Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Benign hyperplasia and carcinoma are the most common prostatic disorders and have an increasing importance in an ageing population. Inflammation and infection of the prostate ( prostatitis ) is a less common condition that occurs in a younger age group and is rather poorly defined clinically.
The normal prostate gland is about 3 cm long and 3 cm in diameter and weighs 20 to 25 g. The gland is situated immediately below the bladder neck so that the first 3 cm of the urethra lies within the gland ( Fig. 35.1 ), so the proximal urethral walls (the prostatic urethra ) are composed of glandular tissue. This contributes to the continence mechanism in men and is referred to as the internal sphincter mechanism. The urethra then passes through the pelvic floor muscle sheet, where it becomes the membranous urethra, and at this level is surrounded by the external urethral sphincter muscle. Prostatic hyperplasia or carcinoma may cause local urethral obstruction and carcinoma may invade and disrupt the sphincter mechanism.
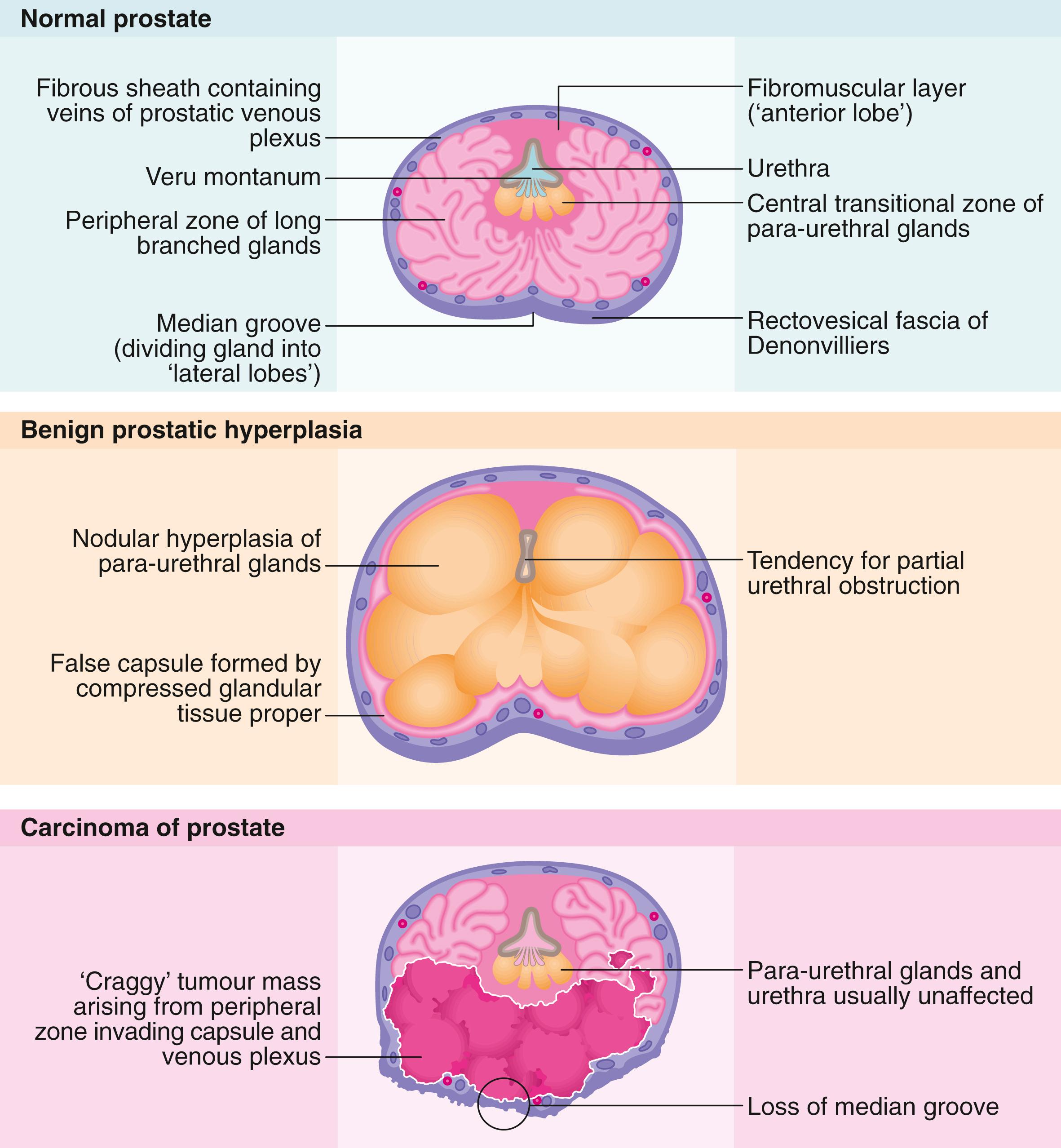

The posterior aspect of the gland is palpable rectally (see Fig. 35.1 ) and a median groove can usually be identified. This groove (or sulcus) is described as dividing the gland into two lateral lobes and tends to be obliterated in advanced prostatic cancer but is usually exaggerated in benign prostatic enlargement.
