Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
All disorders of malabsorption are associated with diminished intestinal absorption of one or more dietary nutrients. Malabsorption can result from a defect in the nutrient digestion in the intestinal lumen or from defective mucosal absorption. Malabsorption disorders can be categorized into generalized mucosal abnormalities usually resulting in malabsorption of multiple nutrients ( Table 364.1 ) or malabsorption of specific nutrients (carbohydrate, fat, protein, vitamins, minerals, and trace elements) ( Table 364.2 ). Almost all the malabsorption disorders are accompanied by chronic diarrhea, which further worsens the malabsorption ( Chapter 367 ).
| MUCOSAL DISORDERS |
|
| PROTEIN-LOSING ENTEROPATHY |
|
| CONGENITAL BOWEL MUCOSAL DEFECTS |
|
| IMMUNODEFICIENCY DISORDERS |
|
| ACQUIRED IMMUNE DEFICIENCY |
|
| AUTOIMMUNE ENTEROPATHY |
|
| MISCELLANEOUS |
|
| CARBOHYDRATE MALABSORPTION |
|
| FAT MALABSORPTION |
|
| PROTEIN/AMINO ACID MALABSORPTION |
|
| MINERAL AND VITAMIN MALABSORPTION |
|
| DRUG INDUCED |
|
The clinical features depend on the extent and type of the malabsorbed nutrient. The common presenting features, especially in toddlers with malabsorption, are diarrhea, abdominal distention, and failure to gain weight, with a fall in growth chart percentiles. Physical findings include abdominal distention, muscle wasting, and the disappearance of the subcutaneous fat, with subsequent loose skinfolds ( Fig. 364.1 ). The nutritional consequences of malabsorption are more dramatic in toddlers because of the limited energy reserves and higher proportion of calorie intake being used for weight gain and linear growth. In older children, malnutrition can result in growth retardation, as is commonly seen in children with late diagnosis of celiac disease (CD). If malabsorption is left untreated, linear growth slows, and with prolonged malnutrition, death can follow (see Chapter 57 ). This extreme outcome is usually restricted to children living in the developing world, where resources to provide enteral and parenteral nutrition support may be limited. Nonetheless, monogenetic causes often produce failure to thrive in all countries. Specific findings on examination can guide toward a specific disorder; edema is usually associated with protein-losing enteropathy (PLE), digital clubbing with cystic fibrosis and CD, perianal excoriation and gaseous abdominal distention with carbohydrate malabsorption, perianal and circumoral rash with acrodermatitis enteropathica, abnormal hair with Menkes syndrome, tricho-hepato-enteric syndrome (THE) and the typical facial features diagnostic of the Johanson-Blizzard syndrome.
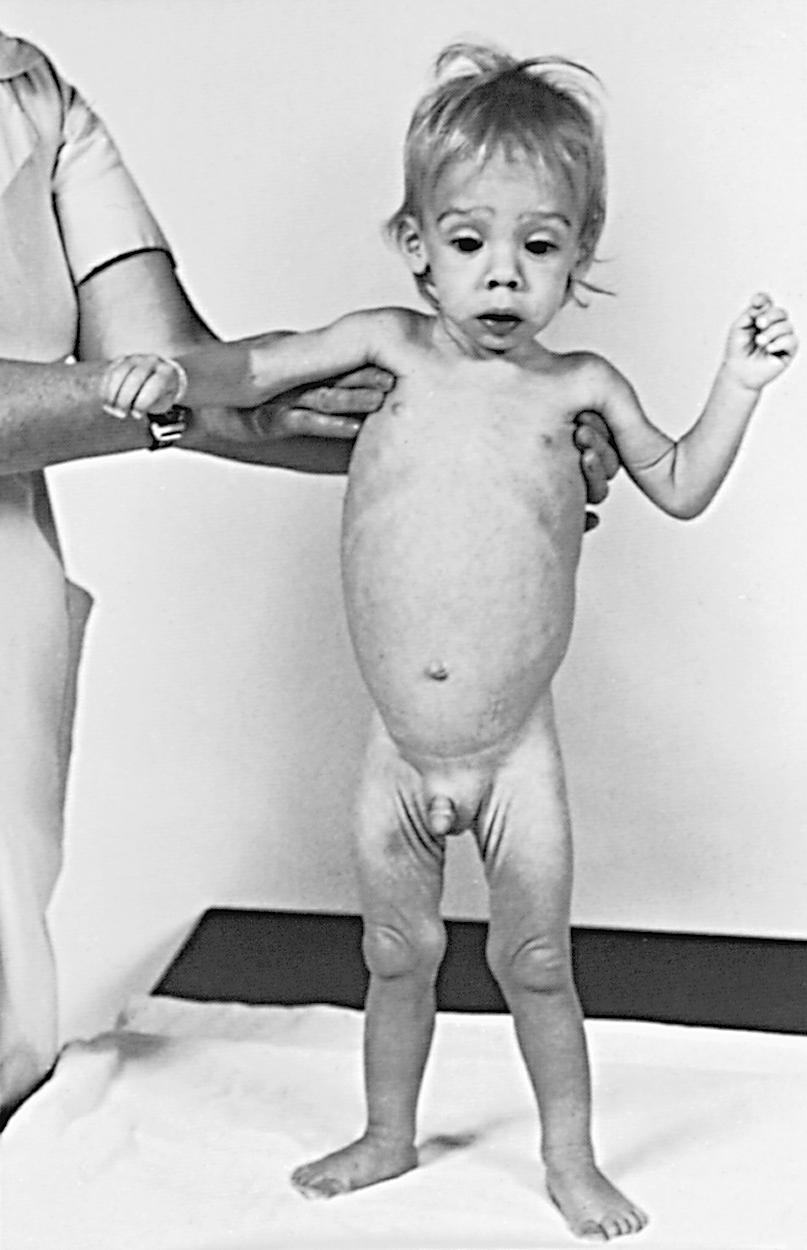
Many children with malabsorption disorders have a good appetite as they try to compensate for the fecal protein and energy losses. In exocrine pancreatic insufficiency, fecal losses of up to 40% of ingested protein and energy do not lead to malnutrition, as long as they are compensated by an increased appetite. In conditions associated with villous atrophy or inflammation (CD, postinfectious enteropathy), fecal protein and energy losses are usually modest, but associated anorexia and reduced food intake results in malnutrition.
The nutritional assessment is an important part of clinical evaluation in children with malabsorptive disorders (see Chapter 55 ). Long-term calcium and vitamin D malabsorption can lead to reduced bone mineral density and metabolic bone disease (often resistant to oral vitamin D), with increased risk of bone fractures. Vitamin K malabsorption, irrespective of the underlying mechanism (fat malabsorption, mucosal atrophy), can result in coagulopathy. Severe PLE is often associated with malabsorption syndromes (CD, congenital disorders of glycosylation, intestinal lymphangiectasia) and causes hypoalbuminemia and edema. Other nutrient deficiencies include iron malabsorption causing microcytic anemia and low reticulocyte count, low serum folate levels in conditions associated with mucosal atrophy, especially in the proximal part of the small intestinal tract and low serum vitamin A and vitamin E concentrations in fat malabsorption.
