Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
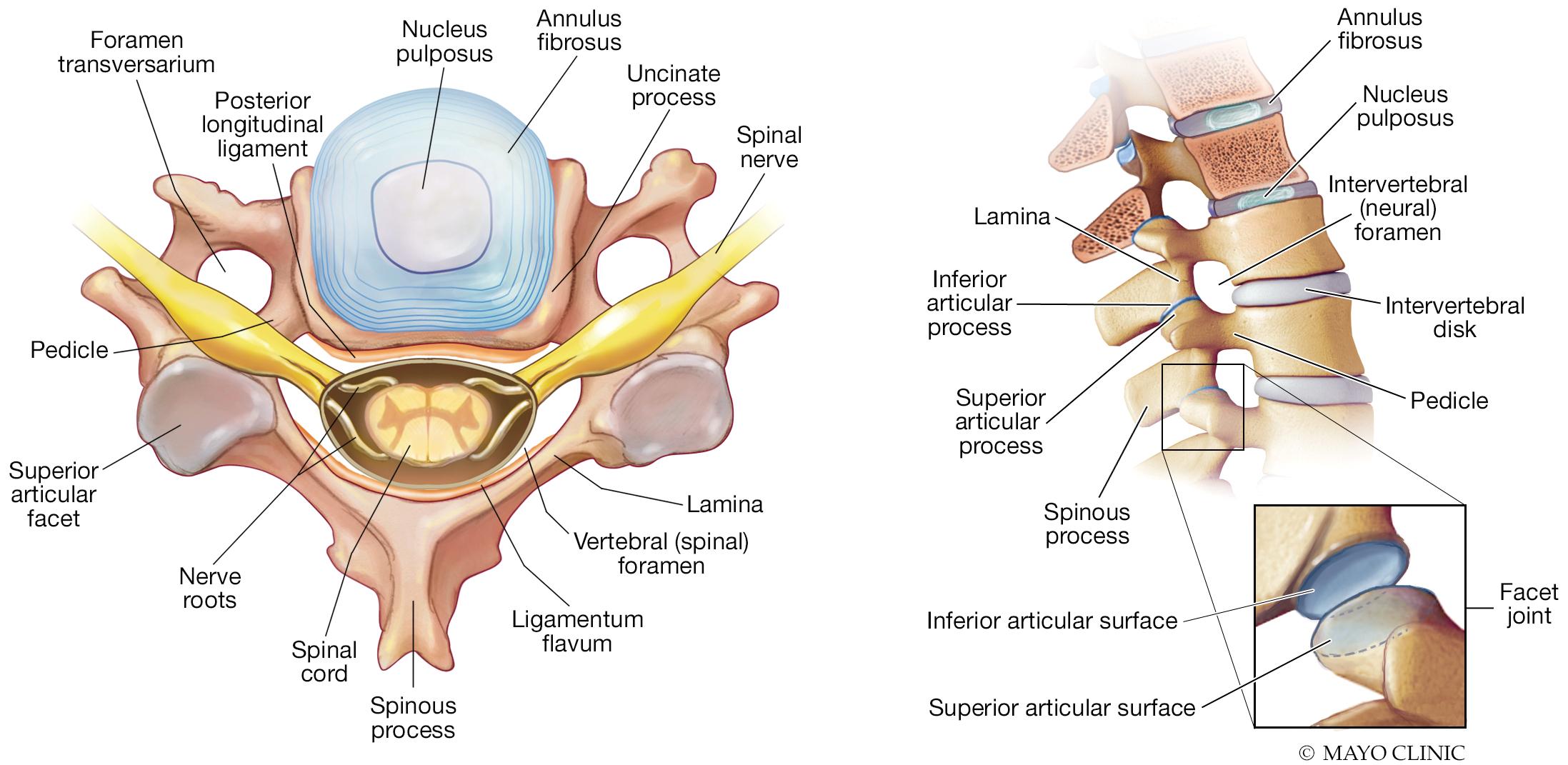
The vertebral column consists of 24 individual vertebrae (7 cervical, 12 thoracic, and 5 lumbar), in addition to the sacrum and coccyx. Basic anatomy of individual vertebrae and the vertebral column is illustrated in Fig. 14.1 . Briefly, the anterior section of vertebrae consists of a body and adjacent vertebral bodies that are separated in intervertebral disks. Posteriorly, vertebrae consist of one midline spinous process and two lateral transverse processes. The lamina is the region of bone between spinous and transverse processes and the pedicle is the segment of bone connecting transverse processes with the vertebral body. Posteriorly, adjacent vertebrae articulate at facet joints. The spinal cord is contained within the bony confines of the central canal of the vertebral column.