When the prostatic urethra is examined cystoscopically (see Fig. 35.3 , p. 475), an important landmark is an elongated mound on the ventral (or posterior) wall of the prostatic urethra known as the verumontanum (urethral crest), which can be variable in size and prominence. At its midpoint is a small depression, sometimes visible, into which the two ejaculatory ducts open. The posterior part of the gland above the ejaculatory ducts is known as the median lobe . If this becomes hypertrophied it may extend into the floor of the bladder (the surgical ‘middle lobe’); this may act as a flap valve and obstruct the bladder outlet.
As seen in Fig. 35.1 , the bulk of the normal prostate consists of up to 50 peripheral glandular lobules . These converge into about 20 separate ducts opening into the prostatic urethra, lateral to the verumontanum. As well as this glandular tissue proper, there is a zone of small paraurethral glands adjacent to the urethra, the transition zone . From middle age onwards, the transition zone tends to enlarge to cause benign prostatic hyperplasia ( BPH ). At the same time, the peripheral glandular tissue is compressed to form a fibrous outer ‘surgical capsule’. In contrast, prostate cancer arises most often in the peripheral glandular tissue, tending to spread outwards into bordering structures more often than obstructing the centrally located urethra. Even after transurethral prostatectomy, cancer can arise in the residual peripheral zone.
The normal prostate gland is surrounded by a filmy true capsule of little surgical significance; external to this, is a rich venous plexus which, in turn, is invested by a dense fascial sheath. During radical or endoscopic prostatectomy, it is important not to disturb this venous plexus, as it is a common source of bleeding during and after the operation. There are direct venous connections between the plexus and the vertebral extradural plexus, which provide a direct route for blood-borne dissemination of prostate cancer. Posteriorly, the prostatic fascial sheath is fused with the dense fascia of Denonvilliers . This provides a barrier against direct spread of cancer from the prostate to the rectum and vice versa.
Clinically-evident growth of the prostate, which may be associated with voiding (obstructive) urinary symptoms is termed benign prostatic enlargement (BPE) . BPH is the histological diagnosis and the cause of the enlargement, and the terms are often used interchangeably. This condition affects half of all men over 50 years, and the proportion increases with advancing age so that BPH is almost universal at 70 years. Approximately half of those with BPH are asymptomatic or have only mild symptoms. In about 50% of men over 60 years, however, hyperplasia produces enough symptoms for treatment to be considered.
In pathological terms, the paraurethral transition zone glands undergo nodular hyperplasia . This causes progressive symmetrical enlargement of the gland up to several times its normal size.
The prostate volume cannot be reliably estimated by digital examination and is best assessed by ultrasound. However, there is little relationship between prostatic volume and symptoms; the presence of a large prostate without symptoms is not an indication for treatment. Urine flow rate is determined by the calibre and length of the prostatic urethra and by detrusor contractility, not by prostatic bulk. Prostatic urethroscopy provides further anatomical detail, but no functional information.
Symptoms and signs of bladder outflow obstruction (summarised in Box 35.1 ) are usually gradual in onset. Benign causes are prostatic hyperplasia and the apparently independent disorder of bladder neck hypertrophy and fibrosis . Acute retention of urine may occur suddenly at any time and is commonly precipitated by bladder overfilling after excessive fluid intake. It is also a risk after general surgical or orthopaedic operations on older men, and also of any pelvic or perineal operations after adolescence. In some patients, the severity of prostatic symptoms fluctuates from month to month (and even perhaps with the season), making it difficult to decide whether an operation is necessary.