The evaluation of a child with malabsorption should be proceed in a stepwise manner. Clinical history alone might not be sufficient to make a specific diagnosis, but it can direct the pediatrician toward a more structured and rational investigative approach. Diarrhea is the main clinical expression of malabsorption. The onset of diarrhea in early infancy suggests a congenital defect ( Table 364.3 ). In secretory diarrhea caused by disorders such as congenital chloride diarrhea (CCD) and microvillus inclusion disease (MVID), the stool is watery and voluminous and can be mistaken for urine (see Chapter 367 ). The onset of symptoms after the introduction of a particular food into a child's diet can provide diagnostic clues, such as with sucrose in sucrase-isomaltase deficiency. The nature of the diarrhea may be helpful: explosive watery diarrhea suggests carbohydrate malabsorption; loose, bulky stools are associated with CD; and pasty and yellowish offensive stools suggest an exocrine pancreatic insufficiency. Stool color is usually not helpful; green stool with undigested “peas and carrots” can suggest rapid intestinal transit in toddler's diarrhea, which by itself is a self-limiting condition unassociated with failure to thrive.
| CONDITION | CLINICAL FEATURES |
|---|---|
| Congenital enteropathy | |
| Microvillus inclusion disease Tufting enteropathy |
Secretory diarrhea Secretory diarrhea |
| Congenital intestinal transport defect | |
| Congenital glucose–galactose malabsorption Congenital bile acid malabsorption Congenital chloride diarrhea Congenital sodium diarrhea ( GUCY2C mutation) |
Acidic diarrhea Steatorrhea Secretory diarrhea, metabolic alkalosis Hydramnion, secretory diarrhea |
| Congenital isolated enzyme deficiency | |
| Congenital lactase deficiency Congenital enterokinase deficiency Congenital trypsinogen deficiency Congenital lipase and/or colipase deficiency |
Acidic diarrhea Failure to thrive, edema Failure to thrive, edema Failure to thrive, oily stool |
| Enteric anendocrinosis ( NEUROG 3 mutation) | Hyperchloremic acidosis, failure to thrive |
| Immunodeficiency and autoinflammatory diseases (see Table 362.6 ) | Failure to thrive, opportunistic infections, eczema |
Following medical history, physical examination and laboratory testing (see next Chapter 364.1 ), intestinal biopsies may assist in the diagnosis. This is usually done for chronic rather than acute diseases (that can be self-limited). Generalized mucosal villous atrophy (flat mucosa) may be associated with malabsorption of multiple macronutrients and micronutrients and has a wide range of differential diagnoses (see Chapter 364.2 ).
Steatorrhea
protein-losing enteropathy
carbohydrate malabsorption
The investigation is guided by the history and physical examination. In a child presenting with chronic or recurrent diarrhea, the initial work-up should include stool cultures and antibody tests for parasites; stool microscopy for ova and parasites such as Giardia ; and fecal leukocytes and calprotectin or lactoferrin to exclude inflammatory disorders. Stool pH and reducing substances for carbohydrate malabsorption, stool osmolality to differentiate between osmotic and secretory diarrhea and quantitative stool fat examination and α 1 -antitrypsin to demonstrate fat and protein malabsorption, respectively, should also be determined. Fecal stool elastase-1 can determine exocrine pancreatic insufficiency.
A complete blood count, including peripheral smear for microcytic anemia, lymphopenia (lymphangiectasia), neutropenia (Shwachman syndrome), and acanthocytosis (abetalipoproteinemia) is useful. If CD is suspected, serum immunoglobulin (Ig) A and tissue transglutaminase (TG2) antibody levels should be determined. Depending on the initial test results, more specific investigations can be planned.
The measurement of carbohydrate in the stool for pH and the amount of reducing substances is a simple screening test when available. An acidic stool with >2+ reducing substance suggests carbohydrate malabsorption. Sucrose or starch in the stool is not recognized as a reducing sugar until after hydrolysis with hydrochloric acid, which converts them to reducing sugars.
Breath hydrogen test is used to identify the specific carbohydrate that is malabsorbed. After an overnight fast, the suspected sugar (lactose, sucrose, fructose, or glucose) is administered as an oral solution (carbohydrate load up to 2 g/kg, maximum total of 25 g, depending on the specific carbohydrate type). In malabsorption, the sugar is not digested or absorbed in the small bowel; it passes on to the colon and is metabolized by the normal gut microflora. One of the products of this process is hydrogen gas, which is absorbed through the colon mucosa and excreted in the breath. Increased hydrogen concentration in the breath samples suggests carbohydrate malabsorption. A rise in breath hydrogen of 20 ppm above the baseline preferably with associated symptoms is considered a positive test. The child should not be on antibiotics at the time of the test, because colonic flora is essential for fermenting the sugar.
Small bowel mucosal biopsies can measure mucosal disaccharidase (lactase, sucrase, maltase, palatinase) concentrations directly. In primary enzyme deficiencies the mucosal enzyme levels are low and small bowel mucosal morphology is normal. Primary enzymatic deficiencies can be diagnosed by genetic testing (see Chapters 364.9 and 367 ). Partial or total villous atrophy due to disorders such as CD, or following acute rotavirus gastroenteritis can result in secondary disaccharidase deficiency and transient lactose intolerance (see Chapter 364.2 for differential diagnosis of villous atrophy). The disaccharidase levels revert to normal after mucosal healing.
The presence of fat globules in the stool suggests fat malabsorption. The ability to assimilate fat varies with age; a premature infant can absorb only 65–75% of dietary fat, a full-term infant absorbs almost 90%, and an older child absorbs more than 95% of fat while on a regular diet. Quantitative determination of fat malabsorption requires a 3-day stool collection for evaluation of fat excretion and determination of the coefficient of fat absorption:
where fat intake and fat losses are in grams. Because fecal fat balance studies are cumbersome, expensive, and unpleasant to perform, simpler tests are often preferred. Among these stool tests, the acid steatocrit test is the most reliable. When BA deficiency is suspected of being the cause of fat malabsorption, the evaluation of BA levels in duodenal fluid aspirate may be useful. Intestinal mucosal abnormalities affect not only fat absorption, but also steatorrhea, and are usually far less severe in intestinal mucosal disorders (CD, cow's milk protein enteropathy) than in exocrine pancreatic insufficiency.
Exocrine pancreatic insufficiency and other fat malabsorption disorders (see Table 364.2 ) are usually associated with deficiencies of fat-soluble vitamins A, D, E, and K. Serum concentrations of vitamins A, D, and E can be measured. A prolonged prothrombin time is an indirect test to assess vitamin K malabsorption and subsequent deficiency.
Dietary and endogenous proteins secreted into the bowel are almost completely absorbed and minimal amounts of protein from these sources passes into the colon. The majority of the stool nitrogen is derived from gut bacterial proteins. Excessive bowel protein loss usually manifests as hypoalbuminemia. Because the most common cause of hypoalbuminemia in children is a renal disorder, urinary protein excretion must be determined. Other potential causes of hypoalbuminemia include acute infection, liver disease (reduced production) and inadequate protein intake. Very rarely hypoalbuminemia can result from an extensive skin disorder (burns) causing protein loss via the skin. Measurement of stool α 1 -antitrypsin is a useful screening test for PLE. This serum protein has a molecular weight similar to albumin; however, unlike albumin it is resistant to digestion in the gastrointestinal (GI) tract. Excessive α 1 -antitrypsin excretion in the stool should prompt further investigations to identify the specific cause of gut or stomach (Menetrier disease) protein loss.
