Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Cerebral blood flow (CBF) is modulated by cerebral metabolic rate, cerebral perfusion pressure (CPP, the difference between the mean arterial pressure [MAP] and intracranial pressure [ICP]), and arterial blood carbon dioxide (Paco 2 ) and oxygen (Pao 2 ) tensions. Various drugs and intracranial pathologies can also impact CBF. During normal physiologic conditions, CBF is autoregulated over a range of CPPs. With intact autoregulation, normal CBF in an awake person is approximately 50 mL/100 g brain tissue per minute. In the past, CBF was thought to be autoregulated over a range of CPPs of 50 to 150 mm Hg in chronically normotensive patients. However, more recent data suggest that the lower limit of autoregulation may be greater than 50 mm Hg in normotensive individuals. Also, the autoregulatory range may be dynamic, changing in response to physiologic factors (e.g., sleep-wake cycles) and likely varies among individuals. Given that a normal adult brain weighs approximately 1500 g and normal cardiac output is 5 L/min, CBF is therefore 750 mL/min or 15% of cardiac output during the awake state.
Normal cerebral metabolic rate, generally measured as rate of oxygen consumption (CMRO 2 ), is 3.0 to 3.8 mL O 2 /100 g brain tissue per minute. During awake resting conditions, total body oxygen consumption is approximately 250 mL O 2 /min. Therefore total brain oxygen consumption is 18% to 23% of total body oxygen consumption. CMRO 2 can be decreased by temperature reductions and various anesthetic drugs and increased by temperature increases and seizures.
Anesthetic and intensive care management of neurologically impaired patients relies heavily on manipulation of intracranial volume and pressure. These in turn are influenced by cerebral blood volume (CBV) and CBF. CBF and CBV do not always change in parallel. For example, vasodilatory anesthetics and hypercapnia may produce parallel increases in CBF and CBV. Conversely, moderate systemic hypotension still within the brain’s autoregulatory capacity can produce minimal change in CBF but, as a result of compensatory vessel dilation, an increase in CBV. Similarly, partial occlusion of an intracranial artery, such as occurs in embolic stroke, may reduce regional CBF. However, vessel dilation distal to the occlusion, which is an attempt to restore circulation, can produce an increase in CBV.
Variations in Paco 2 produce corresponding changes in CBF ( Fig. 13.1 ). As a guideline, CBF increases by 1 to 2 mL/100 g/min (or ~15 mL/min for a 1500-g brain) for every 1 mm Hg increase in Paco 2 . A similar decrease occurs during hypocapnia when Paco 2 is acutely decreased. The impact of Paco 2 on CBF is mediated by variations in the pH of the cerebrospinal fluid (CSF). Decreased CSF pH causes cerebral vasodilation, and increased CSF pH results in vasoconstriction. Paco 2 can also modulate CBV. The extent of CBV reduction is dependent on the anesthetic being used. In general, vasoconstricting anesthetics tend to attenuate the effects of Paco 2 on CBV.
