Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The clinical recognition of neuromuscular transmission disorders ( Table 390-1 ) is critically important because all are treatable. Afflicted patients have the potential to improve substantially or even return to normal function, whereas undiagnosed or untreated disease leads to severe morbidity and death. Each of these disorders compromise the postsynaptic potential, thereby reducing the force generated by muscles. Myasthenia gravis and Lambert-Eaton syndrome are autoimmune diseases. The much less common genetic disorders mimic the autoimmune diseases clinically but do not respond to immunotherapies. Toxic and infectious disorders of neuromuscular transmission can be more easily identified because of coexistent autonomic findings and a history of exposure, which sometimes is obvious but sometimes requires a detailed evaluation.
| DISEASE | TARGET | PATHOBIOLOGY |
|---|---|---|
| AUTOIMMUNE | ||
| Myasthenia gravis
Transient neonatal myasthenia |
AChRs MuSK LRP4 AChRs MuSK |
Antibodies to AChR in 85% reduce AChR numbers and EPP amplitude Antibodies to MuSK in 5 to 8% reduce MuSK–LRP4 binding Antibodies to LRP4 in a small proportion of patients reduce LRP4–agrin binding Maternal antibodies cause transient disease in neonate |
| Arthrogryposis | Fetal AChR | Maternal antibodies that inhibit fetal AChR function resulting in paralysis in the fetus in utero, leading to multiple joint contractures |
| Lambert-Eaton myasthenic syndrome | VGCCs | Antibodies to VGCC in 90% reduce VGCC numbers, acetylcholine release and EPP amplitude |
| Acquired neuromyotonia | VGKC-complex | Antibodies to VGKC-complex (mainly CASPR2) in 40% lead to increased and spontaneous ACh release |
| GENETIC | ||
| Presynaptic | ChAT, ChT, SYT2, SNAP25 | Different mutations (mostly recessive) reduce acetylcholine release. ChAT mutations are the most common |
| Synaptic | ColQ, laminin ß2 | Mutations in the collagen tail ( COLQ ) that anchor AChE at the neuromuscular junction cause absence of AChE
|
| AChR | Recessive mutations in AChR-subunits (mostly in the ε-subunit) cause reduced AChR expression | |
| Postsynaptic | AChR | Dominant or recessive mutations in AChR-subunits cause kinetic defects: “slow” and “fast” channel syndromes |
| Na ν 1.4 | Mutations in SCN4A sodium channel reduce muscle fiber excitability | |
| Defects in end-plate development and maintenance | Rapsyn, DOK7, agrin, MuSK, LRP4 | Recessive mutations cause structural alterations of the end plate. DOK7 and rapsyn defects are the most common |
| Glycosylation defects | GFPT1, DPAGT1, ALG2, ALG14 | Recessive mutations cause glycosylation defects of end-plate proteins. GFPT1 defect is the most common |
| Arthrogryposis, multiple pterygium, Escobar syndrome | Rapsyn, AChR δ- and γ-subunits, DOK7, MuSK, GFPT1 | Recessive mutations cause a prenatal defect of neuromuscular transmission with fetal akinesia |
| NEUROTOXIC | ||
| Botulism | SNARE (SNAP) receptor) proteins | Botulinum toxin gains entry into the presynaptic motor nerve and cleaves proteins involved in ACh release mechanism |
| Envenomation following bites from snakes, spiders, scorpions, etc. | Varied sites of action | Neurotoxins specific for VGCCs, VGKCs, AChE, AChRs, voltage-gated sodium channels, and other targets are frequent in many animal venoms and generally inhibit function |
| Drugs and insecticides (unintentional or intentional poisoning) | Varied sites of action | Muscle relaxants and other drugsMany antibiotics and quinine-related drugs can alter neuromuscular transmission at high dose |
| Organophosphates block AChE and have complicated acute and chronic actions (Novichok, common pesticides) | ||
All neuromuscular transmission disorders are rare. Myasthenia gravis has an estimated annual incidence of about three to four per million, with a prevalence of 200 to 400 per million. Myasthenia gravis occurs more commonly after age 50 years and in women, but at least 30% of patients, especially older patients, are men. Japanese and Chinese populations appear to have higher frequencies of myasthenia gravis restricted to the ocular muscles, whereas muscle-specific kinase myasthenia gravis appears to be more common in Blacks compared with Whites and is more common in European countries closer to the equator. The estimated prevalence of Lambert-Eaton syndrome is about one-tenth that of myasthenia gravis. Congenital myasthenic syndromes have an estimated prevalence of 10 per million. Botulism cases depend on outbreaks of foodborne infections, with 100 cases, about 25% of which are foodborne, reported annually in the United States. Toxic exposures to organophosphates and other chemicals are sporadic and vary based on the scale of exposure. Except for myasthenia gravis, these diseases are sufficiently uncommon that most information is based on expert opinion, retrospective studies, or small case series.