Cystic fibrosis ( Chapter 432 ) is the most common cause of exocrine pancreatic insufficiency in children; therefore, a sweat chloride test must be performed before embarking on invasive tests to investigate possible exocrine pancreatic insufficiency ( Fig. 364.2 ). Many cases of cystic fibrosis are detected by neonatal genetic screening programs; occasional rare mutations are undetected.
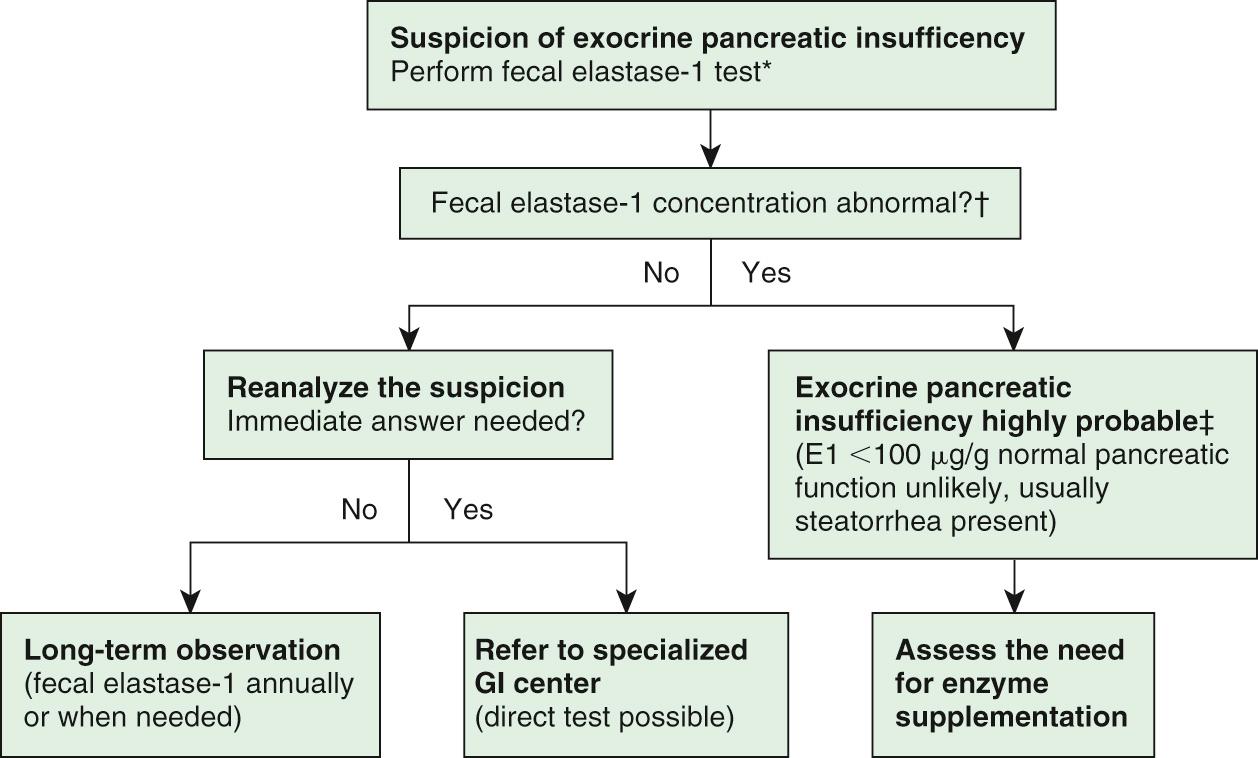
Fecal elastase-1 estimation is a sensitive test to assess exocrine pancreatic function in chronic cystic fibrosis and pancreatitis. Elastase-1 is a stable endoprotease unaffected by exogenous pancreatic enzymes. One disadvantage of the fecal elastase-1 test is the lack of full differentiation between primary exocrine pancreatic insufficiency and exocrine pancreatic dysfunction secondary to intestinal villous atrophy. The proximal small bowel is the site for pancreozymin/cholecystokinin production; the latter is the hormone that stimulates enzyme secretion from the exocrine pancreas. Mucosal atrophy can lead to diminished pancreozymin/cholecystokinin secretion and subsequently to exocrine pancreatic insufficiency. Fecal elastase-1 can also give a false-positive result during acute episodes of diarrhea.
Serum trypsinogen concentration can also be used as a screening test for exocrine pancreatic insufficiency. In cystic fibrosis, the levels are greatly elevated early in life, and then they gradually fall, so that by 5-7 yr of age, most patients with cystic fibrosis with pancreatic insufficiency have subnormal levels. Patients with cystic fibrosis and adequate exocrine pancreatic function tend to have normal or elevated levels. In such patients, observing the trend in serial serum trypsinogen estimation may be useful in monitoring exocrine pancreatic function. In Shwachman syndrome, another condition associated with exocrine pancreatic insufficiency, the serum trypsinogen level is low.
Other tests for pancreatic insufficiency (nitroblue tetrazolium–paraaminobenzoic acid test and pancreolauryl test) measure urine or breath concentrations of substances released and absorbed across the mucosal surface following pancreatic digestion. These tests lack specificity and are rarely used in clinical practice.
The gold standard test for exocrine pancreatic function is direct analysis of duodenal aspirate for volume, bicarbonate, trypsin, and lipase upon secretin, and pancreozymin/cholecystokinin stimulation. This involves duodenal intubation (see Chapter 375 ).
Establishing a specific diagnosis for malabsorption often requires histologic examination of small bowel mucosal biopsies. These are obtained during endoscopy, which allows multiple biopsies to be performed, because mucosal involvement can be patchy, especially in CD. Periodic acid–Schiff (PAS) staining of mucosal biopsies and electron microscopy are necessary in congenital diarrhea to assess congenital microvillus atrophy. Bowel mucosal lesions can also be segmental in cases of IL. In these situations, radiographic small bowel series, repeated ultrasonographies or lymphoscintigraphy can identify a region of thickened bowel responsible for protein loss. Intestinal biopsies can detect infectious agents such as Giardia lamblia . During endoscopy, mucosal biopsies can be obtained to measure mucosal disaccharidase activities. Duodenal aspirates can be performed to measure pancreatic enzyme concentration as well as quantitative bacterial cultures.
Plain radiographs and barium contrast studies might suggest a site and cause of intestinal motility disorders. Although flocculations of barium and dilated bowel with thickened mucosal folds have been attributed to diffuse malabsorptive lesions such as CD, these abnormalities are nonspecific. Diffuse fluid-filled bowel loops during sonography also suggest malabsorption.
celiac disease
gluten
prolamines
HLA DQ2
DQ8
tissue transglutaminase
small bowel biopsy
gluten free diet
CD is an immune-mediated systemic disorder elicited by gluten in wheat and related prolamines from rye and barley in genetically susceptible individuals, and is characterized by the presence of a variable combination of gluten-dependent clinical manifestations, CD–specific antibodies, human leukocyte antigen (HLA)-DQ2 or DQ8 haplotypes, and enteropathy. CD–specific antibodies comprise autoantibodies against TG2 including endomysial antibodies (EMAs), and antibodies against deamidated forms of gliadin peptides.