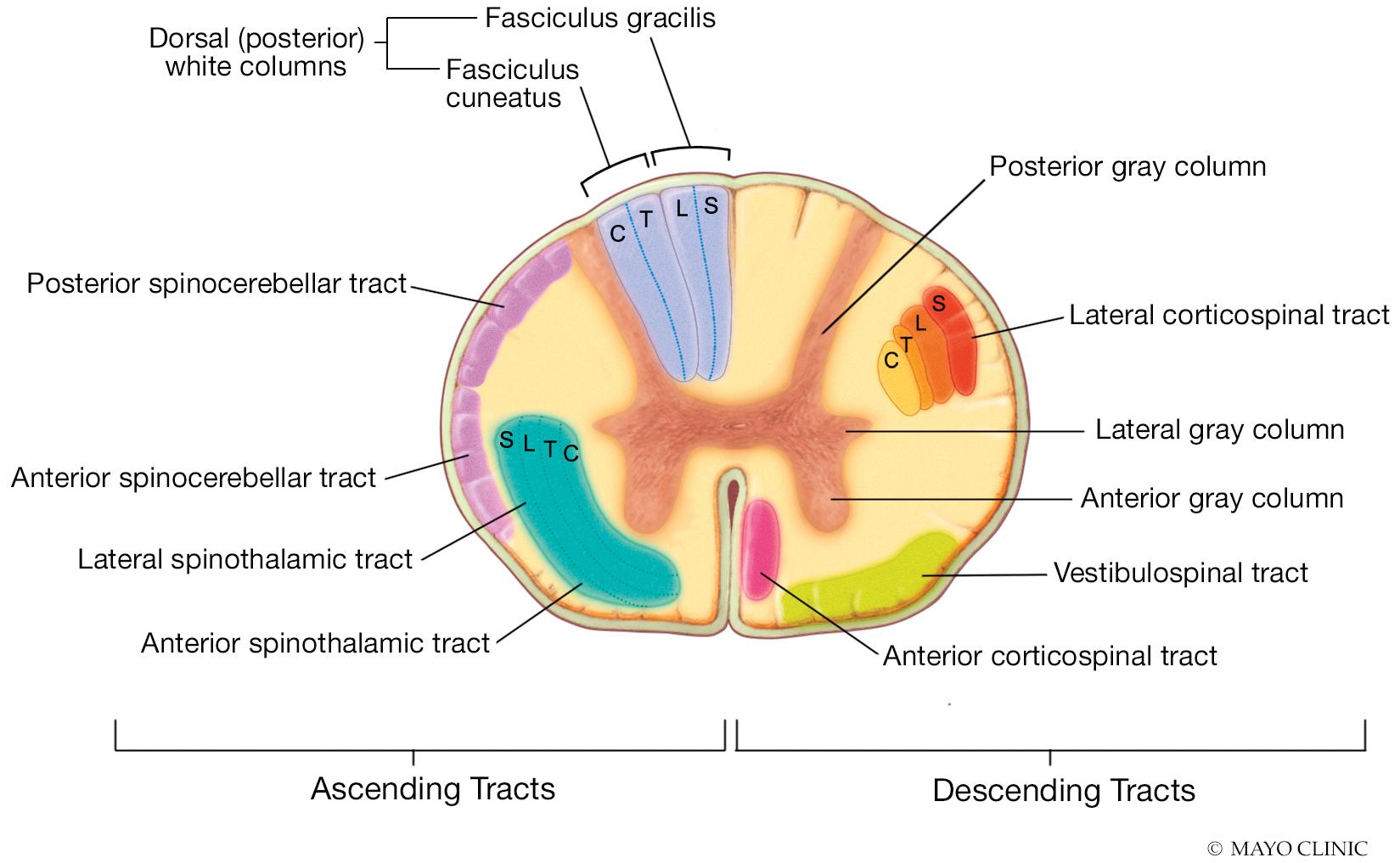
The spinal cord extends from the medulla oblongata of the brainstem to the lumbar region of the vertebral column. The cross-sectional anatomy of the spinal cord is illustrated in Fig. 14.2 . At each vertebral level, the spinal cord gives off bilateral nerve roots. Posterior nerve roots carry afferent information from the periphery to the spinal cord. Deep touch and vibration sensation is carried by the spinal cord to the brain via the posterior columns, whereas pain and temperature information is carried to the brain via the lateral spinothalamic tracts. The corticospinal tract carries efferent information from the brain through the spinal cord, and this information leaves the anterior roots of the spinal cord. Innervation of muscles by spinal roots is summarized in Table 14.1 . Blood is supplied to the spinal cord by two posterior spinal arteries that predominantly supply the posterior spinal roots and posterior columns, and an anterior spinal artery that supplies the remainder of the spinal cord.
| Muscle | Action | Roots | Nerve |
|---|---|---|---|
| Serratus anterior | Anterior movement of shoulder | C5, C6, C7 | Long thoracic |
| Rhomboids | Scapula adduction | C4, C5 | Dorsal scapular |
| Deltoid | Arm abduction | C5, C6 | Axillary |
| Biceps brachii | Forearm flexion and supination | C5, C6 | Musculocutaneous |
| Flexor carpi ulnaris | Hand flexion | C7, C8, T1 | Ulnar |