Neuromuscular transmission involves propagation of an action potential along a motor nerve, thereby leading to an electrical potential change at the nerve terminal and the triggering of calcium influx through voltage-gated calcium channels. The calcium influx signals a series of synaptic proteins to fuse the synaptic vesicles that contain acetylcholine to the neuronal cell membrane, thereby allowing their contents to be released into the narrow cleft between the nerve ending and the postsynaptic muscle surface. Acetylcholine rapidly diffuses across the cleft and binds to highly concentrated acetylcholine receptors, which trigger the ion channel to open and generate an end plate potential that, if of adequate magnitude, activates an action potential, which then propagates along the muscle fiber to prompt muscle contraction. The postsynaptic folds and concentration of sodium channels at their base serve to increase the postsynaptic potential. Diffusion of acetylcholine and its hydrolysis by acetylcholinesterase, which is anchored to the basal lamina of the synaptic cleft, serve to terminate postsynaptic activation ( E-Fig. 390-1 ).
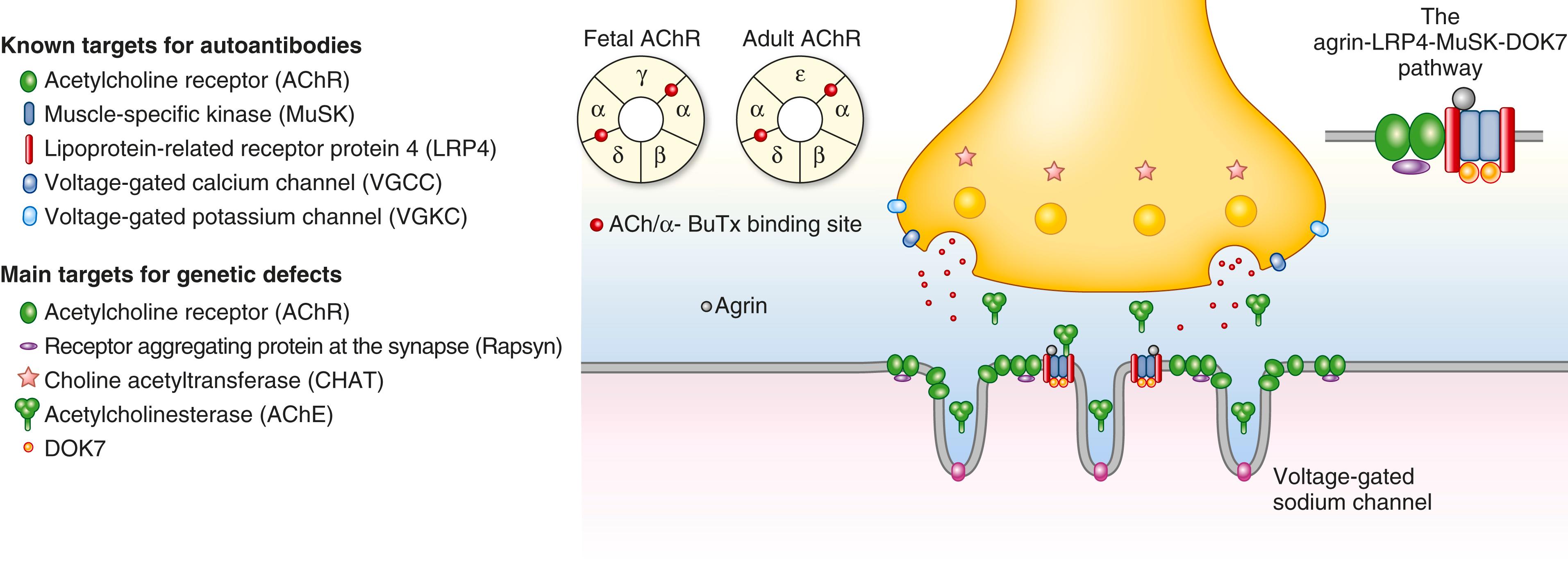
Disorders of neuromuscular transmission produce clinical manifestations by compromising or eliminating the difference between the postsynaptic potential and the minimum magnitude action potential that is necessary to signal muscle contraction. In myasthenia gravis, autoantibody–mediated injury of the postsynaptic surface leads to a reduction of acetylcholine receptors; if severe enough, this reduction will lead to such a low postsynaptic potential that an action potential cannot be generated. In contrast, Lambert-Eaton syndrome and botulinum toxin reduce the release of acetylcholine, thereby leading to a compromised activation of the normal postsynaptic surface. Congenital myasthenic syndromes and toxins compromise neuromuscular transmission by a variety of mechanisms.
