Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
A wide variety of diseases can affect the great thoracic arteries. They include entities resulting from long-standing atherosclerotic degeneration (e.g., aortic aneurysms) that as such can go undetected for a prolonged time, as well as diseases presenting with acute severe and potentially life-threatening symptoms, like pulmonary embolism and acute aortic dissection. As clinical signs and other preliminary tests are often unreliable to establish a correct diagnosis, patients suspected of having thoracic vascular disease are in general promptly referred for imaging examinations to establish presence and extent of disease. In clinical practice, a wide variety of modern imaging techniques are generally available, including echocardiography, computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET), the latter sometimes combined with computed tomography (PET-CT).
Given the amount of morphological and functional information that can be derived from these imaging modalities, the acquired data imaging is increasingly not only used for diagnostic purposes, but forms also an important cornerstone in the pre-procedural planning of a variety of interventional procedures. In this chapter, we will provide an overview of the most important diseases of the thoracic great vessels and discuss the specific contributions of different imaging modalities.
The term “acute aortic syndrome” (AAS) is often used to refer to a number of potentially life-threatening diseases, which can all present with acute chest pain [ ]. Traditionally, AAS encompasses three entities: classic aortic dissection (AD), intramural hematoma (IMH), and penetrating atherosclerotic ulcer (PAU). While these components of AAS have historically been considered separate entities, this view has been recently challenged. Improvements in CT imaging, including the introduction of ECG-triggered acquisitions, have increased the available imaging detail of the aortic wall leading to a better understanding of the underlying pathophysiological mechanism [ ]. It has also been demonstrated that the different AAS entities have both discriminating and overlapping features, which can dynamically evolve over time [ ]. This is especially true for classic aortic dissection and intramural hematoma, which can be considered two different presentations of the same pathologic process arising from the aortic media. Conversely, a PAU has a completely different etiology, as it is the consequence of an atherosclerotic degeneration of the intimal layer.
The clinical presentation of AAS is often non-specific, with a common denominator of severe chest pain. In acute aortic dissection this pain is often described as intense, acute, shearing/tearing, throbbing, or sometimes migratory. When the ascending aorta is involved, pain can be referred to the anterior chest, throat, neck, and jaw. When the abdominal aorta is also affected, patients can have back and abdominal pain. Furthermore, patients can develop ischemic symptoms due to stenosis and occlusion of side branches of the aorta by the dissection flap.
Given the myriad of potential clinical complaints, clinical features are as such unreliable to differentiate between the different etiologies of AAS and other important entities such as myocardial infarction and pulmonary embolism [ ]. Additional non-invasive imaging is therefore needed to establish the correct diagnosis and initiate the appropriate treatment.
Two classifications of aortic dissection exist: the DeBakey classification and the Stanford classification [ , ]. The DeBakey classification distinguishes three different types of dissection. Type I involves the ascending aorta, the arch, and a variable length of the thoracic and abdominal aorta. Type II is restricted to the ascending aorta. Type IIIA is confined to the descending thoracic aorta and type IIIB extends in the abdominal aorta to the bifurcation/iliac vessels.
The Stanford classification only distinguishes between two types: type A and B aortic dissection. Type-A is defined as involving the ascending aorta, regardless of further involvement of the aortic arch and descending aorta. A Stanford type-B dissection includes every dissection in which the ascending aorta is not involved.
Many centers routinely use the Stanford classification as the distinction between a dissection with its origin proximal or distal from the ostium of the left subclavian artery to quickly differentiate between different treatment strategies (in general urgent surgery for type-A dissection vs. strict hypertension control and follow-up for uncomplicated type-B dissections).
