolet (UV) light, are threats from the external environment ( Fig. 11.1 ). One pronounced example is exposure to genotoxic compounds in cigarette smoke, which currently are responsible for the most common cancers in Western countries. Most active chemotherapeutic agents function by damaging DNA through alkylation, cross-linking, and other means, and mechanisms to repair these lesions determine the sensitivity and resultant resistance of a tumor to such treatments. Damage to DNA can cause genetic mutations, and these mutations can lead to the development of cancer. A complex set of cellular surveillance and repair mechanisms has evolved to reverse or limit potentially deleterious DNA damage. Some of these DNA repair systems are so important that life cannot be sustained without them. An increasing number of human hereditary diseases that are characterized by severe developmental problems or a predisposition to cancer have been linked to deficiencies in DNA repair (see Table 11.2 ).
The results of DNA damage are diverse and usually adverse. Acute effects arise from disturbed DNA metabolism, triggering cell cycle arrest or cell death. Long-term effects result from irreversible mutations contributing to oncogenesis and inherited genetic disorders. Many lesions block transcription, which has elicited the development of a dedicated repair system, transcription-coupled repair (TCR), which displaces or removes the stalled RNA polymerase and ensures preferential repair of lesions within the transcribed strand of expressed genes. Transcriptional stress due to DNA lesions that block RNA polymerase, and DNA strand breaks caused by DNA damage or stalled replication forks, constitute two major signals for DNA damage–inducible responses, including apoptosis, through both p53-dependent and p53-independent mechanisms.
Lesions also may interfere with DNA replication. A class of more than 17 specialized DNA polymerases that are devoted to overcoming damage-induced replication stress has been discovered. These special polymerases take over temporarily from the stalled replicative DNA polymerases. Although translesion polymerases protect the genome, this solution to replication blocks comes at the expense of a higher replicative error rate, and mutations in some of these polymerases cause cancer susceptibility. Therefore, detection of DNA lesions may occur by blocked transcription, replication, or specialized sensors.
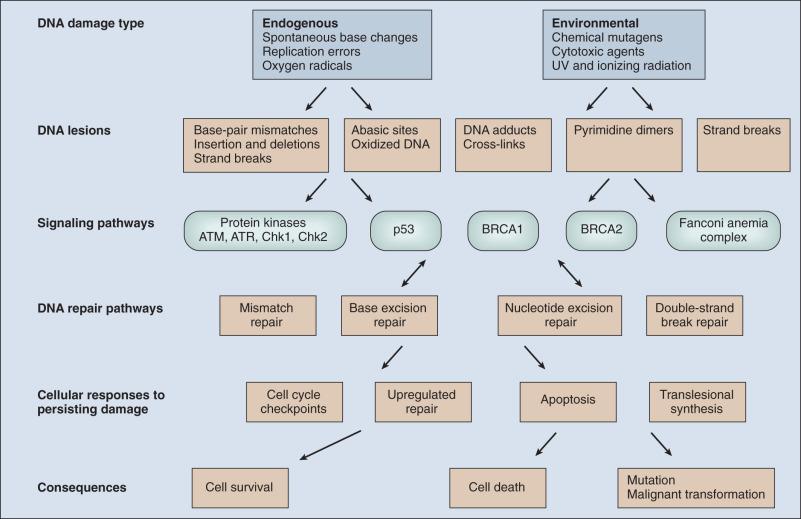
DNA damage checkpoints initially were defined as regulatory pathways that control the ability of cells to arrest the cell cycle in response to DNA damage, perhaps allowing time for repair or other cellular functions. However, in addition to controlling cell cycle arrest, proteins involved in these pathways have been shown to control the activation of DNA repair pathways, the movement of DNA repair proteins to sites of DNA damage, and activation of transcriptional responses. When damage is too significant, a cell may opt for the ultimate mode of rescue by initiating apoptosis to benefit the organism at the expense of losing a cell. As the DNA damage response pathway has been better defined at a molecular level, it has been seen as a network of interacting pathways that together execute the response. Initial recognition of DNA damage occurs by a variety of damage-specific DNA binding proteins that, either by themselves or together with complexes of associated proteins not directly involved in DNA repair, signal the DNA damage response. Initiation of DNA damage signaling pathways often is carried out by the phosphoinositide-3-kinase-related proteins which include ataxia-telangiectasia mutated (ATM) and ATM-Rad3-related (ATR) proteins, DNA-PK, the checkpoint kinases CHEK1 and CHEK2, and others. ATM plays a critical role in DNA damage signaling originating at double-strand breaks (DSBs), whereas ATR responds to single-strand DNA regions. Many of these protein kinases are themselves targets for phosphorylation and activation; they then further target downstream gene products critical to oncogenesis such as p53 and BRCA1. The ultimate targets of this highly regulated DNA damage response include mechanisms for DNA repair, and although much of DNA repair is constitutive, a number of regulatory connections between the DNA damage response pathway and DNA repair have emerged. In mammals, a large number of genes involved in DNA repair are transcriptionally upregulated in response to DNA damage, suggesting that many facets of repair are inducible. In fact, the p53 tumor suppressor gene is a central mediator of the DNA damage–inducible transcriptional response in humans, and p53 -mutant mammalian cells are deficient in several aspects of DNA repair. Therefore the mammalian DNA damage–inducible response pathway is highly regulated, and fine-tuned to determine if a particular cell type proceeds to a cell cycle checkpoint and DNA repair, or cell death, after a significant damage insult. Defects at any level of these pathways can alter repair and result in carcinogenesis ( Fig. 11.2 ).