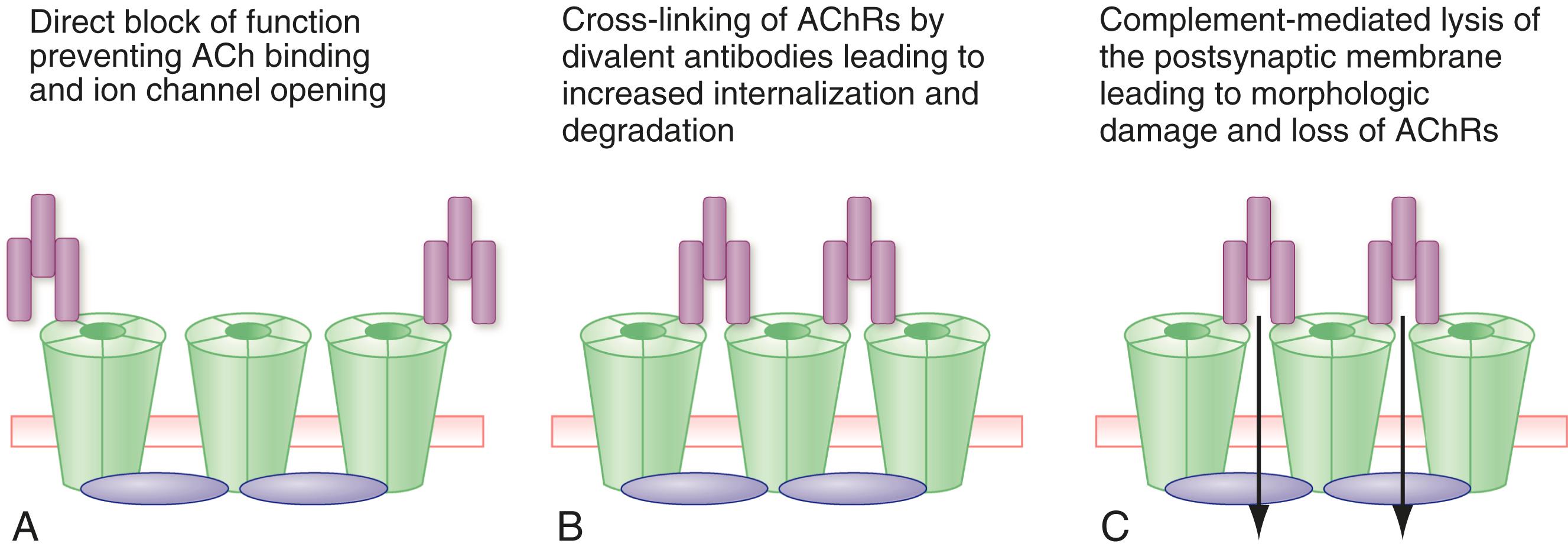
Myasthenia gravis is an autoimmune disorder caused by antibodies that are directed at postsynaptic proteins, primarily the skeletal muscle nicotinic acetylcholine receptors, muscle-specific kinase, lipoprotein receptor–related protein-4, and perhaps other autoantigens. Antibodies to the acetylcholine receptor act through activation of complement, thereby leading to destruction of the post-junctional membrane; antigenic modulation by cross-linking receptors and removal of the acetylcholine receptors from the muscle membrane; and blockade of the function of the acetylcholine receptors ( E-Fig. 390-2 ). Antibodies to muscle-specific kinase are primarily immunoglobulin (Ig)G4 and act by inhibiting the signals that lead to the concentration of acetylcholine receptors on the postsynaptic surface. The activity of low-density lipoprotein receptor-related protein 4 antibodies is less well understood, but they likely also interfere with signals that maintain the integrity of the neuromuscular junction. Other putative antigenic targets include agrin, cortactin, and other proteins that are concentrated in the neuromuscular junction.