| Adductor pollicis | Thumb adduction | C8, T1 | Ulnar |
| Pronator teres | Forearm pronation | C6, C7 | Median |
| Abductor pollicis | Thumb metacarpal abduction | C8, T1 | Median |
| Triceps brachii | Forearm extension | C6, C7, C8 | Radial |
| Extensor carpi radialis | Hand extension | C5, C6 | Radial |
| Iliopsoas | Hip flexion | L1, L2, L3 | Femoral |
| Quadriceps femoris | Knee extension | L2, L3, L4 | Femoral |
| Adductor longus | Thigh adduction | L2, L3, L4 | Obturator |
| Gluteus medius | Thigh abduction and medial rotation | L4, L5, S1 | Superior gluteal |
| Gluteus maximus | Thigh abduction | L5, S1, S2 | Inferior gluteal |
| Biceps femoris | Leg flexion | L5, S1, S2 | Sciatic |
| Tibialis anterior | Foot dorsiflexion | L4, L5, S1 | Deep peroneal |
| Tibialis posterior | Foot plantar flexion | L4, L5 | Tibial |
| Gastrocnemius | Knee flexion and foot plantar flexion | S1, S2 | Tibial |
| Soleus | Foot plantar flexion | S1, S2 | Tibial |
| Rectal sphincter | Rectal sphincter contraction | S2, S3, S4 | Pudendal |
The mobility of the cervical spine makes it vulnerable to injury, especially hyperextension injury. It is estimated that cervical spine injury occurs in 1.5% to 3% of all major trauma victims. Approximately 4% to 5% of patients with traumatic head injury have a concurrent injury of the spine, typically occurring in the upper cervical spine (i.e., C1–C3). Trauma can also injure the thoracic and lumbar spinal cord segments.