Symptoms formally assessed using the International Prostate Symptom Score (I-PSS). Each symptom is graded from 0 to 5 for the previous month. The total indicates the severity of symptoms: 0–7=mild; 8–19=moderate; 20–35=severe.
Incomplete bladder emptying after urination
Frequency—need for urination again after less than 2 hours
Intermittent flow—stopping and starting during urination
Urgency—difficult to postpone urination
Weak stream—often made worse by a full bladder or by straining
Straining to begin urination
Nocturia—number of times needing to urinate per night
It also includes a separate question on how this problem affects the patient’s quality of life, graded from 0 (delighted) to 6 (terrible).
Other symptoms (not scored)
Hesitancy, worse with a full bladder or at night
Postmicturition dribbling
Double micturition (‘pis-à-deux’)
Prostatic obstruction can progressively interfere with the patient’s ability to empty his bladder, but only 20% to 30% of patients have progressive symptoms, and 50% remain unchanged over 5 years. In progressive cases, the volume of residual urine gradually increases over weeks and months (i.e., chronic retention) and in a minority, this leads to a rise in intravesical pressure. In the latter, the threshold for the voiding reflex is reached more quickly and calls to void become more frequent. The stagnant residual urine is prone to infection, which exacerbates the symptoms. In chronic retention, the bladder becomes vastly distended and atonic, which can lead to overflow incontinence . In other cases, the detrusor muscle undergoes hypertrophy in an attempt to overcome the outflow obstruction ( Fig. 35.2 ). The normally smooth bladder lining then becomes trabeculated. Eventually, muscle fibre bundles are replaced by noncontractile fibrous tissue; this may explain why some patients fail to improve after obstruction is relieved. With a further rise in pressure, the depressions between the muscle bands deepen (sacculation) and eventually form bladder diverticula . Urinary stasis in the diverticula predisposes to stone formation.
A small proportion of patients with bladder outlet obstruction experience few local symptoms. In these, rising intravesical pressure can be transmitted back into the ureters and kidneys causing hydronephrosis and progressive renal parenchymal damage . Patients often present with systemic illness or symptoms, such as anorexia, apparently of non-renal origin. The renal failure may be accompanied by anaemia, dehydration, acidosis and infection. Bladder outflow obstruction in these patients is easily overlooked unless the bladder is examined for distension and plasma creatinine and urea measured. The symptom of nocturnal enuresis (i.e., incontinence at night) is a warning sign for high-pressure chronic retention. Emergency intervention is catheterisation, with later definitive treatment being bladder outlet surgery.
The principles of management of bladder outlet obstruction believed to be caused by BPE are outlined in Box 35.2 .
Assess the symptoms and the likely need for treatment from the history, particularly how much the symptoms bother the patient.
Estimate the severity of bladder outlet obstruction by ultrasound and by measuring urine flow rate (± urodynamics).
Investigate any disturbance of upper tract function and structure with renal function tests and ultrasound.
Exclude urinary tract infection by urine microscopy and culture.
Exclude prostatic carcinoma clinically, biochemically (prostate specific antigen) and by transrectal diagnostic ultrasound; if necessary, perform guided-needle biopsy of abnormal areas.
Treat renal failure and other systemic problems.
Consider whether catheter drainage of the bladder is required.
Cystoscope the patient to rule out other pathology and to define the anatomical problem.
Discuss with the patient what can be offered and at what risk, that is, drug treatment is first-line followed by bladder outlet surgery if symptoms fail to improve (transurethral resection of prostate or holmium laser enucleation of the prostate), or, as a last resort, long-term catheterisation.
Implement appropriate nonsurgical treatments.
If operation becomes necessary, diagnose the cause and extent of obstruction by cystoscopy, then either:
Resect or laser benign prostatic hyperplasia, divide bladder neck hypertrophy transurethrally (bladder neck incision), or obtain biopsy material by transurethral resection of prostate if carcinoma seems likely and prior confirmation has been negative.
or
Consider any other alternative operative measures, such as Urolift implants to expand the prostatic urethral cavity for small to moderate sized prostates, open simple (Millen) prostatectomy for large prostates, or excision of diverticula (once bladder outlet obstruction has been treated).