Myasthenia gravis consists of distinct subgroups: acetylcholine receptor antibody-positive, early-onset disease; acetylcholine receptor antibody–positive, late-onset disease, which generally occurs after age 50 years; muscle-specific kinase antibody-positive; thymoma-associated myasthenia gravis; and seronegative myasthenia gravis without detectable autoantibodies. Patients with early-onset myasthenia gravis are more often women with thymic hyperplasia, whereas the late-onset patients skew toward being men with an atrophic thymus. In about 10% of patients, and especially in men, thymomas trigger myasthenia gravis, but removal of the thymoma does not cure the myasthenia gravis. Women with early-onset myasthenia gravis are more likely to be human leukocyte antigen (HLA)-A1, B8, or DRw3-positive, whereas men with late-onset disease are more likely to have HLA-A3, B7, or DRw2.1.
Myasthenia gravis has a genetic predisposition, with close to 5% of patients having a family member with the disease. Twin studies indicate a heritability index of 0.65 for myasthenic gravis, similar to that of Alzheimer disease. Little is understood about whether environmental factors lead to myasthenia gravis.
Across all subgroups, autoantibodies are produced by B cells, supported by T cells and pro-inflammatory cytokines, but with largely unknown triggers, and the mechanisms that maintain autoreactive B cells are poorly characterized. In patients with early-onset disease, thymic hyperplasia with active germinal centers suggests that the breakdown in immune tolerance is initiated in the thymus, but the autoimmune reaction persists after thymectomy. Thymoma-associated myasthenia gravis is marked by a deficiency of the autoimmune regulatory protein AIRE, which appears to lead to a deficiency of regulatory T cells and a resultant increase in autoreactivity. Some autoreactive T cells may be activated in association with the thymoma because of local expression of muscle antigens, including the acetylcholine receptors, and the lack of AIRE. These autoreactive T cells can then stimulate peripheral B cells to produce antibodies. Triggers for non–acetylcholine receptor antibody myasthenia gravis are not known. Patients with muscle-specific kinase antibodies have a normal-appearing thymus.
Myasthenia gravis is characterized by fluctuating, painless muscle skeletal weakness, which can worsen over minutes to hours. Symptoms can vary across muscle groups and over time, with spontaneous remissions. This variability can lead to diagnostic challenges, especially when patients lack findings during clinical examinations. The majority of patients present with ocular muscle involvement that causes drooping eyelids or double vision ( Fig. 390-1 ). Ptosis may be unilateral or bilateral, and it often is asymmetrical. The pupils are always normal, an important finding that distinguishes myasthenia gravis from other ocular motility disorders and causes of ptosis, (e.g., third nerve palsy and Horner syndrome). Some patients have only isolated ocular muscle weakness (so-called ocular myasthenia), some patients have isolated weakness of bulbar muscles (which manifests as slurred speech, difficulty swallowing, and facial weakness), but the majority of patients will develop general weakness, especially in the proximal arms and legs and in respiratory muscles. Isolated bulbar weakness is particularly seen in patients who have muscle-specific kinase antibodies and who also may have muscle atrophy; these presentations can be confused with motor neuron disease ( Chapter 387 ). Some patients have remarkably acute, lateralized weakness that mimics a stroke ( Chapters 376 and 377 ) or distal weakness that may be confused with peripheral nerve injury ( Chapter 388 ). Myasthenic crisis is defined as the need for ventilatory support for respiratory muscle weakness.