An aortic dissection is caused by a primary intimal tear. While the exact etiology of this entry tear is often unknown, several predisposing conditions exist which can affect the integrity of the media ( Table 20.1 ). One of the most common risk factors is long-standing arterial hypertension, which increases shearing forces on and augments stiffness of the aortic wall. Other causes include connective tissue disorders like Marfan and Ehlers-Danlos syndrome, and traumatic aortic deceleration injuries.
| Acquired |
| Arterial hypertension |
| Pregnancy |
| Inflammatory disease (Giant cell aortitis, Takayashu, SLE) |
| Syphilitic aortitis |
| Traumatic deceleration injury |
| Cocaine abuse |
| Iatrogenic (i.e., percutaneous coronary angiography) |
| Inherited |
| Marfan syndrome |
| Ehlers-Danlos syndrome |
| Adult polycystic disease |
| Turner syndrome |
| Noonan syndrome |
| Osteogenesis imperfecta |
| Polycystic kidney disease |
| Congenital |
| Bicuspid aortic valve |
| Aortic coarcation |
Once an intimal tear has developed, this acquired entry point allows blood from the aorta to disseminate in the media layer and extend both proximally and distally with inward displacement of the intima ( Figure 20.1 ). The entry tear is typically located at the site of highest wall tension, most often a few centimeters above the aortic valve or at the attachment site of the ligamentum arteriosum just distally from the left subclavian artery origin ( Figure 20.1 ).
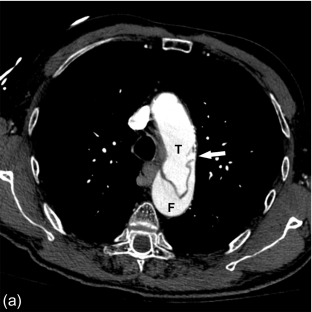
This intramural blood flow and the consequent dissection of the aortic wall commonly lead to a double-barrel lumen consisting of two channels: a true and a false lumen. The false lumen is completely contained within the aortic media, and divided from the true lumen by a dissection flap consisting of intima and a portion of the media layer.
In a classic dissection , both an entry and exit site exists for the intramural flowing blood. Consequently, both true and false lumina contain flowing blood, with faster flow in the true lumen. As pressure builds in the false lumen, the cross-sectional diameter of the false lumen decreases and often becomes the smallest channel ( Figure 20.2 ).
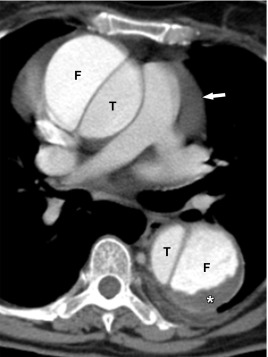
Conversely, an intramural hematoma is best understood as a dissection in which there is a clear intramural entry site, but no clear or adequate exit site for the blood back in the true lumen. As such, the false lumen contains thrombosed blood, and remains the smallest channel next to a larger true lumen ( Figure 20.3 ). It is important to realize that over time, an exit site can develop, allowing an intramural hematoma to transgress into a channel with flowing blood and reaching the configuration of a classic dissection ( Figure 20.4 ).
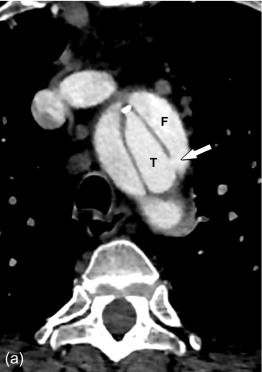
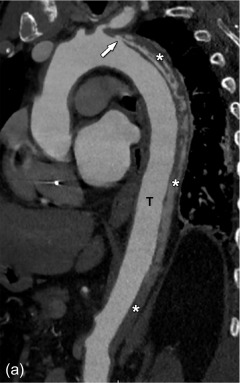
Many imaging techniques can be used to visualize an aortic dissection, each with their specific strengths and weaknesses. An overview is given in Table 20.2 .