The clinical manifestations of acute spinal cord injury depend on both the extent and the site of injury. Acute spinal cord injury initially produces a state of spinal shock that is characterized by flaccid muscle paralysis with loss of sensation below the level of injury. It should be noted that spinal shock refers to the loss of neurologic function following spinal cord injury, whereas neurogenic shock refers to the manifestations of autonomic nervous system impairment that can result following central nervous system injury.
The extent of injury is commonly described in terms of the American Spinal Injury Association (ASIA) classification system ( Table 14.2 ), which characterizes the injury in terms of both motor and sensory impairment. A score of A indicates a “complete” injury in which all motor and sensory function is lost below the level of the lesion, including function at the lower sacral segments of S4 and S5. Lower sacral neurologic function is determined by assessing rectal tone and sensation. Scores of B through D are assigned to “incomplete” lesions in which some degree of spinal cord integrity is maintained below the level of injury. A score of E indicates “normal” spinal cord function.
| Category | Description | Definition |
|---|---|---|
| A | Complete | No motor function below level of lesion or in sacral segments S4 and S5 |
| B | Incomplete | Sensory but not motor function is preserved below neurologic level and includes S4–S5 segments |
| C | Incomplete | Motor function is preserved below level of injury and more than half of key muscles below neurologic level have a grade less than 3 |
| D | Incomplete | Motor function is preserved below level of injury and more than half of key muscles below neurologic level have a grade of 3 or more |
| E | Normal | Sensory and motor function are intact |
The extent of physiologic effects from spinal cord injury depends on the level and extent of injury, with the most severe physiologic derangements occurring with complete injury to the cervical cord and lesser perturbations occurring with less complete injury and more caudal cord injuries. Reductions in blood pressure are common, especially with cervical cord injury, and are influenced by (1) loss of sympathetic nervous system activity and a decrease in systemic vascular resistance, and (2) bradycardia resulting from loss of the T1 through T4 sympathetic innervation to the heart (i.e., loss of cardiac accelerator innervation). Hypotension can also occur with thoracic and lumbar cord injuries, although typically it is less severe than with cervical injuries. With cervical and upper thoracic cord injury, the major cause of morbidity and mortality is alveolar hypoventilation combined with an inability to clear bronchial secretions. Respiratory muscles are not affected with lumbar and low thoracic injuries, so minimal respiratory impairment can be expected with these injuries. Aspiration of gastric contents, pneumonia, and pulmonary embolism can also occur.
Cervical spine radiographs are obtained for a large percentage of patients who come for treatment of various forms of trauma and are intended to identify both suspected and occult cervical spine injuries. However, the probability of cervical spine injury is minimal in patients younger than 60 years of age who meet the following five criteria: (1) no midline cervical spine tenderness, (2) no focal neurologic deficits, (3) normal sensorium, (4) no intoxication, and (5) no painful distracting injury. Patients who meet these criteria do not require routine imaging studies to rule out occult cervical spine injury.
An estimated two-thirds of trauma patients have multiple injuries that can interfere with cervical spine evaluation. Evaluation ideally includes computed tomography (CT) or magnetic resonance imaging (MRI), but imaging may not be practical in some cases because of the risk of transporting patients in an unstable condition. For this reason, standard radiographic views of the cervical spine, often taken with a portable x-ray machine, are frequently relied upon to evaluate for the presence of cervical spine injury and associated instability. For cervical spine imaging to have greatest utility, the entire cervical spine (including the body of the first thoracic vertebra) must be visible. Images are analyzed for alignment of the vertebrae (lateral view) and presence of fractures (all views), and the disk and soft tissue spaces are evaluated. The sensitivity of plain radiographs for detecting cervical spine injury is less than 100%, so the likelihood of cervical spine injury must be interpreted in conjunction with other clinical signs, symptoms, and risk factors. If there is any doubt, it is prudent to treat all acute cervical spine injuries as potentially unstable until proven otherwise.