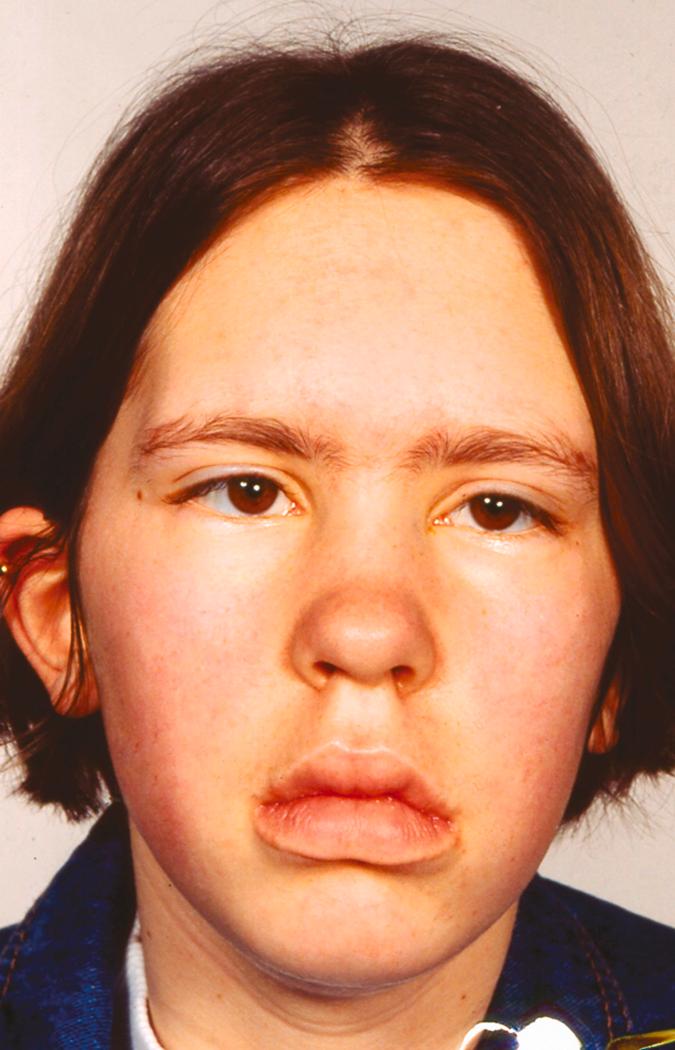
Patients do not have sensory abnormalities, reflexes are normal, and bladder function is normal. Nevertheless, patients typically report a more substantial decrement in their quality of life than their physicians suspect.
Serologic testing is indicated when the clinical diagnosis of myasthenia gravis is suggested by ocular findings, especially when coupled with weakness or fatigue as documented by asking patients to maintain upgaze or hold their arms outstretched for 30 seconds to a minute. Provocative testing with edrophonium, ice packs, and rest tests should be performed only by individuals with extensive experience to avoid false positives.
Myasthenia gravis can be confirmed by serologic detection of acetylcholine receptor antibodies in about 50% of patients with isolated ocular myasthenia and upwards of 90% of patients with generalized disease. Muscle-specific kinase antibodies are present in about one-third of patients who do not have acetylcholine receptor antibodies, and very occasional patients may have both antibodies. False positives are exceedingly rare, but acetylcholine receptor antibodies can be detected in patients with thymoma and in asymptomatic family members of patients with myasthenia gravis. Low-density lipoprotein receptor–related protein 4 antibodies may be present in 1 to 4% of patients without other autoantibodies, but they are also detected in patients with motor neuron disease ( Chapter 387 ).
In patients without serologic evidence of myasthenia gravis, electrodiagnostic studies can confirm a disorder of neuromuscular transmission. Approximately 75% of patients will have a decremental response to repetitive nerve stimulation. Single-fiber electromyography, which records action potentials generated by individual muscle fibers, has an estimated sensitivity over 95% when performed by experts (but is not likely to be that high in general practice). However, single fiber examination is so sensitive that it will detect abnormalities produced by previous therapeutic botulinum toxin, thereby leading to the potential for a false positive evaluation. All patients should have chest imaging to detect a possible thymoma.
If found on imaging, a thymoma should be removed. Postoperative radiation is recommended for completely resected stage II or III thymomas. Unfortunately, this procedure does not eliminate the autoimmune disease and is not effective in reducing symptoms. For patients who do not have a thymoma, who are acetylcholine receptor antibody–positive, and who are younger than 65 years of age, thymectomy reduces weakness and the cumulative required medication dose over time. , Video-assisted thoracoscopic removal is as good as open surgery. For patients who have muscle-specific kinase antibodies or do not have serum autoantibodies, thymectomy is not indicated.
For severe exacerbations of myasthenia gravis or myasthenia crisis, an immunoglobulin infusion (intravenous immunoglobulin [IVIG] 1 to 2 g/kg given over 2 to 5 days) or plasmapheresis can provide rapid improvement. The duration of response is usually only 4 to 6 weeks, so chronic therapy also must be instituted.