CD is a common disorder with about 1% prevalence of biopsy-proven disease. It is thought to be rare in Central Africa and East Asia. Although CD develops in genetically susceptible individuals, environmental factors might affect the risk of developing CD or the timing of its presentation. Neither breastfeeding during gluten introduction nor any breastfeeding has been shown to reduce the risk of CD. The earlier introduction of gluten is associated with the earlier development of CD autoimmunity (positive serology) and CD, but the cumulative incidence of each in later childhood is not affected. It is advised to introduce gluten into the infant's diet anytime between 4 and 12 mo of age. Infectious agents have been hypothesized to play a causative role as frequent rotavirus infections were shown to be associated with an increased risk of developing CD. It is plausible that the contact with gliadin at a time when there is an ongoing intestinal inflammation alters intestinal permeability, and the enhanced antigen presentation can increase the risk of developing CD, at least in a subset of persons. The mode of delivery, socioeconomic status, season of birth, and the use of drugs have been associated with the risk of developing CD, but the evidence is contradictory.
A genetic predisposition is suggested by the family aggregation and the concordance in monozygotic twins, which approaches 100%. The strongest association is with HLA-DQ2.5 (1 or 2 copies encoded by DQA1 *05 [for the alpha] and DQB1*02 genes [for the beta chain]). Such a DQ molecule has been found to be present in more than 90% of CD patients. The highly homologous DQ2.2 molecule confers a much lower risk, while the data available on DQ2-negative CD patients indicate that they almost invariably are HLA-DQ8–positive (DQA1*0301/DQB1*0302). A gene dosage effect has been proved in prospective studies, and a molecular hypothesis for such a phenomenon has been proposed, based on the impact of the number and quality of the HLA-DQ2 molecules on gluten peptide presentation to T cells. The HLA locus is the most significant and dominant gene associated with CD; however, other loci known to contribute to CD have been documented. Most have been found to be associated with other autoimmune diseases such as type 1 diabetes. Interestingly, very few polymorphisms associated with CD are in coding regions, as they often are in binding sites for transcription factors, then affecting gene expression.
CD is a T-cell–mediated chronic inflammatory disorder with an autoimmune component. Altered processing by intraluminal enzymes, changes in intestinal permeability and activation of innate immunity mechanisms precede the activation of the adaptive immune response. Immunodominant epitopes from gliadin are highly resistant to intraluminal and mucosal digestion; incomplete degradation favors the immunostimulatory and toxic effects of these sequences. Some gliadin peptides (p31-43) activate innate immunity, in particular they induce interleukin (IL)-15. The latter, but also type 1 interferons, may alter the tolerogenic phenotype of dendritic cells, resulting in lamina propria T-cell activation by other peptides presented in the context of HLA-DQ2 or HLA-DQ8 molecules. Gliadin-specific T-cell responses are enhanced by the action of TG2: the enzyme converts particular glutamine residues into glutamic acid, which results in higher affinity of these gliadin peptides for HLA-DQ2 or HLA-DQ8. The pattern of cytokines produced following gliadin activation is dominated by interferon-γ (T-helper type 1 skewed); IL-21 is also upregulated. In downstream T-cell activation a complex remodeling of the mucosa takes place, involving increased levels of metalloproteinases and growth factors, which leads to the classical histologic finding of a flat mucosa. A severe impairment of intraepithelial lymphocytes (IELs) homeostasis is present in CD. IL-15 is implicated in the expression of natural killer receptors CD94 and NKG2D, as well as in epithelial expression of stress molecules, thus enhancing cytotoxicity, cell apoptosis, and villous atrophy. The most evident expression of autoimmunity is the presence of serum antibodies to TG2. However, the mechanisms leading to autoimmunity are largely unknown, as well as their pathogenetic significance. Potential CD, in which TG2 antibodies can be detected in situ without any histologic abnormality, shows that the production of antibodies does not necessarily lead to intestinal damage. The finding that IgA deposits on extracellular TG2 are not limited to the intestine but can be found in the liver, lymph nodes, and muscles indicates that TG2 is accessible to the gut-derived autoantibodies, turning CD into a systemic disease.
Clinical features of CD vary considerably. Intestinal symptoms are more common in children whose disease is diagnosed within the first 2 yr of life ; failure to thrive, chronic diarrhea, vomiting, abdominal distention, muscle wasting, anorexia, and irritability are present in most cases ( Fig. 364.3 ). Occasionally there is constipation, with cases presenting with intussusception. As the age at presentation of the disease shifts to later in childhood, and with the more extensive use of serologic screening tests, extraintestinal manifestations, without any accompanying digestive symptoms, have increasingly become recognized, affecting almost all organs ( Table 364.4 ). One of the most common extraintestinal manifestation of CD is iron-deficiency anemia, which is usually unresponsive to iron therapy. Osteoporosis may be present; in contrast to adults, it can be reversed by a gluten-free diet, with restoration of normal peak bone densitometric values. Other extraintestinal manifestations include short stature, delayed puberty, arthritis and arthralgia, epilepsy with bilateral occipital calcifications, peripheral neuropathies, isolated hypertransaminasemia, dental enamel hypoplasia, and aphthous stomatitis. The mechanisms responsible for the severity and the variety of clinical presentations remain obscure. Nutritional deficiencies or abnormal immune responses have been advocated. Silent CD is recognized, mainly in asymptomatic first-degree relatives of CD patients and in subjects affected by diseases associated with CD ( Table 364.5 ). Small bowel biopsy in silent/subclinical CD reveals severe mucosal damage consistent with CD. Potential CD is defined when patients have positive CD–specific antibodies, but without documented small bowel damage ( Table 364.6 ).