Treatment of a cervical fracture or dislocation entails immediate immobilization to limit neck motion. Soft neck collars have little effect in limiting movement of the neck. Hard neck collars limit neck flexion and extension, but only by about 25%; therefore halo-thoracic devices are often needed to provide effective immobilization and traction to prevent cervical spine movement. During direct laryngoscopy with manual inline stabilization (MILS), any cervical collars are removed and an assistant’s hands are placed on each side of the patient’s face while downward pressure is applied against a firm table surface to hold the head immobile in a neutral position and minimize cervical flexion and extension. MILS can be done with the assistant either behind the patient adjacent to the intubator or with the assistant orthogonal to the patient and the assistant’s forearms resting on the patient’s chest or clavicles. Hard collars tend to limit mouth opening, but with their removal and application of MILS 56% of patients will improve their Cormack-Lehane grade. Attention must be paid while grasping the head not to apply traction and elongate the cord, which may compromise cord perfusion. Not only can movement of the neck in the presence of cervical spine injury cause mechanical deformation of the spinal cord, but there is an even greater risk that neck motion that elongates the cord will compromise spinal cord blood supply as a result of narrowing the longitudinal blood vessels. In fact, maintenance of spinal cord perfusion pressure may be of more importance than positioning for prevention of spinal cord injury in the presence of cervical spine injury.
Patients with acute spinal cord injury often require special precautions during airway management. When laryngoscopy is performed, neck movement must be minimized and hypotension avoided so that spinal cord perfusion pressure can be maintained. However, fear of possible spinal cord compression must not prevent necessary airway interventions. Extensive clinical experience supports the use of direct laryngoscopy for orotracheal intubation provided that (1) maneuvers are taken to stabilize the head during the procedure and thus to avoid hyperextension of the neck, (2) prior evaluation of the airway did not suggest the likelihood of any associated technical difficulties, and (3) adequate blood pressure and oxygenation are maintained during airway management. Otherwise, video laryngoscopes are reasonable alternates to direct laryngoscopy. Awake fiberoptic laryngoscopy with topical anesthesia is another alternative to direct laryngoscopy if the patient is cooperative and airway trauma—with associated blood, secretions, and anatomic deformities—does not preclude visualization with the fiberscope. It is important to remember that coughing during topical anesthetization of the airway and fiberoptic intubation may result in cervical spine movement. It is reasonable to have an assistant maintain MILS during all airway manipulations. There is no evidence of increased neurologic morbidity after elective or emergency orotracheal intubation of anesthetized or awake patients who have an unstable cervical spine if appropriate steps are taken to minimize neck movement. Awake tracheostomy is reserved for the most challenging airway conditions, in which neck injury, combined with facial fractures or other severe anomalies of airway anatomy, makes securing the airway by nonsurgical means difficult or unsafe. Airway management in the presence of cervical spine injury should be dictated by common sense, not dogmatic approaches. Certainly, clinical experience supports the safety of a variety of airway management techniques.
The absence of compensatory sympathetic nervous system responses in patients with cervical or high thoracic spinal cord injury makes these patients particularly vulnerable to significant decreases in blood pressure following changes in body position, blood loss, or positive pressure ventilation. To minimize these effects, liberal intravenous infusion of crystalloid solutions may be necessary to maintain intravascular volume, which has been compromised by vasodilation. Acute blood loss should be treated promptly. Electrocardiographic abnormalities are common during the acute phase of spinal cord injury, especially with cervical cord injuries. Breathing is best managed by mechanical ventilation, since abdominal and intercostal muscle weakness or paralysis is exacerbated by general anesthesia and increases the likelihood of respiratory failure with ensuing hypoxemia and hypercapnia. Body temperature should be monitored and manipulated because patients tend to become poikilothermic in dermatomes below the level of the spinal cord lesion. Maintenance of anesthesia is targeted at ensuring physiologic stability and facilitating tolerance of the endotracheal tube. Volatile and intravenous anesthetics are both satisfactory. Nitrous oxide should be used with great caution, if at all, given concerns for coexisting trauma and air entrainment in closed spaces, as can occur with basilar skull fracture or rib fracture. Arterial hypoxemia is common following spinal cord injury, which emphasizes the need for continuous pulse oximetry and oxygen supplementation.