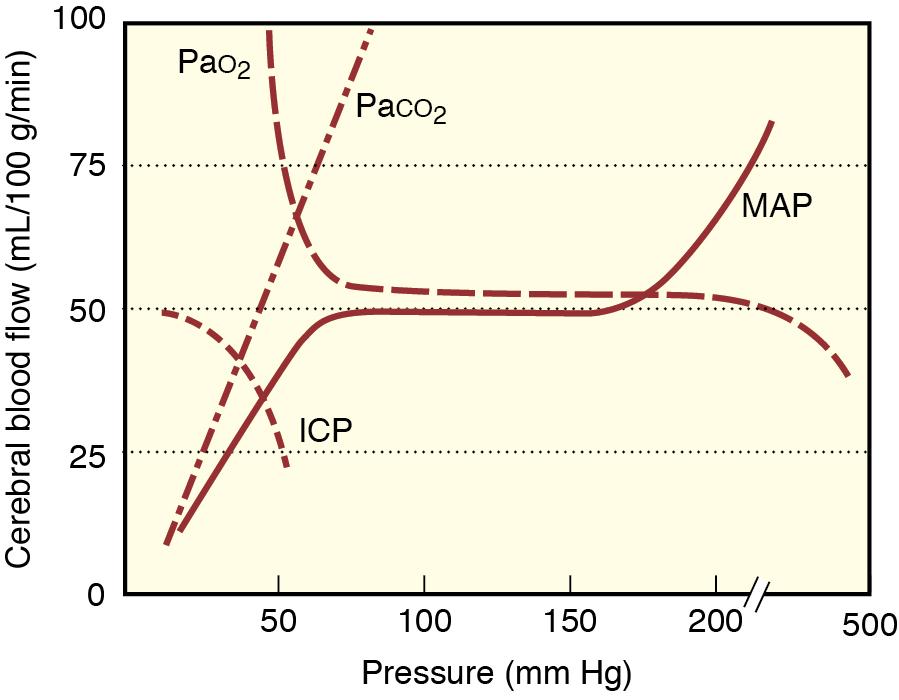
The ability of hypocapnia to acutely decrease CBF, CBV, and ICP is fundamental to the practice of clinical neuroanesthesia. Significant acute hypocapnia can increase risk for cerebral ischemia due to excessive vasoconstriction. The ability of hypocapnia to decrease CBV, and thus ICP, is attenuated by the return of CSF pH to normal after approximately 6 hours of hypocapnia. This reduces the effectiveness of induced hypocapnia as a means of long-term control of intracranial hypertension.
Decreased Pao 2 does not significantly affect CBF until a threshold value of approximately 50 mm Hg is reached (see Fig. 13.1 ). Below this threshold, there is abrupt cerebral vasodilation, and CBF increases. Hyperoxia has minimal, if any, effect on CBF.
The ability of the brain to maintain CBF at constant levels despite changes in CPP is known as autoregulation (see Fig. 13.1 ). Autoregulation is an active vascular response characterized by (1) arterial constriction when CPP is increased and (2) arterial dilation in response to decreases in CPP. When CPP is below the lower limit of autoregulation, cerebral blood vessels are maximally dilated, and CBF decreases. CBF becomes directly related to CPP. As CPP is further decreased, cerebral ischemia may ensue, causing nausea, dizziness, and altered consciousness. When CPP is increased above the upper limit of autoregulation, cerebral arterioles are maximally constricted, and CBF varies proportionally with CPP. If CPP increases further, fluid may be forced across blood vessel walls into the brain parenchyma, producing cerebral edema. The risk of cerebral hemorrhage also increases.
In the setting of chronic hypertension, the autoregulation curve is displaced to the right so that pressure dependence of CBF occurs at a higher CPP at both the lower and upper limits of autoregulation. In those with chronic hypertension, risk for cerebral ischemia can occur at systemic blood pressures that would be tolerated by normotensive individuals. Gradual treatment of hypertension can restore the autoregulation curve to normal. Acute hypertension can produce signs of central nervous system dysfunction at MAP values that are well tolerated in chronically hypertensive patients. Similarly, an acute hypertensive response associated with direct laryngoscopy or surgery may exceed the upper limit of autoregulation in chronically normotensive patients. Autoregulation of CBF may be lost or impaired during a variety of conditions, including the presence of intracranial tumors or head trauma and the administration of volatile anesthetics. Increased impairment of autoregulation leads to greater dependence of CBF on systemic blood pressure such that the autoregulation curve is no longer flat.
Increases in intracranial venous blood pressure can impede venous drainage from the brain and may predispose the patient to cerebral edema and cerebral ischemia, the latter due to increases in ICP, which in turn reduces CPP. Impaired venous drainage can increase brain bulk and complicate intracranial surgery. Examples of situations that can cause impaired venous drainage include superior vena cava syndrome, cerebral venous thrombosis, or jugular vein compression, as can occur with improper neck positioning during surgery. With coughing against a partially closed glottic opening, increases in intrathoracic pressure result in transient increases in cerebral venous pressure. However, if a coughing or bucking patient is tracheally intubated, the glottis is stented open by the endotracheal tube, and the effects of a cough or buck on cerebral venous pressure will be decreased compared to those encountered in nonintubated patients.