| Imaging modality | Sensitivity | Specificity | Availability | Examination time | Evaluation of aortic sinus & ascending aorta | Evaluation of aortic arch and more distal aorta segments | Evaluation of aortic side branch involvement | Overall benefits | Overall disadvantages |
|---|---|---|---|---|---|---|---|---|---|
| Chest radiography | − | − | +++ | Short | − | − | − | Quick initial chest review | Very non-specific |
| Transthoracic echocardiography | +/− | +/− | +++ | Variable | ++ | − | − | Good evaluation possible of aortic root & ascending aorta, bedside examination possible, hemodynamic evaluation of aortic valve | Operator-dependent, no complete aorta evaluation possible |
| Transesophageal echocardiography | +/− | +/− | ++ | Variable | +++ | − | + | ||
| CT | +++ | +++ | +++ | Short | +++ | +++ | +++ | Modality of choice for acute initial evaluation, detection & follow-up of complications | Use of iodinated-contrast material, radiation exposure |
| MR | +++ | +++ | + | Long | +++ | +++ | +++ | Can be used when iodinated contrast agents are contra-indicated, or in patients with impaired renal function | Longer examination time, more patient cooperation required, more sensitive to artifacts in critically ill patients |
| Angiography | ++ | ++ | − | Long | +++ | +++ | +++ | Intervention possible (endografts) in specific cases | Less suitable for evaluation of intramural hematoma, operator-dependent technique, more prone to procedure-related complications, use of iodinated contrast-agent, radiation exposure |
While a chest radiograph is often the first imaging examination performed on a patient with acute chest pain, its value in the evaluation of a potential acute aortic event is very limited. Several signs, including widening of the mediastinum and aortic contour, disparity in size between the ascending and descending aorta, changes in aortic configuration on serial examinations, and displacement of aortic calcification by at least 10 mm, have been described but remain non-specific.
Ultrasound techniques available for the detection of AAS include transthoracic and transoesophageal echocardiography (TTE/TOE).
Both TOE and TTE are non-invasive, widely available, and relatively inexpensive imaging modalities. They can also be performed bedside in a critically ill patient.
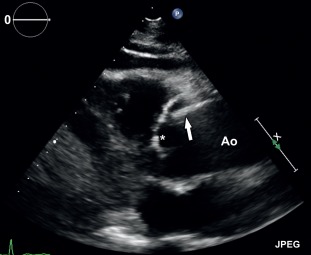
TTE is a valid imaging choice to detect complications involving the aortic valve, for example, aortic regurgitation. However, a large part of the ascending aorta and aortic arch are often not visualized with TTE due to poor acoustic window. TOE is superior in depicting the ascending aorta and therefore useful in the further evaluation of a potential type-A dissection ( Figure 20.5 ). Also, it can simultaneously visualize complications such as aortic regurgitation (in a dissection involving the aortic sinus), pericardial effusion, coronary artery involvement, and left ventricular function. It is less suitable for evaluation of the distal ascending aorta and proximal aortic arch. TOE can also detect localized wall thickening in patients with intramural hematoma.
Nevertheless, both TTE and TOE are operator-dependent techniques, which are unable to visualize the complete thoracic aorta, and can produce false-positive results due to reverberation artifacts. As such, they are rarely relied upon as the sole diagnostic imaging modality in patients with suspected acute aortic syndrome and have a more complementary function.
Computed tomography angiography (CTA) is the imaging technique of first choice in patients with acute aortic syndrome. CTA is the only imaging modality that can provide an accurate and complete assessment of the whole thoraco-abdominal aorta and side-branches in a very short examination time, with a reported sensitivity and specificity for aortic dissection of more than 95%. Furthermore, it can simultaneously evaluate all other non-vascular structures (such as the pulmonary parenchyma and visceral abdominal organs), thus allowing detection of an alternative etiology for the acute clinical presentation.