Consensus treatment guidelines emphasize a trial of cholinesterase inhibitors (e.g., pyridostigmine [starting at 30 to 60 mg four times daily]) as first-line therapy to enhance neuromuscular transmission and reduce symptoms over the 3 to 4 hours after treatment. Unfortunately, cholinesterase inhibitors typically do not eliminate weakness, and they may cause gastrointestinal side-effects (bloating, nausea, and diarrhea) as well as increased respiratory secretions that can worsen reactive airway disease. Furthermore, they are much less effective in patients with muscle-specific kinase antibodies.
The most consistently effective treatment is high-dose prednisone (e.g., prednisone escalating to a maximum of 60 mg daily) that then should be tapered to the lowest effective dose, which may be maintained for years. All patients have some adverse effects ( Chapter 28 ), with 30% being severe, ranging from weight gain, diabetes, and hypertension to severe insomnia and osteoporosis. For the 20% or so of patients who do not respond to prednisone or have unacceptable adverse effects, immunosuppressive treatments are warranted and can permit tapering of the prednisone dose. Unfortunately, IVIG does not appear to be effective as a steroid-sparing medication.
Rozanolixizumab, (a neonatal Fc receptor blocker under expert supervision) can provide meaningful improvements in symptoms in patients who have generalized muscle-specific kinase antibody positive myasthenia gravis. Rituximab (e.g., 375 mg/m 2 repeated weekly for 4 weeks) eliminates CD20 cells and appears to be effective for patients who have muscle-specific kinase antibodies, but its efficacy in patients with acetylcholine receptor antibodies is less clear. Efgartigimod (an IgG1 antibody at 10 mg/kg as four weekly intravenous infusions, with a maximum dose of 1200 mg for patients who weigh more than 120 kg) is effective for generalized myasthenia gravis. Other immunosuppressive options include azathioprine (e.g., 2.5 mg/kg/day), mycophenolate (e.g., 2000 mg/day), tacrolimus (e.g., 3 mg/day), and less commonly methotrexate (e.g., 5 to 15 mg weekly). Each of these agents may require many months of therapy before they have biologic and clinical efficacy and may have significant toxicity. Intravenous eculizumab (e.g., 1200 mg every 2 weeks), which inhibits the activation of complement, is effective and well-tolerated in patients with acetylcholine receptor antibodies, but it is currently reserved for patients who have not responded to prednisone or immunosuppressive agents, in part because of its very high price. Other currently experimental immunotherapies include zilucoplan (a complement inhibitor, given subcutaneously at 0.3 mg/kg daily for 2 weeks). , The precise roles of these newer therapies continues to evolve, but these drugs do not eliminate the production of autoantibodies and, therefore, are unlikely to provide a continuous remission of weakness after they are discontinued. Experimental treatments include B cell ablation, cytokine inhibitors, and CAR-T cells.
Patients should be advised to be as physically active as possible once severe weakness is effectively treated. Exercise limits corticosteroids and may have further positive effects on the immune system.
If not recognized and treated, myasthenic crisis will lead to death. There is no cure for myasthenia gravis. Effective therapies can eliminate symptoms in most patients, but more than 30% of patients have a poor response or intolerable adverse effects to first-line therapy. Patients suffer significant disability, with limitations in employment and compromised quality of life. With modern treatment, however, the lifespan of patients with myasthenia gravis is similar to that of the general population.
Lambert-Eaton syndrome is caused by autoantibodies against the voltage-gated calcium channel on the presynaptic nerve terminal. The antibodies cross-link the calcium channels, thereby leading to increased internalization, which reduces the influx of calcium when the nerve terminal depolarizes and, as a result, reduces the vesicular release of acetylcholine. If the amount of acetylcholine is insufficient to activate the required number of acetylcholine receptors, no action potential will be generated to signal muscle contraction. If a nerve is repetitively stimulated during electrophysiologic testing, calcium will accumulate in the nerve terminal, can reach a level that is sufficient to overcome the defect, and will allow the release of acetylcholine. This accumulation is the basis of the incremental response observed in diagnostic testing, a response that is just the opposite of the fatigue seen with myasthenia gravis.
About 60% of patients with Lambert-Eaton syndrome have small cell lung cancer ( Chapter 177 ), which expresses calcium channels that become the target of autoantibodies, whereas the remainder of patients have a primary autoimmune disorder. These latter patients tend to be younger, are more likely to be female, and often to have other autoimmune diseases. The specific stimulus that produces Lambert-Eaton syndrome in these patients is not known.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here