During normal physiologic conditions, changes in CMRO 2 usually lead to parallel changes in CBF, a phenomenon known as CBF-CMRO 2 coupling . The volatile anesthetics, isoflurane, sevoflurane, and desflurane, particularly when administered in concentrations greater than 0.6 to 1.0 minimum alveolar concentration (MAC), are potent direct cerebral vasodilators that produce dose-dependent increases in CBF despite concomitant decreases in cerebral metabolic oxygen requirements. Below 1 MAC, volatile anesthetics alter CBF minimally, in part because any direct effects of the anesthetics are counterbalanced by CBF-CMRO 2 coupling. When volatile anesthetic–induced CMRO 2 depression is maximized, concomitant with maximal depression of cerebral electrical activity, larger dosages of volatile anesthetic will dilate cerebral blood vessels. This vascular dilation can lead to increases in CBF, CBV, and possibly ICP. With halothane, which at clinically relevant dosages does not induce the extent of CMRO 2 depression that is seen with other volatile anesthetics (isoflurane, sevoflurane, desflurane), direct vasodilatory effects predominate, which results in greater increases in CBV at equipotent doses compared with other commonly used volatile anesthetics. The effect of volatile anesthetics on CBF can be attenuated by hypocapnia or cerebral vasoconstrictive drugs, such as propofol.
Nitrous oxide also causes an increase in CBF, but, in contrast to volatile anesthetics, nitrous oxide does not appear to interfere with autoregulation. The initiation of nitrous oxide administration after closure of the dura or use of nitrous oxide in a patient with intracranial air, such as those who underwent recent craniotomy, should be with caution as nitrous oxide can diffuse into the gas space. This leads to an increase in the size and pressure of the air pocket. Clinically, tension pneumocephalus usually presents as delayed emergence from general anesthesia after craniotomy.
Like the volatile anesthetics, ketamine is considered to be a cerebral vasodilator. Propofol, barbiturates, and etomidate are potent cerebral vasoconstrictors capable of decreasing CBF, CBV, and ICP. Opioids are also cerebral vasoconstrictors, assuming that opioid-induced ventilatory depression is controlled and no increase in Paco 2 is allowed. Drugs that produce cerebral vasoconstriction predictably decrease CBV and ICP.
Administration of nondepolarizing neuromuscular blocking drugs does not meaningfully alter ICP. However, muscle relaxation may help prevent acute increases in ICP resulting from movement or coughing during direct laryngoscopy. The use of succinylcholine in the setting of increased ICP may temporarily raise ICP. The mechanism for this effect is most likely due to increases in muscle afferent activity, a process somewhat independent of visible muscle fasciculations. This can lead to cerebral arousal (which can be seen on electroencephalography [EEG]) and corresponding increases in CBF and CBV. These cerebral effects of succinylcholine can be attenuated by prior induction of deep anesthesia with a cerebral vasoconstricting anesthetic or a defasciculating dose of a nondepolarizing neuromuscular blocking drug.