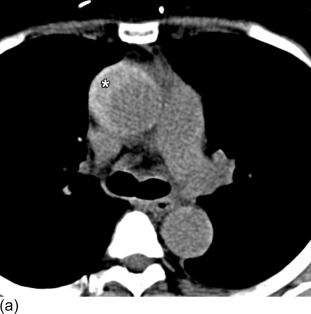
In practice, all CTA examinations are invariably performed with the use of intravenous contrast administration for optimal vascular enhancement and evaluation. However, an initially performed non-enhanced CT-scan can have incremental value in patients with suspected aortic dissection, as it can depict a hyperdense acute intramural hematoma ( Figure 20.6 ), that may not otherwise be seen. Our recommendation is to add an unenhanced examination to your institutional CT protocol for evaluation of patients with suspected aortic dissection.
Despite all the clear advantages of CTA over other imaging modalities, its use of an iodinated contrast agent and associated radiation exposure limits its application in patients with impaired renal function and known intolerance to iodinated dye. Radiation exposure is especially an important issue, as patients with a stable type-B dissection require follow-up imaging over years to monitor for the development of (among others) a post-dissection aneurysmal dilatation of the affected aortic segments. Consequently, and especially in young patients, many centers often use MRI for follow-up of stable patients with a type-B dissection. However, the recent introduction of iterative reconstruction techniques has made radiation dose less of a concern [ , ].
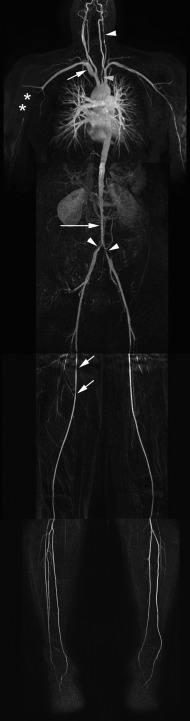
Both non-contrast (phase-contrast and time-of-flight techniques) and contrast-enhanced acquisitions are available to visualize the thoracic aorta with MRI. However, non-contrast techniques have significant drawbacks, including a longer examination time, a shorter anatomic coverage, and greater difficulty in differentiating between slow-flow effects and the presence of thrombus. Consequently, contrast-enhanced MR angiography (MRA) is the method of choice to evaluate the thoracic aorta for the presence and extent of dissection.
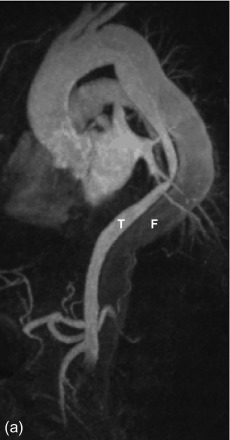
MRA has similar advantages over echocardiography as CT, including a comparable high sensitivity and specificity (> 95%) to diagnose aortic dissection, a large anatomic coverage, and the evaluation of the relation of the dissection flap with aortic side branches. Additionally, given the lack of radiation exposure, multiple acquisitions are possible to visualize the dynamics of contrast enhancement of the true and false lumen with no compromise to the patient’s safety ( Figure 20.7 ). The use of Gadolinium-chelates as a contrast agent makes MRA also the technique of choice in patients with impaired renal function. It is also a good choice for repeated follow-up of patients with a known and stable (type-B) dissection to monitor for the presence and evolution of a post-dissection aortic aneurysm. Finally, it has increased sensitivity compared with CT to detect an intramural hematoma, providing as such additional value to CT in equivocal cases.
However, it is a technically more complex study with a longer examination time, requiring a higher level of patient cooperation compared with CT. Therefore, it is less suitable for critically ill patients. Also, while the anatomic coverage acquired during a single study is generally large enough to cover the thoracic and supra-renal aorta, contrary to CT, most MR systems cannot cover the whole aorta and iliac arteries in a single examination. These disadvantages make MRA rarely the first imaging choice in a patient with a suspected aortic dissection.
In every mainstream radiology practice, the excellent sensitivity and specificity of CT and MRI for diagnosing acute aortic dissection have completely obliterated the need for invasive angiography for this indication. While both direct and indirect signs of aortic dissection can be distinguished using angiography (including visualization of the intima and lumina, inward displacement of the true lumen by the intima flap, and the presence of aortic valve regurgitation), it has virtually no place in the initial evaluation. Furthermore, and despite the high sensitivity and specificity for the detection of dissection, it only visualizes the lumen and is not suitable for detection of an intramural hematoma, peri-aortic complications, and total size of the aorta.