Muscle relaxant use should be based on the operative site and the level of spinal cord injury. Succinylcholine does not provoke excessive release of potassium during the first few hours after spinal cord injury. Use of a nondepolarizing relaxant, with mask ventilation and possible cricoid pressure, is another alternative to airway management during anesthetic induction and before laryngoscopy. A nondepolarizing relaxant may also facilitate patient positioning.
Sequelae of chronic spinal cord injury include impaired alveolar ventilation, autonomic hyperreflexia, chronic pulmonary and genitourinary tract infections, renal stones and possible renal dysfunction, anemia, and altered thermoregulation. Injuries that occur more rostral along the spinal cord tend to have more significant systemic effects. Chronic urinary tract infection reflects the inability to empty the bladder completely and predisposes to calculus formation. As a result, renal failure may occur and is a common cause of death in patients with chronic spinal cord injury. Prolonged immobility leads to osteoporosis, skeletal muscle atrophy, and decubitus ulcers. Immobility also predisposes patients to deep venous thrombosis, so prophylactic measures such as use of compression stockings, low-dose anticoagulant therapy, and insertion of inferior vena cava filters may be indicated. Pathologic fractures can occur when these patients are moved. Pressure points should be well protected and padded to minimize the likelihood of trauma to the skin and the development of decubitus ulcers.
Chronic pain and depression are common problems following spinal cord injury. Nerve root pain is localized at or near the level of injury. Visceral pain is produced by distention of the bladder or bowel. Phantom pain can occur in areas of complete sensory loss. There may also be a loss of lifestyle and ability that frustrates and depresses many. As a result, many patients are often treated with antidepressants and analgesics, including opioids that require attention when anesthetic management is planned (e.g., avoidance of serotonin syndrome by judicious use of serotonergic agents such as ondansetron or fentanyl for patients on selective serotonin reuptake inhibitors [SSRIs] or monoamine oxidase inhibitors [MAOIs]).
Several weeks after acute spinal cord injury, spinal cord reflexes gradually return, and patients enter a more chronic stage characterized by overactivity of the sympathetic nervous system and involuntary skeletal muscle spasms. Baclofen, which potentiates the inhibitory effects of γ-aminobutyric acid (GABA), is useful for treating spasticity. Abrupt cessation of baclofen therapy, as may occur with hospitalization for an unrelated problem, may result in withdrawal reactions that can include seizures. Diazepam and other benzodiazepines also facilitate the inhibitory effects of GABA and may have utility in the management of a patient receiving baclofen. Spasticity refractory to pharmacologic suppression may require surgical treatment via dorsal rhizotomy or myelotomy, but usually implantation of a spinal cord stimulator or subarachnoid baclofen pump will be undertaken before rhizotomy is considered.
Spinal cord injury at or above the fifth cervical vertebra may result in apnea caused by denervation of the diaphragm (“C three, four, and five to keep the diaphragm alive ”). With function of the diaphragm intact, tidal volumes are likely sufficient, but coughing and secretion clearance are often impaired because of decreased expiratory reserve volumes from denervation of intercostal and abdominal muscles. Indeed, acute spinal cord injury at the cervical level is accompanied by marked decreases in vital capacity. Arterial hypoxemia is a consistent early finding following cervical spinal cord injury. Tracheobronchial suctioning has been associated with bradycardia and even cardiac arrest in these patients, so it is important to optimize arterial oxygenation before suctioning the airway.
Anesthetic management in patients with chronic spinal cord injury should focus on preventing autonomic hyperreflexia. When general anesthesia is selected, administration of muscle relaxants is useful to facilitate tracheal intubation and prevent reflex skeletal muscle spasms in response to surgical stimulation. Nondepolarizing muscle relaxants are the primary choice in this circumstance, since succinylcholine may provoke hyperkalemia, most commonly during the initial 6 months after spinal cord injury. Indeed, it seems reasonable to avoid succinylcholine after 24 hours from spinal cord injury.