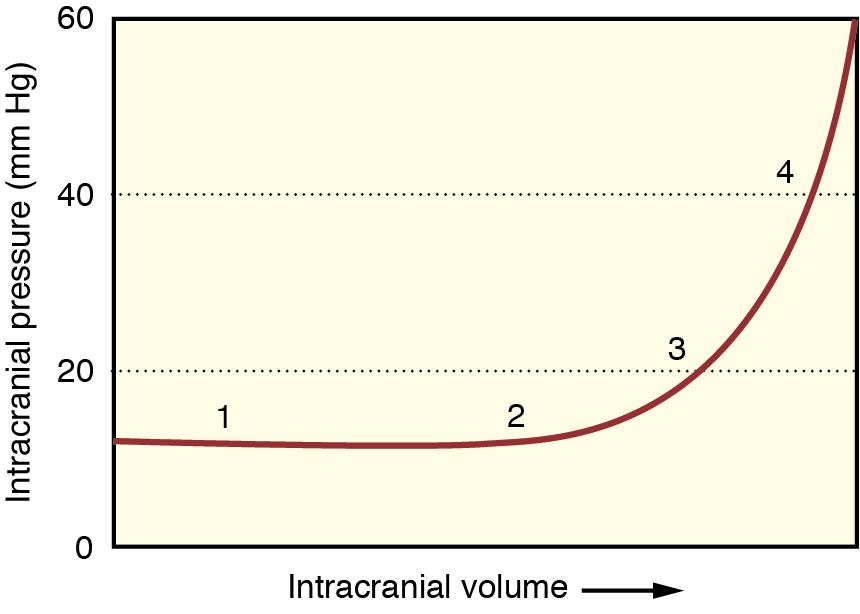
The intracranial and spinal vault contains neural tissue (brain and spinal cord), blood, and CSF, and is enclosed by the dura mater and bone. During normal conditions, brain tissue, intracranial CSF, and intracranial blood have a combined volume of approximately 1200 to 1500 mL, and normal ICP is usually 5 to 15 mm Hg (or 7–20 cm H 2 O). Any increase in one component of intracranial volume must be offset by a decrease in the volume of another intracranial component to prevent an increase in ICP. During normal physiologic conditions, changes in one component are well compensated for by changes in other components, but eventually a point can be reached at which even a small change in intracranial contents results in a large change in ICP ( Fig. 13.2 ), as described by the Monro-Kellie hypothesis. Since ICP is one of the determinants of CPP, homeostatic mechanisms work to increase MAP to help support CPP despite increases in ICP, but eventually compensatory mechanisms can fail, and cerebral ischemia will result.
Factors leading to alterations in CSF flow or its absorption into the vasculature can often lead to increased ICP. CSF is produced by two mechanisms: (1) ultrafiltration and secretion by the cells of the choroid plexus and (2) the passage of water, electrolytes, and other substances across the blood-brain barrier. CSF is therefore a direct extension of the extracellular fluid compartment of the central nervous system. CSF is produced at a constant rate of 500 to 600 mL/day in adults and is contained within the ventricular system of the brain, the central canal of the spinal cord, and the subarachnoid space, as well as the extracellular compartment of the central nervous system. CSF is absorbed from microscopic arachnoid villi and macroscopic arachnoid granulations within the dura mater and bordering venous sinusoids and sinuses, and at the blood-brain barrier.
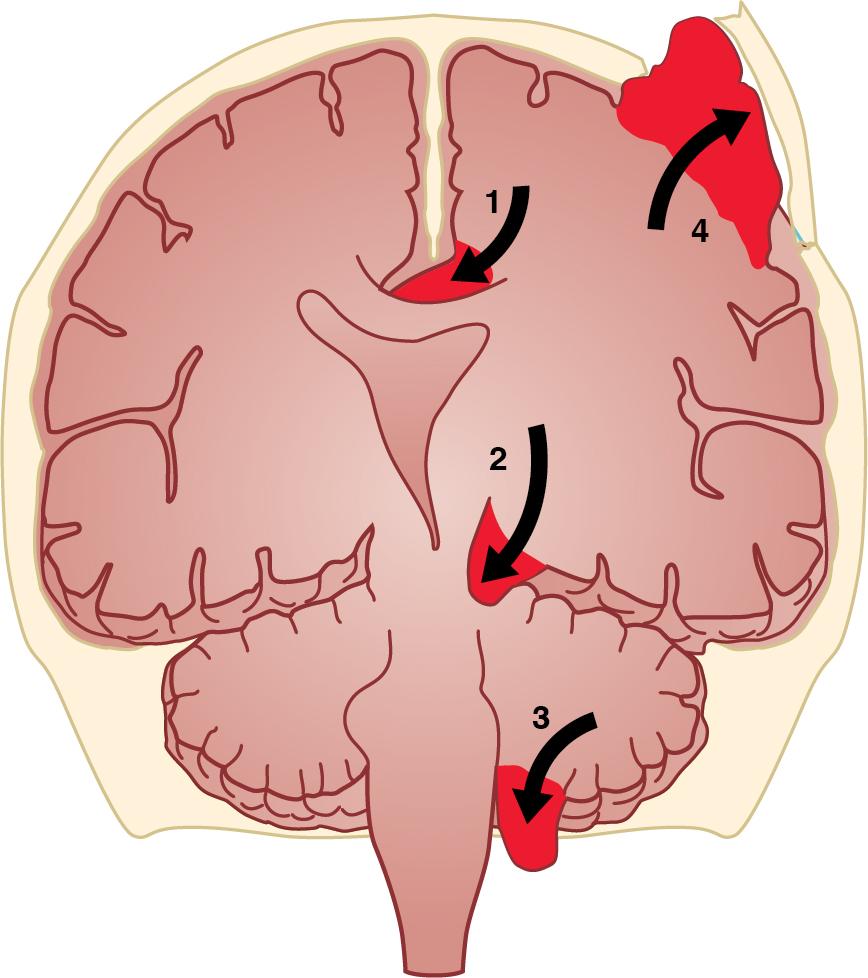
It is important to note that the intracranial vault is considered to be compartmentalized. Specifically, there are various meningeal barriers within the intracranial vault that functionally separate the contents: the falx cerebri (a reflection of dura mater that separates the two cerebral hemispheres) and the tentorium cerebelli (a reflection of dura mater that lies rostral to the cerebellum and marks the border between the supratentorial and infratentorial spaces). Increases in the contents of one region of brain may cause regional increases in ICP, and in extreme instances, the contents of that compartment can move (herniate) into a different compartment. Various types of herniation syndromes are categorized based on the region of brain affected ( Fig. 13.3 ). Herniation of cerebral hemispheric contents under the falx cerebri is referred to as subfalcine herniation. Typically, this condition leads to compression of