However, the continuous development of percutaneous intervention techniques has increased their potential application in the treatment of selected patients with type-B dissection, in recent years reflected as an increased number of endovascular stenting procedures in specialized centers. While not commonplace, highly specialized imaging techniques like cine-angiography of different segments of the aorta and intravascular or phased-array linear ultrasound may additionally provide a supporting role during these aortic interventions. These adjuvant techniques may as such further contribute to a better distinction between true from false lumen, detect side branch compromise, and further facilitate interventions such as flap-fenestration.
Communication of the imaging findings to the surgeon and clinician is facilitated when using a logically structured report. A summary of the most important points to evaluate can be found in Table 20.3 .
The following items should be looked for and discussed in the different imaging reports.
|
Generally, echocardiography will be used to evaluate the presence and extent of a dissection in the aortic root and ascending aorta. It also provides excellent assessment of the functional cardiac repercussions in general, and more specifically at the level of the aortic valve.
A more complete and accurate evaluation of an aortic dissection can be delivered with contrast-enhanced CT or MR. Here, the presence and extent of the dissection must be accurately evaluated, as well as the involvement of the supra-aortic and splanchnic side branches.
Finally, the possibility of treatment using an endovascular approach in type-B dissection can be evaluated, discussing entry and exit sites and potential sealing locations.
A broad variety of diseases can lead to symptomatic inflammatory changes in the arterial wall. In clinical practice, the distinction is made between large vessel vasculitis (i.e., giant cell arteritis and Takayasu arteritis), and medium vessel and small vessel vasculitis [ ]. In this chapter, we will focus on thoracic aortitis, which can have different infectious and non-infectious causes ( Table 20.4 ).
| Non-infectious | Infectious |
|---|---|
| Takayasu arteritis Giant cell arteritis Behçet’s disease Ankylosing spondylitis Relapsing polychondritis Rheumatoid arthritis Idiopathic isolated aortitis |
Pyogenic infection Tuberculous aortitis Syphilitis aortitis |
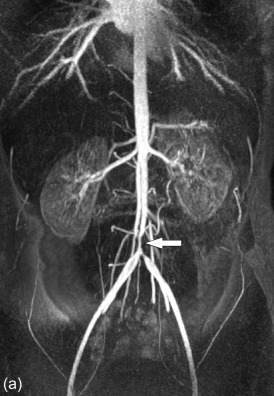
The initial clinical presentation of aortitis is often characterized by non-specific symptoms like persistent fever without a clear origin, malaise, night sweating, and arthralgia. In patients with a late clinical presentation symptoms related to complications (stroke, myocardial infarction, heart failure, aortic rupture, and ischemia distal to a stenosis) can often be observed [ ]. In patients with giant cell arteritis, a minority (15%) show involvement of extracranial vessels. In this subgroup of patients, amaurosis fugax may be the presenting symptom if the ophthalmic artery is involved. In contrast, Takayasu arteritis develops characteristically in the thoracic aorta with involvement of the subclavian arteries. It can extend into the abdominal aorta/iliac and femoral vessels and cause hypertension due to occlusion/stenosis of the renal arteries ( Table 20.5 ).
| Giant cell arteritis | Takayasu arteritis |
|---|---|
| Age at onset of disease > 50 years | Age at onset of disease < 40 years |
| New headache | Claudication of an extremity |
| Associated with stiffness and pain of the joints (polymyalgia rheumatica) | Decreased brachial artery pulse |
| Temporal artery abnormality | Difference in systolic blood pressure between arms |
| Elevated erythrocyte sedimentation rate | A bruit over the subclavian arteries or the aorta |
| Abnormal findings on biopsy of temporal artery | Imaging criteria:
|
Several imaging modalities are used and suitable for evaluation of patients with (suspected) thoracic aortitis. The advantages and disadvantages of the different techniques are listed in Table 20.6 .