| MANIFESTATION | PROBABLE CAUSE(S) |
|---|---|
| CUTANEOUS | |
| Ecchymoses and petechiae | Vitamin K deficiency; rarely, thrombocytopenia |
| Edema | Hypoproteinemia |
| Dermatitis herpetiformis | Epidermal (type 3) tTG autoimmunity |
| Follicular hyperkeratosis and dermatitis | Vitamin A malabsorption, vitamin B complex malabsorption |
| ENDOCRINOLOGIC | |
| Amenorrhea, infertility, impotence, delayed puberty | Malnutrition, hypothalamic-pituitary dysfunction, immune dysfunction |
| Secondary hyperparathyroidism | Calcium and/or vitamin D malabsorption with hypocalcemia |
| HEMATOLOGIC | |
| Anemia | Iron, folate, vitamin B 12 , or pyridoxine deficiency |
| Hemorrhage | Vitamin K deficiency; rarely, thrombocytopenia due to folate deficiency |
| Thrombocytosis, Howell-Jolly bodies | Hyposplenism |
| HEPATIC | |
| Elevated liver biochemical test levels Autoimmune hepatitis |
Lymphocytic hepatitis Autoimmunity |
| MUSCULAR | |
| Atrophy | Malnutrition due to malabsorption |
| Tetany | Calcium, vitamin D, and/or magnesium malabsorption |
| Weakness | Generalized muscle atrophy, hypokalemia |
| NEUROLOGIC | |
| Peripheral neuropathy | Deficiencies of vitamin B 12 and thiamine; immune-based neurologic dysfunction |
| Ataxia | Cerebellar and posterior column damage |
| Demyelinating central nervous system lesions | Immune-based neurologic dysfunction |
| Seizures | Unknown |
| SKELETAL | |
| Osteopenia, osteomalacia, and osteoporosis | Malabsorption of calcium and vitamin D, secondary hyperparathyroidism, chronic inflammation |
| Osteoarthropathy | Unknown |
| Pathologic fractures | Osteopenia and osteoporosis |
| OTHER | |
| Enamel hypoplasia | Vitamin D, calcium malabsorption |
| Anxiety, schizophrenia | Unknown, uncertain |
| Pulmonary hemosiderosis | Unknown, uncertain |
| Aphthous stomatitis | Unknown |
| CELIAC TESTING RECOMMENDED |
|
| CELIAC TESTING SHOULD BE CONSIDERED |
|
| SYMPTOMATIC |
|
| SILENT |
|
| LATENT |
| Subjects who have a normal intestinal histology, but at some other time have shown a gluten-dependent enteropathy |
| POTENTIAL |
| Subjects with positive celiac disease serology but without evidence of altered intestinal histology. Patients may or may not have symptoms and signs of disease and may or may not develop a gluten-dependent enteropathy later |
Some diseases—many with an autoimmune pathogenesis—are found with a higher-than-normal incidence in CD patients. Among these are type 1 diabetes, autoimmune thyroid disease, Addison disease, Sjögren syndrome, rheumatoid arthritis, autoimmune cholangitis, autoimmune hepatitis, and primary biliary cholangitis. Such associations have been interpreted as a consequence of the sharing of identical HLA haplotypes, but a direct role of gluten in promoting autoimmunity cannot be excluded. The relation between CD and other autoimmune diseases is poorly defined; once those diseases are established, they are not influenced by a gluten-free diet. Other associated conditions include selective IgA deficiency and Down, Turner, and Williams syndromes.
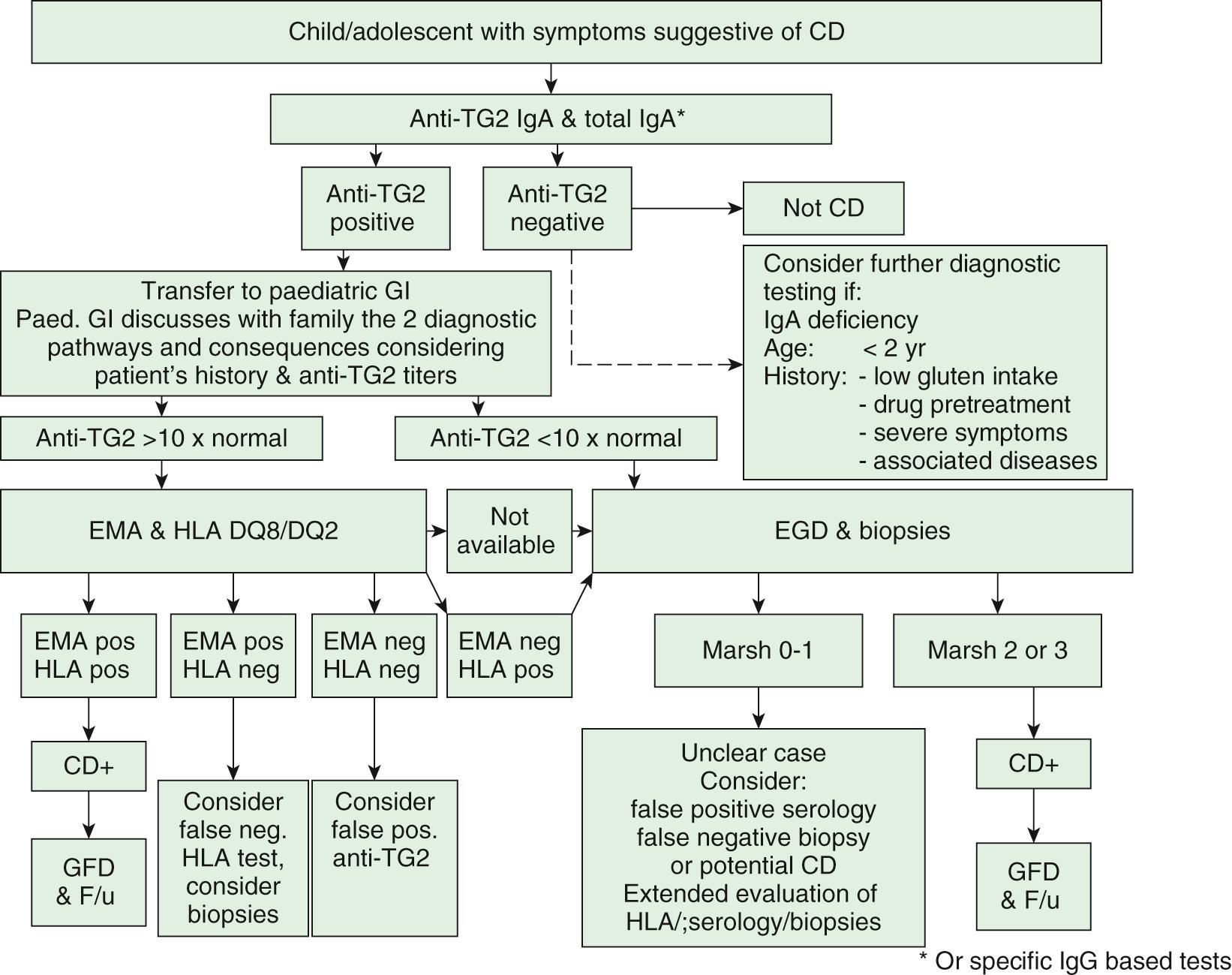
The diagnosis of CD is based on a combination of symptoms, antibodies, HLA status, and duodenal histology. The initial approach to symptomatic patients is to test for anti-TG2 IgA antibodies and for total IgA in serum to exclude IgA deficiency. If IgA anti-TG2 antibodies are negative, and serum total IgA is normal for age, CD is unlikely to be the cause of the symptoms. If anti-TG2 antibody testing is positive the patients should be referred to a pediatric gastroenterologist for further diagnostic workup, which depends on the serum antibody levels.
IgA anti-TG2 decline if the patient is on a gluten free diet. In patients with selective IgA deficiency, testing is recommended with IgG antibodies to TG2.