branches of the anterior cerebral artery and is evident on radiographic imaging as midline shift. Herniation of the supratentorial contents past the tentorium cerebelli is referred to as transtentorial herniation, in which evidence of brainstem compression occurs in a rostral to caudal manner, resulting in altered consciousness, defects in gaze and afferent ocular reflexes, and, finally, hemodynamic and respiratory compromise followed by death. The uncus (i.e., the medial portion of the temporal lobe) may herniate over the tentorium cerebelli, which results in a subtype of transtentorial herniation referred to as uncal herniation. A specific sign is ipsilateral oculomotor nerve dysfunction because the oculomotor nerve is compressed against the brainstem; this results in pupillary dilatation, ptosis, and lateral deviation of the affected eye, which occurs before evidence of brainstem compression and death. Herniation of the cerebellar tonsils can occur in the setting of elevated infratentorial pressure, which leads to extension of these cerebellar structures through the foramen magnum. Typical signs are those indicating medullary dysfunction, including cardiorespiratory instability and subsequently death.
Nonspecific signs and symptoms of increased ICP include headache, nausea, vomiting, and papilledema. As ICP further increases and cerebral perfusion becomes limited, decreased levels of consciousness and possibly coma can be observed.
Increased ICP is often diagnosed clinically based on the symptoms described earlier, by radiographic means, and by direct measurement of ICP. Typically, computed tomography (CT) or magnetic resonance imaging (MRI) will help identify the cause of an increase in ICP. For example, a large mass or hematoma may be evident. If aqueductal stenosis is present, the third, but not fourth, ventricle is enlarged.
Several methods are currently available to measure and monitor ICP. The choice of technique depends on the clinical situation. The gold standard technique is the ventriculostomy that allows not only for ICP measurement but also the removal of CSF for analysis or as a treatment of intracranial hypertension. A catheter can also be placed in the CSF in the lumbar region of the spine to monitor ICP and to remove CSF. However, because of the compartmentalization of the intracranial contents, lumbar CSF pressure may not accurately reflect ICP in all circumstances. In certain clinical settings, such as a brain tumor, there is also a risk of herniation of the cerebellar tonsils when CSF is drained using the lumbar subarachnoid approach. ICP can also be measured via transducers placed in the epidural or subdural spaces as well as in brain parenchyma, but these latter techniques do not allow for CSF removal. Other techniques, such a measurement of optic nerve sheath diameter via ultrasonography, may provide a noninvasive way to assess ICP.