A detailed history is first taken to assess the nature of the symptoms and how much they interfere with the patient’s life. The International Prostate Symptom Score sheet helps in assessing the overall impact of symptoms in a standardised way (see Box 35.1 ). This, and the patient’s general condition, are the principal factors determining whether treatment is needed. The abdomen is examined for an enlarged bladder and the prostate palpated rectally. These clinical examinations, however, reveal only gross abnormalities.
The next step is to investigate the effects of outlet obstruction on the bladder by measuring urinary flow rate and estimating the volume of residual urine using ultrasound. This is reliable, quick, noninvasive, safe and cheap. When urinary symptoms are severe but residual volume is insignificant, the alternative diagnosis of an overactive bladder should be considered. Twin-channel urodynamic studies are more complex and involve measuring the filling and emptying pressures of the bladder, but may be valuable if diagnostic doubts remain.
Renal function is assessed by estimating plasma urea, creatinine and electrolytes. If these are abnormal, further metabolic investigations may be necessary and renal tract ultrasound is mandatory.
A midstream specimen of urine should be examined by microscopy and culture as urinary infection alone may be responsible for the symptoms or may have precipitated an episode of urinary retention. In addition, if surgery is intended, it is important that infection is eradicated to minimise risk of perioperative infection and secondary haemorrhage.
If the prostate feels nodular on palpation, cancer should be suspected, particularly if the serum prostate-specific antigen (PSA) is elevated. Transrectal ultrasound scanning (TRUS) and needle biopsy should be performed even if prostatectomy is planned, because a preoperative diagnosis of cancer is likely to alter the plan of management. Magnetic resonance imaging (MRI) is an additional investigation used to diagnose, stage and target biopsies for prostate cancer (although this tends to be reserved for guiding repeat biopsies, where previous results have been benign but clinical suspicion persists). Marked elevation of serum PSA is diagnostic of prostatic cancer but a mildly elevated PSA may be caused by benign disease or infection. A normal result does not, however, exclude cancer.
Immediate catheterisation should be offered to patients presenting with large volume urinary retention (750 mL or more) associated with abnormal renal function or upper tract dilatation on renal tract ultrasound. Bladder drainage allows any reversible component of renal failure to self-correct. This commonly corrects in days, but in some chronic cases, it may take up to 3 weeks to improve biochemical renal function tests. After that, spontaneous improvement is unlikely. Initially, fluid and electrolyte balance is monitored and normalised, if necessary by intravenous fluids. In patients with chronic outflow obstruction and obstructive renal failure, catheterisation may produce a massive diuresis and this should be anticipated and treated appropriately. Where there has been any evidence of renal compromise caused by bladder outlet obstruction, the catheter must not be removed until definitive treatment has been performed (i.e., bladder outlet surgery) or the patient accepts a long-term catheter.