Patients with positive anti-TG2 antibody levels <10 times the upper limit of normal should undergo upper endoscopy with multiple biopsies. In patients with positive anti-TG2 antibody levels at or >10 times the upper limit of normal, blood should be drawn for HLA and EMA testing. If the patient is positive for EMA antibodies and positive for DQ2 or DQ8 HLA testing, the diagnosis of CD is confirmed, a life-long gluten-free diet is started and the patient is followed for the improvement of symptoms and the decline of antibodies. HLA testing is almost always positive; thus, it is possible that HLA testing will not be necessary in the future to establish diagnosis. In the rare case of negative results for HLA and/or anti-EMA in a child with TG2 antibody titers >10 times the upper limits of normal, the diagnostic workup should be extended, including repeated testing and duodenal biopsies ( Fig. 364.4 ). In asymptomatic persons belonging to high-risk groups, CD should always be diagnosed using duodenal biopsies ( Fig. 364.5 ). When biopsies are indicated, at least 4 fragments should be obtained from the descending part of the duodenum and at least 1 from the duodenal bulb. The diagnosis is confirmed by an antibody decline and preferably a clinical response to a gluten-free diet. CD is not the only cause for villous atrophy ( Table 364.7 ). Gluten challenge and biopsies will only be necessary in selected cases in which diagnostic uncertainty remains.
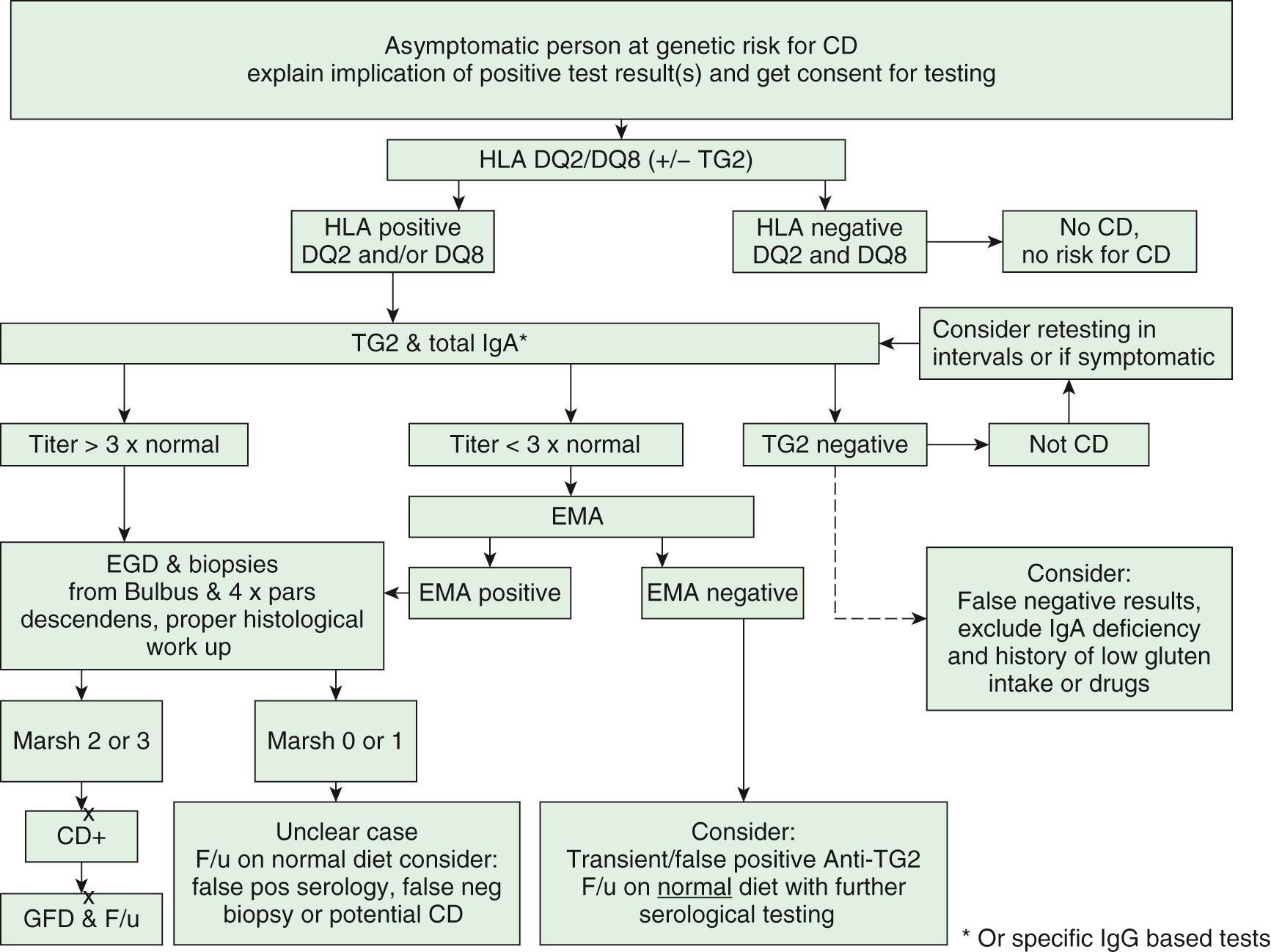
|
The only treatment for CD is lifelong strict adherence to a gluten-free diet. This requires a wheat-, barley-, and rye-free diet ( Tables 364.8 and 364.9 ). Despite evidence that oats are safe for most patients with CD, there is concern regarding the possibility of contamination of oats with gluten during harvesting, milling, and shipping. Nevertheless, it seems wise to add oats to the gluten-free diet only when the latter is well established, so that possible adverse reactions can be readily identified. There is a consensus that all CD patients should be treated with a gluten-free diet regardless of the presence of symptoms. However, whereas it is relatively easy to assess the health improvement after treatment of CD in patients with clinical symptoms of the disease, it proves difficult in persons with asymptomatic CD. The nutritional risks, particularly osteopenia and increased risk for other autoimmune disorders, are those mainly feared for subjects who have silent CD and continue on a gluten-containing diet. Little is known about the health risks in untreated patients with potential CD.
|
|
Some patients do not respond to a gluten free diet; refractory or nonresponsive CD requires a systematic approach to determine the correct diagnosis, compliance, and therapeutic options ( Fig. 364.6 ).