| Imaging modality | Advantages | Disadvantages |
|---|---|---|
| Ultrasound |
|
|
| MRI/MRA |
|
|
| CTA |
|
|
| 18 FDG PET-CT |
|
|
Radiography can demonstrate very non-specific findings such as a dilated aorta or mediastinal widening due to dilatation of aortic branch vessels. In the late phase, calcifications can be present in the aortic arch and the descending aorta. Radiography can be of value in ruling out alternative explanations of non-specific vascular complaints such as cervical ribs in patients with thoracic outlet syndrome.
In patients with aortitis, ultrasound can demonstrate a hypoechogenic halo around the arterial lumen, stenosis, and occlusions in 93% of the cases [ ]. Differentiation between arterial wall inflammation and atherosclerosis is crucial and clinically very relevant. Several echographic characteristics are associated with arterial wall inflammation: concentric and long segment involvement, the presence of minimal amount of plaque content, and the location of the lesion (atherosclerosis has typical preferential locations) [ ]. Furthermore, a luminal halo is 100% specific for wall inflammation and is absent in patients with atherosclerosis [ ]. TTE can be useful to evaluate the ascending aorta and the presence of aortic regurgitation. TOE can evaluate a larger section of the thoracic aorta.
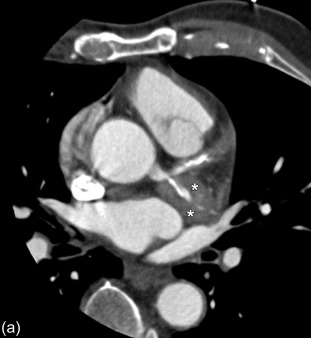
Computed tomography (CT) is often the preferred initial imaging modality. Unenhanced CT images can demonstrate mural calcifications. CT angiography can demonstrate vessel wall thickening, representing vessel wall inflammation. Typically a double-ring pattern is present with a poor enhancing ring centrally and well-enhancing ring peripherally. Furthermore, a hypodense ring around the vessel wall (vessel wall thickening) representing peri-vasculitis can be visualized as well. Besides this vessel wall thickening, peri-vascular infiltration can be a presenting sign in patients with vasculitis. Moreover, CTA can reveal the presence of mural thrombi. Furthermore, CTA can very accurately (sensitivity 93% and specificity 98%) demonstrate findings typical for the late phase of the disease: arterial stenosis, occlusions, dilatations, and aneurysm formation [ , ] ( Figure 20.8 ).
MRI is a superior imaging technique compared to CTA and ultrasound in evaluating early and late arterial wall inflammation. MRI has additional value in evaluating the large arteries in patients with large vessel arteritis, especially Takayasu arteritis [ , , ]. However, in patients with giant-cell arteritis, the role for MRI is limited. MRI can accurately depict vessel wall inflammation and mural thickening. Furthermore, MRI can also demonstrate peri-vasculitis as soft tissue around the vessel wall. T2 inversion recovery weighted images can depict mural oedema as hyperintense signal, which correlates to active early disease [ ]. Although experimental, diffusion-weighted MRI has shown promise to detect inflammatory changes in the thoracic aorta. MR angiography is suitable for the detection of late findings in aortitis such as stenosis, dilatations and aneurysms ( Figures 20.9 and 20.10 ). Furthermore, MRI can reveal involvement of the aortic valve and quantify concomitant aortic valve regurgitation or stenosis. Edema imaging is very sensitive (sensitivity up to 94%) in detection of large-vessel vasculitis [ ]. Furthermore, dynamic contrast-enhanced (DCE) MRI can accurately (sensitivity up to 86%) monitor inflammation activity with a comparable accuracy to FDG-PET examinations [ , ]. Enhancement of the vessel wall decreases in case of lower disease activity.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here