The anesthesiologist must be aware of the potential for altered hemodynamics, especially with cervical and high thoracic cord lesions. These can manifest as wide alterations in both blood pressure and heart rate. In chronically immobile patients, the index of suspicion for pulmonary thromboembolism, which can manifest as alterations in hemodynamics and oxygenation, must be high. If intercostal muscle function is impaired, patients may be at high risk of postoperative hypoventilation and may have an impaired cough and a corresponding accumulation of secretions. Baclofen and benzodiazepines should be continued throughout the perioperative period to avoid withdrawal symptoms. Patients with impaired renal function may require close attention to fluid administration, serum electrolyte concentrations, and potential altered pharmacology of drugs eliminated by the kidney. Prophylaxis against deep venous thrombosis should be continued.
Autonomic hyperreflexia appears following spinal shock and in association with return of spinal cord reflexes. This reflex response can be initiated by cutaneous or visceral stimulation below the level of spinal cord injury. Surgery and distention of a hollow viscus such as the bladder or rectum are common stimuli.
Stimulation below the level of spinal cord injury initiates afferent impulses that enter the spinal cord ( Fig. 14.3 ). Because of reflexes entirely within the spinal cord itself, these impulses elicit an increase in sympathetic nervous system activity along the splanchnic outflow tract. In neurologically intact individuals, this outflow would be modulated by inhibitory impulses from higher centers in the central nervous system, but in the presence of a spinal cord lesion this outflow is isolated from inhibitory impulses from above, so generalized vasoconstriction occurs below the level of the spinal cord injury. Because of the intense vasoconstriction, reflex bradycardia and cutaneous vasodilation occur above the level of the spinal cord injury, which is often inadequate to overcome the increased blood pressure. Nasal stuffiness reflects the vasodilation, while headaches and blurred vision reflect severe hypertension. This increase in blood pressure can result in cerebral, retinal, or subarachnoid hemorrhage as well as increased operative blood loss. Loss of consciousness and seizures may also occur, and cardiac arrhythmias are often present. Pulmonary edema reflects acute left ventricular failure resulting from dramatically increased afterload.