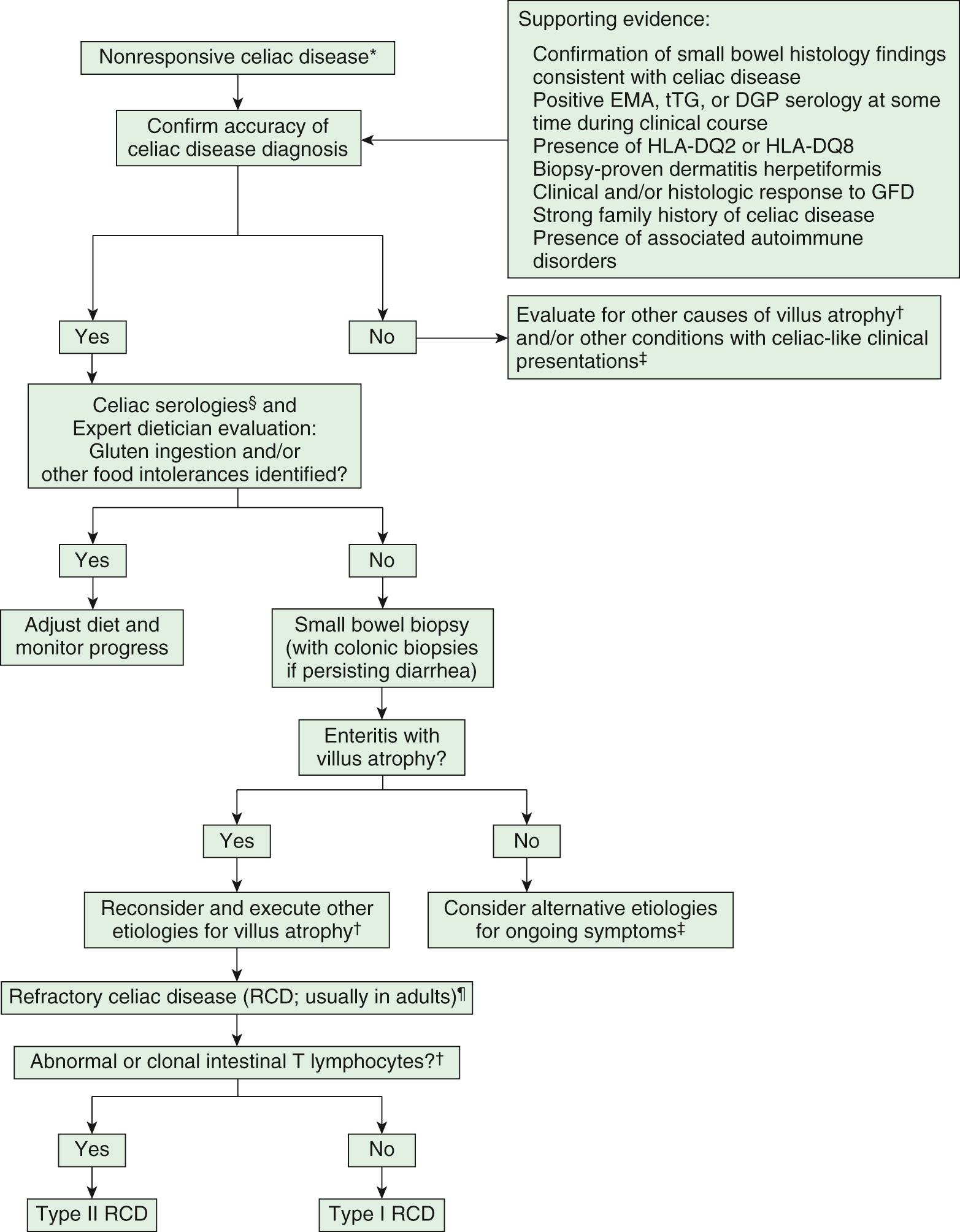
The Codex Alimentarius Guidelines define gluten-free food item for food containing <20 ppm (equivalent to 20 mg gluten in 1 kg of product); however, although analytical methods for gluten detection have reached a satisfactory degree of sensitivity, more information is needed on the daily gluten amount that may be tolerated by CD patients. The data available so far seem to suggest that the threshold should be set to <50 mg/day, although individual variability makes it difficult to set a universal threshold.
It is important that an experienced dietician with specific expertise in CD counseling educates the family and the child about dietary restriction. Compliance with a gluten-free diet can be difficult, especially in adolescents. It is recommended that children with CD be monitored with periodic visits for assessment of symptoms, growth, physical examination, complete blood count, thyroid diseases, and adherence to the gluten-free diet. Periodic measurements of TG2 antibody levels to document reduction in antibody titers can be helpful as indirect evidence of adherence to a gluten-free diet, although they are insensitive to slight dietary transgressions. If compliance is uncertain, bone health should be assessed.
CD is not the only disorder related to gluten ingestion. Symptoms in IgE-mediated wheat allergy are usually immediate (urticaria, angioedema, asthma, exercise-induced anaphylaxis). Diagnosis is based on dietary challenge, in vitro assay for specific IgE and skin testing.
Non-celiac gluten sensitivity (NCGS) is a poorly understood condition. Diagnosis is suspected in patients who do not have CD or wheat allergy, and yet show GI and non-GI symptoms upon ingestion of gluten- or wheat-containing food. In the general population, the incidence of self-reported gluten avoidance varies from 0.5 to 13%. Similar symptoms are often experienced by patients with irritable bowel syndrome (IBS), and some patients with IBS respond positively to a gluten-free diet.
Microvillus inclusion disease
Secretory diarrhea
Congenital tufting enteropathy
Tricho-hepatic-enteric syndrome
Enteric anendocrinosis
Proprotein convertase 1/3
Mitchell-Riley syndrome
Autoimmune enteropathy
Abetalipoproteinemia
Steatorrhea
Hypobetalipoproteinemia
Chylomicron retention disease
DGAT1
Wolman disease
Adrenal calcification
Lysosomal acid lipase
Tangier disease
Sitosterolemia
Primary bile acids malabsorption
Congenital diarrhea
Protein-losing enteropathy
Intestinal lymphangiectasia
This group mainly includes 2 conditions characterized by typical histological and ultrastructural lesions in the intestinal biopsies, microvillus inclusion disease (MVID) and congenital tufting enteropathy (CTE). Tricho-hepato-enteric syndrome (THE) or syndromic/phenotypic diarrhea is also usually classified in this group.
MVID is an autosomal recessive disorder, which manifests at birth with profuse watery secretory diarrhea . A late-onset variant, with onset 2-3 mo postnatal has also been described. It is the most severe cause of congenital diarrhea involving the development of the intestinal mucosa. Light microscopy of the small bowel mucosa demonstrates diffuse thinning of the mucosa, with hypoplastic villus atrophy and no inflammatory infiltrate. Diagnosis is performed with light microscopy using PAS and CD10 staining, which shows a very thin or absent brush border, together with positive PAS and CD10 intracellular inclusions. Electron microscopy shows enterocytes with absent or sparse microvilli. The apical cytoplasm of the enterocytes contains electron-dense secretory granules; the hallmark is the presence of microvilli within involutions of the apical membrane ( Fig. 364.7 ). Polyhydramnios is observed on prenatal sonography, and neonates usually present very early onset of severe watery diarrhea (up to 200-330 mL/kg/day) causing dehydration and failure to thrive. Despite parenteral nutrition, diarrhea continues, and initial fluid management is difficult. MVID and Fanconi syndrome that have been described in two patients may complicate management because of the additional features of a renal tubular acidosis, phosphaturia, rickets, and renal fluid losses. Mutations of the MYO5B gene coding for a nonconventional motor protein, myosin Vb, are associated with MVID in a cohort of patients suffering from early-onset MVID.