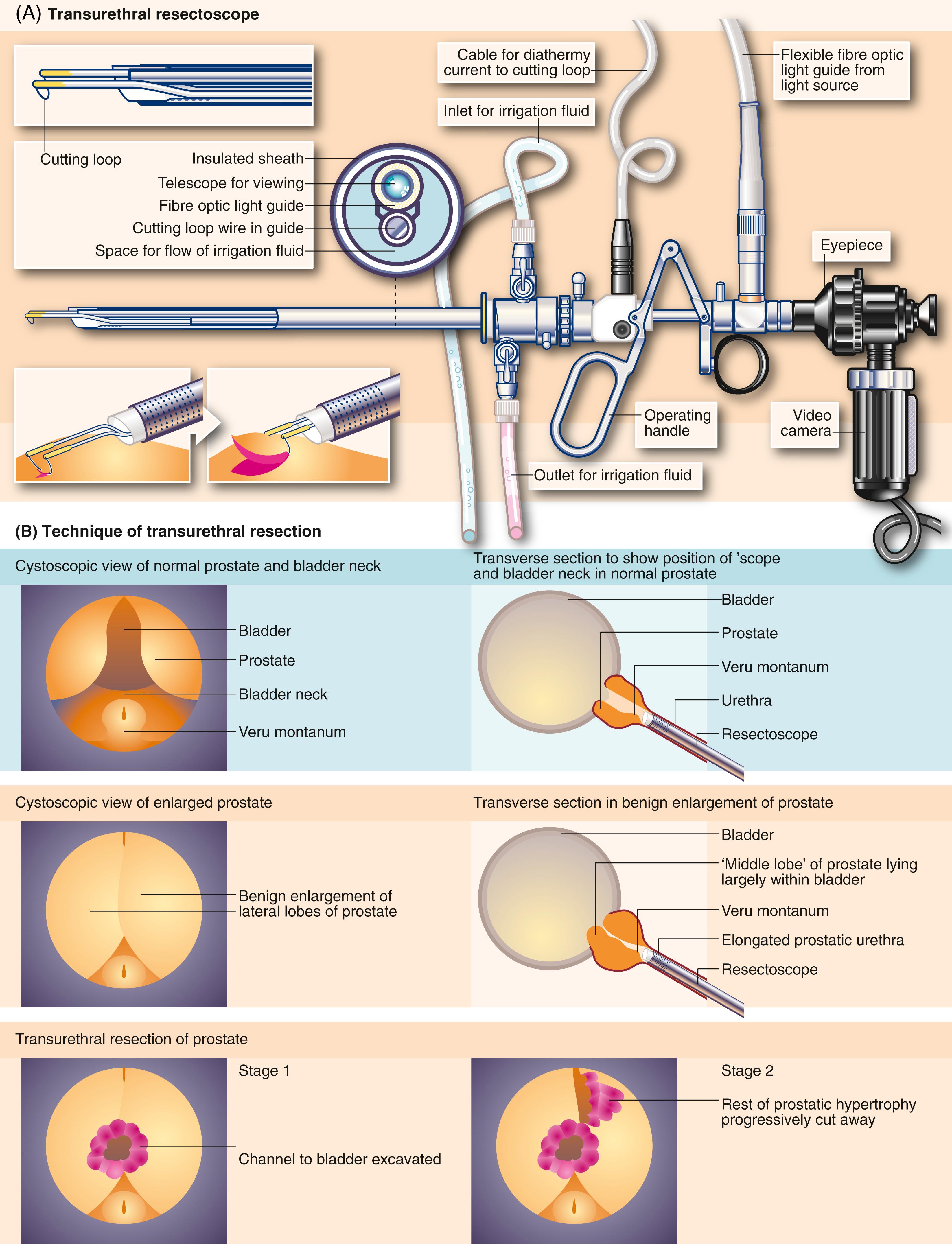
The anatomical nature of the bladder outlet obstruction can be further assessed by direct cystoscopic examination. It also provides an opportunity to examine for other problems, such as trabeculation, diverticula, tumours and stones. In patients with complications from bladder outlet obstruction or severe symptoms not responding to medical treatment, transurethral resection or holmium laser enucleation of the prostatic obstruction is performed, under the same anaesthetic (see transurethral resection of prostate [TURP], later). In elderly or unfit patients, placement of a urethral stent may be considered, however, they are rarely used, as these devices are prone to displacement, haemorrhage, local irritation and blockage. Only very occasionally are patients too unfit for some form of intervention. It is now rarely necessary to leave a patient with a long-term catheter, but in this event, a suprapubic catheter is preferable to a urethral catheter, because of the ease of changing it, greater patient comfort, and avoidance of urethral trauma, which can result in traumatic hypospadias long term.
Alpha-adrenergic A 1 receptors are present in the bladder neck and prostate. Selective alpha-1a subtype adrenergic blocking drugs ( tamsulosin and alfuzosin ) enable the prostatic urethra to open more readily by blocking prostatic smooth muscle contraction, so relieving symptoms.
Finasteride and dutasteride block the enzyme 5-alpha reductase from converting testosterone to dihydrotestosterone (a more potent androgen) and thus reduce the size of hyperplastic prostate glands. A 6-month trial of treatment is required; if successful, symptoms may improve to the extent that surgery can be delayed or avoided. Some herbal remedies, such as saw palmetto , contain naturally occurring 5-alpha reductase inhibitors. Combination therapy with alpha-adrenergic blockers and 5-alpha reductase inhibitors may be beneficial in patients with larger glands.
Prostate artery embolisation is not commonly offered but does provide an alternative option for those who have failed medical therapy and wish to avoid surgery. Under fluoroscopy guidance and using local anaesthetic, interventional radiologists access the femoral artery to selectively block the prostate artery using microparticles. This results in prostatic necrosis and a reduction in prostate size. Risks include urinary retention, pain, and dysuria. Whilst urinary symptoms are improved and sexual function is generally preserved, results are inferior to TURP.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here