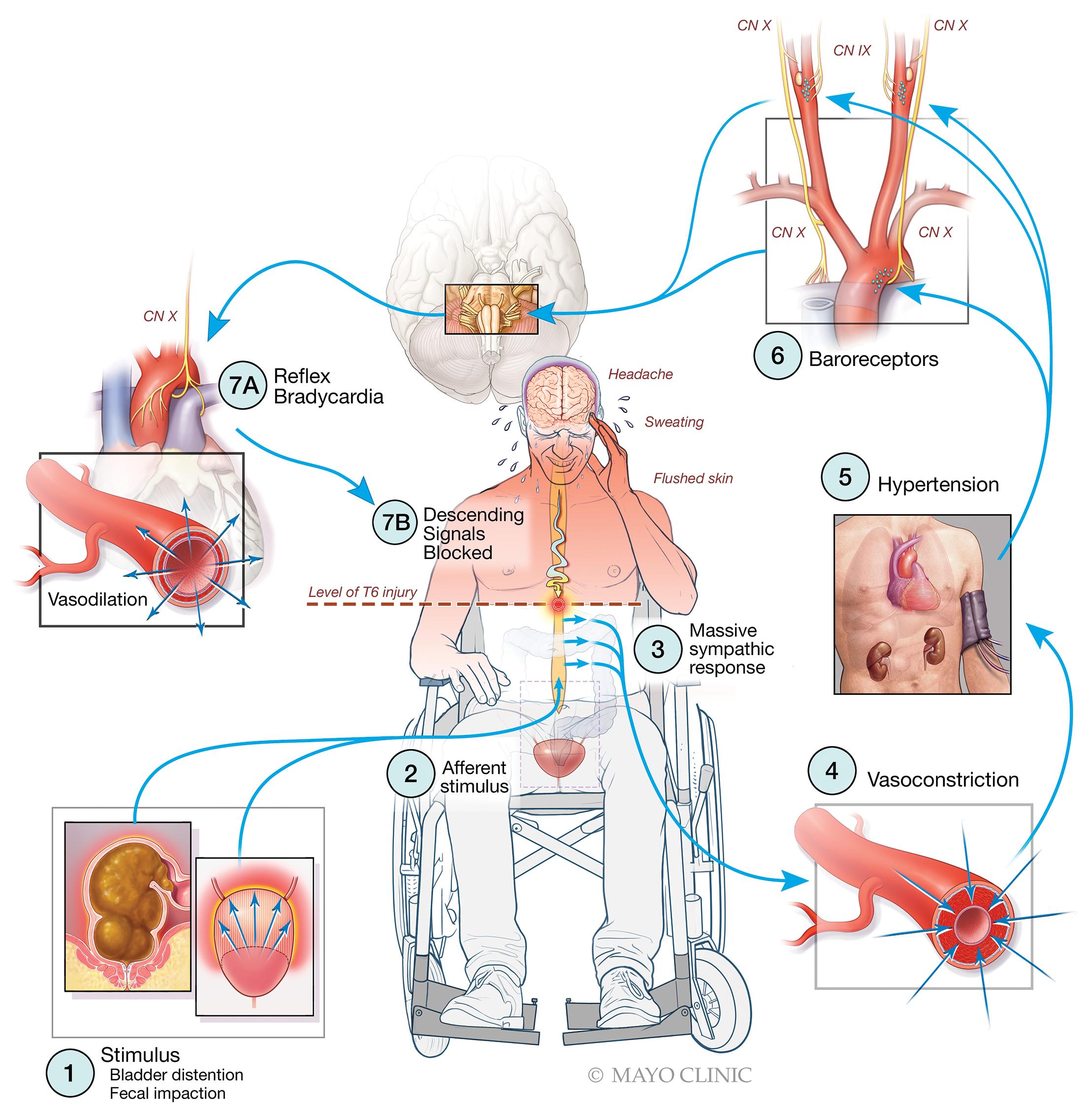
The incidence of autonomic hyperreflexia depends on the level of spinal cord injury. Approximately 85% of patients with lesions above T6 exhibit this reflex. It is unlikely to be associated with spinal cord lesions below T10. Also, in patients with cervical or high thoracic spinal cord lesions, those with complete lesions are more likely to exhibit autonomic hyperreflexia than those with incomplete lesions. The incidence and severity also diminish the further out from injury.
Management of patients at risk should begin with efforts to prevent the development of autonomic hyperreflexia. Patients who have no history of this reflex are still at risk of its occurrence during surgery simply because of the intense stimuli that surgery can produce. Before surgical or other stimulation is initiated in locations that lack sensory innervation, general, neuraxial, or regional anesthesia should be instituted. Epidural anesthesia has been described for the treatment of autonomic hyperreflexia provoked by uterine contractions during labor. However, epidural anesthesia may be less effective than spinal anesthesia in preventing autonomic hyperreflexia because of its relative sparing of the sacral segments and lesser block density. Blocking afferent pathways with topical local anesthetics applied to the urethra for a cystoscopic procedure does not prevent autonomic hyperreflexia because this form of anesthesia does not block the bladder muscle proprioceptors that are stimulated by bladder distention.
Regardless of the anesthesia technique selected, vasodilator drugs having a short half-life (e.g., clevidipine) should be readily available to treat sudden-onset severe hypertension. Persistence of hypertension requires continuous infusion of vasodilators, perhaps supplemented with longer-acting drugs such as hydralazine. It is important to note that autonomic hyperreflexia may first manifest postoperatively when the effects of the anesthetic drugs begin to wane.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here