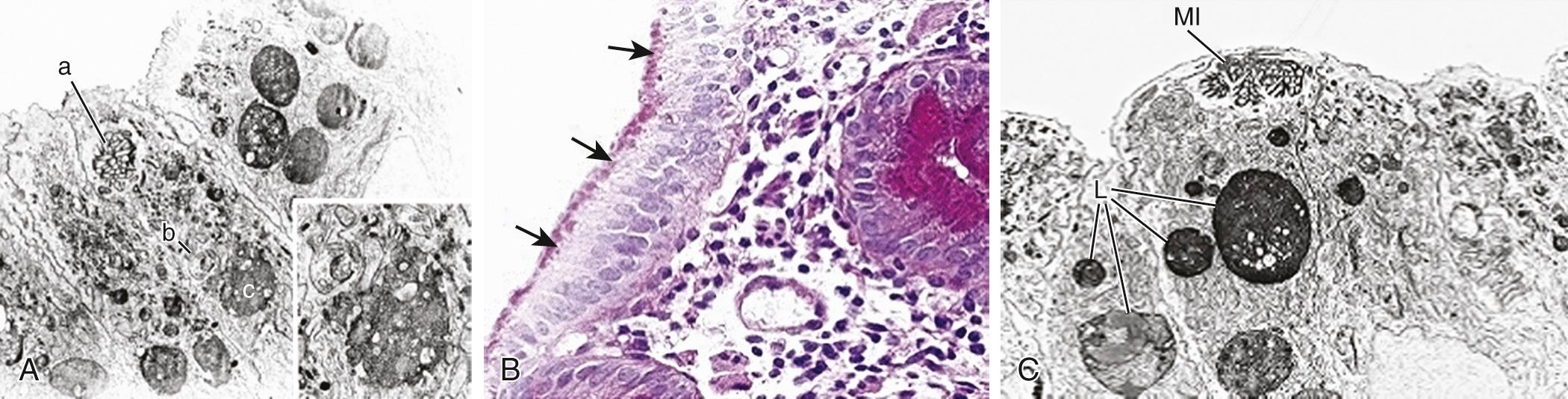
MYO5B mutations result in mislocalization of apical proteins and disrupted enterocyte polarization, leading to MVID. Another gene, the t-SNARE syntaxin3 (STX3) , has been described in patients with MVID and a milder phenotype. Patients with mutations in the STX3 binding protein STXBP2/Munc18-2 , causing familial hemophagocytic lymphohistiocytosis type 5, also show microvillus atrophy and histologic findings reminiscent of MVID. Loss of STX3 or Munc18-2 inhibits the fusion of vesicles with the apical membrane, resulting in the intracellular retention of apical proteins. MYO5B mutations have also been identified in several patients with progressive familial intrahepatic cholestasis (PFIC)-like phenotype with normal serum gamma-glutamyl transferase activity and without intestinal disease.
CTE (intestinal epithelial dysplasia) manifests in the first few weeks of life with persistent watery diarrhea. CTE accounts for a small fraction of infants with intractable diarrhea of infancy . The distinctive feature on small intestinal mucosal biopsy is focal epithelial tufts (teardrop-shaped groups of closely packed enterocytes with apical rounding of the plasma membrane) involving 80–90% of the epithelial surface. The typical pathology does not appear immediately after birth; other enteropathies may show tufts on the epithelial surface.
CTE is a phenotypic and genetic heterogenous condition. Genetic studies identified mutations in epithelial cell adhesion molecule (EPCAM) gene in 73% of patients and mutations in hepatocyte growth factor activator inhibitor type 2 SPINT2/HAI2 gene in 21%. No identified mutations are identified in a minority of patients. The phenotype associated with mutations of EPCAM is usually an isolated congenital diarrhea without associated extra digestive symptoms, except late-onset arthritis or superficial punctuate keratitis. In the syndromic form of CTE, diarrhea is associated with 1 or more of these same anomalies: superficial punctate keratitis (100%), choanal atresia (50%), esophageal or intestinal atresia, anal imperforation, hair dysplasia, skin hyperlaxity, bone abnormalities, hexadactylia, and facial dysmorphism.
No specific treatment exists, thus, as for MVID, management requires permanent parenteral nutrition (PN) with possible intestinal transplantation (see Chapter 365 ).
THE, also known as syndromic diarrhea (SD), is a congenital enteropathy manifesting with early onset of severe diarrhea. Patients are born small for gestational age and present with diarrhea starting in the first 6 mo of life. They have an abnormal phenotype, including facial dysmorphism with prominent forehead, broad nose, and hypertelorism with a distinct abnormality of hair, trichorrhexis nodosa . Hairs are woolly, easily removed, and poorly pigmented. Abnormal cutaneous lesions including café-au-lait on the lower limbs may be observed. Liver disease affects about half of the patients with extensive fibrosis or cirrhosis. Cardiac abnormalities and colitis have been reported sporadically, as well as one case involving polyhydramnios, placental abnormalities, and congenital hemochromatosis. Patients may have defective antibody responses despite normal serum Ig levels and defective antigen-specific skin tests despite positive proliferative responses in vitro. Patients with THE can also present as very-early-onset inflammatory bowel disease (IBD). Small bowel biopsies show nonspecific villus atrophy with or without mononuclear cell infiltration of the lamina propria, and without specific histologic abnormalities involving the epithelium. Mutations in either tetratricopeptide repeat domain 37 (TTC37) gene (60%) or SKIV2L (40%) have been identified as cause of THE syndrome. Enterocytes with TTC37 mutations, show reduced expression of brush-border-associated NHE-2 and -3, aquaporin-7, the Na+/I- symporter, and the H+/K+-ATPase or mislocalization relative to their normal pattern. Prognosis of this type of intractable diarrhea of infancy is poor. The long-term follow-up of these children reported that at 15 years about 50% of patients were alive or have been weaned off PN. The main complications are liver disease and infections. Most of the children achieve short final stature and half are slightly developmentally delayed.
This class of congenital diarrheas is characterized by abnormal enteroendocrine cells development or function. The genes causing these disorders encode either transcription factors essential for the development of all or a subset of enteroendocrine cells, or cellular proteins/endopeptidases that are required for the production of active hormones from prohormones. The conditions manifest with osmotic diarrhea and in some, additional systemic endocrine disorders. The treatment is nutritional support and hormonal replacement if needed. Four genes have been associated with the diseases classified in this group: NEUROG3, RFX6, ARX, and PCSK1 .
NEUROG3 is a key transcription factor that controls the fate of endocrine cells in both the pancreas and intestine. Mutations of the NEUROG3 gene produce generalized mucosal malabsorption, vomiting, diarrhea, failure to thrive, dehydration, and a hyperchloremic metabolic acidosis. Oral alimentation with anything other than water produces diarrhea. Villus-crypt architecture in small bowel biopsies is normal, but staining for neuroendocrine cells (e.g., employing antichromogranin antibodies) demonstrates a complete absence of this secretory cell lineage with the preservation of goblet cells and Paneth cells.
Autosomal recessive proprotein convertase 1/3 (PC1/3) deficiency, caused by mutations in the PCSK1 gene, is characterized by severe congenital malabsorptive diarrhea, early-onset obesity, and other endocrine abnormalities. All functional hormones produced by endocrine cells, including those in the gut, are processed by a specific Ca2+-dependent serine endoprotease named proprotein convertase 1/3 (also known as neuroendocrine convertase 1). Chronic watery, neonatal onset diarrhea is described in infants with hyperinsulinism, hypoglycemia, hypogonadism, and hypoadrenalism. A small bowel biopsy reveals a nonspecific enteropathy.
Growth hormone deficiency, adrenal insufficiency, central diabetes insipidus, and hypogonadism are commonly observed.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here