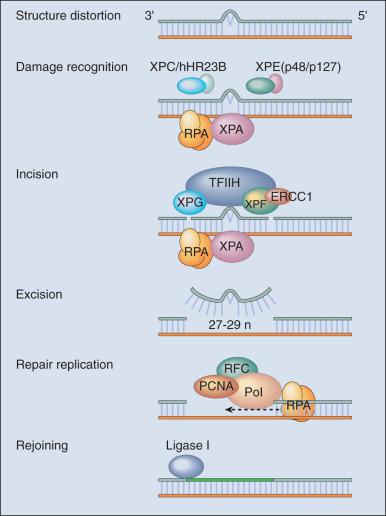
DNA repair may be defined as those cellular responses associated with the restoration of the normal nucleotide sequence after events that damage or alter the genome. Given the wide variety of DNA damage a cell encounters, it is not surprising that there is an equally large number of repair systems available to handle these insults. Indeed, many are broadly overlapping and interacting, with several sharing certain strategies and even specific gene products. In humans, a great deal has been learned regarding DNA repair from the often rare, autosomal recessive hereditary syndromes associated with defects in DNA repair genes.
The most versatile and ubiquitous mechanisms for DNA repair are those in which the damaged or incorrect part of a DNA strand is excised and the resulting gap is filled through repair replication with use of the complementary strand as a template. The redundancy of genetic information provided by the duplex DNA structure is essential to the maintenance of the genome by this “cut and patch” mode known as excision repair. Each DNA strand can serve as a template for replication-based repair of the other strand. Nucleotide excision repair (NER) functions to remove many types of lesions, including bulky base adducts of chemical carcinogens, intrastrand cross-links (ICLs), and UV-induced cyclobutane pyrimidine dimers (CPDs) and 6-4 photoproducts. Such lesions may serve as structural blocks to transcription and replication due to distortion of the helical conformation of DNA, and they also may result in mutations if translesional replication occurs or if they are not repaired correctly. The sequential steps for NER are (1) recognition of the damaged site, (2) incision of the damaged DNA strand near the site of the defect, (3) removal of a stretch of the affected strand containing the lesion, (4) repair replication to replace the excised region with a corresponding stretch of normal nucleotides with use of the complementary strand as a template, and (5) ligation to join the repair patch at its 3′ end to the contiguous parental DNA strand. This excision repair pathway can remove DNA damage from sites throughout the genome and is termed global genomic repair (GGR). The majority of human NER genes have been identified and cloned, and many have been shown to be mutated in hereditary NER-deficient, cancer-prone diseases.
A unique problem arises if a bulky lesion is encountered by a translocating RNA polymerase making messenger RNA before repair enzymes have removed the damage and restored intact DNA. The polymerase may be arrested at the site of the lesion and prevent access to the damage by repair enzymes. Furthermore, the arrest of transcription in human cells is a strong signal for p53 activation and can trigger apoptosis. In this situation, a dedicated excision repair pathway known as transcription-coupled repair comes to the rescue to displace the RNA polymerase, and then efficiently repairs the blocking lesion so that transcription may resume and the cell may survive.
It has become apparent that the GGR subpathway of NER is damage inducible and highly regulated by both transcriptional and posttranslational mechanisms after DNA damage, in concert with damage-inducible cell cycle checkpoints and apoptosis. In fact, the p53 gene, central to maintaining genomic stability in human cells, is required for efficient GGR of UV light– and carcinogen–induced DNA damage, and functions as a DNA damage–activated transcription factor that directly regulates the expression of several NER damage recognition genes. Similarly, several other important cancer-related genes have been shown to transcriptionally regulate the DNA damage recognition NER genes XPC and DDB2, including BRCA1 and E2F1. Therefore the GGR pathway of NER appears relevant to suppressing DNA damage–induced malignancy, and highly regulated by genes involved in tumor suppression.
Despite the plethora of mechanisms for DNA repair described herein, it is likely that the cellular replication machinery will nevertheless encounter lesions in the DNA template strand that block replication during each cell cycle. The solution adapted by cells is DNA damage tolerance, in which DNA is synthesized past the damaged bases and subsequently excised after the replication fork has passed—a process known as translesional synthesis (TLS) and carried out by a class of specialized error-prone DNA polymerases. These Y-family DNA polymerases take over temporarily from the stalled replicative DNA polymerases. They have more flexible base-pairing properties that permit translesion DNA synthesis, with each polymerase probably designed for a specific category of injury. Although translesion polymerases protect the genome, this solution to replication blocks comes at the expense of a higher error rate. For instance, inherited defects in polymerase eta (pol η), encoded for by the XPV/POLH/RAD30 gene, which specializes in relatively error-free bypassing of UV-induced CPDs, cause a variant form of the skin cancer–prone disorder Xeroderma pigmentosum (XP).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here