A normal ICP waveform is pulsatile and varies with the cardiac impulse and spontaneous breathing. An ICP remaining below 15 mm Hg is normal. In patients with increased intracranial elastance, not only may ICP be greater than 15 mm Hg, but abnormal waveforms may also appear. There are three types of Lundberg waves that may appear on an ICP waveform tracing. Lundberg A waves (or “plateau waves”) are abrupt increases in ICP from 20 to 100 mm Hg that can last for up to 20 minutes. Lundberg A waves occur in the setting of increased ICP with impaired oxygen and substrate delivery that results in abrupt vasodilation and an increase in ICP . During these dramatic increases in ICP, patients may become symptomatic and manifest evidence of inadequate cerebral perfusion. Spontaneous hyperventilation or changes in mental status may occur. Lundberg A waves are related to poor outcome. Lundberg B waves are sharp, brief spikes in ICP to 20 to 50 mm Hg occurring approximately every 0.5 to 2 minutes. They also indicate increased intracranial elastance but not to the degree where one may observe Lundberg A waves. Lundberg C waves are rhythmic, very short-duration spikes in ICP up to 20 mm Hg of unknown etiology. Lundberg C waves are considered benign.
Methods to decrease ICP include elevation of the head; hyperventilation; CSF drainage; administration of hyperosmotic drugs, diuretics, corticosteroids, cerebral vasoconstricting anesthetics (such as propofol), and surgical decompression. It is not possible to reliably identify the level of ICP that will interfere with regional CBF or alter cerebral function and well-being in individual patients unless other monitors are employed. These monitors include, but are not limited to, cerebral near-infrared spectroscopy, cerebral microdialysis, and brain tissue oxygen partial pressure monitoring. Improvements in neurologic status, such as improved level of consciousness that occurs with reduction in ICP, can serve as a crude monitor of cerebral well-being. In the absence of these additional monitors or a reliable neurologic exam, a frequent recommendation is to treat any sustained increase in ICP that exceeds 20 mm Hg. Treatment may be indicated even when the ICP is less than 20 mm Hg if the appearance of occasional plateau waves suggests the presence of increased intracranial elastance.
Elevating the patient’s head above heart level encourages venous outflow from the brain and can lower ICP. Extreme flexion or rotation of the head can obstruct the jugular veins and restrict venous outflow from the brain. The head-down position can increase ICP.
Hyperventilation, and hence lowering of the Paco 2 , is an effective method for rapidly reducing ICP. In adults a frequent recommendation is to maintain the Paco 2 near 30 to 35 mm Hg. Lowering the Paco 2 more than this may not meaningfully decrease ICP further but may result in adverse changes in systemic physiology. The effects of hyperventilation will diminish after 6 hours. When prolonged hyperventilation is discontinued, rebound increases in ICP are a potential problem, especially if normocapnia is rapidly restored.
Intravenous infusion of drugs and fluids, such as mannitol and hypertonic saline, are effective at decreasing ICP. These osmotic diuretics produce transient increases in the osmolarity of plasma, which acts to draw water across the blood-brain barrier, thus decreasing the water content of the brain and decrease ICP. An adverse effect from osmotic diuretics is diuresis that can lead to systemic dehydration and subsequent impaired cerebral and other end-organ perfusion, if not rectified. In regions of brain where the integrity of the blood-brain barrier is compromised, osmotic diuretics can enter the brain parenchyma, causing water to follow, and paradoxically increase brain water volume and ICP. The brain eventually adapts to sustained increases in plasma osmolarity, so long-term use of hyperosmotic drugs results in reduced effectiveness.
Mannitol is ideally administered in doses of 0.25 to 0.5 g/kg IV. Larger initial doses have little incremental effect on ICP and may predispose the patient to rebound increases in ICP. Hence it is better to give an initial dose of 0.25 to 0.5 g/kg IV and, if the desired effect is not achieved, either administer another dose or switch to another type of therapy. Also, no further mannitol should be administered if serum osmolarity is greater than 320 mOsm/L. Decreases in ICP occur within 30 minutes with maximum effect observed in 1 to 2 hours and a duration of 6 hours. Mannitol can initially increase intravascular fluid volume and should be used with caution, if at all, in patients who are not tolerant of increases in cardiac preload, such as those with congestive heart failure. Mannitol also has direct vasodilating properties and can produce brief hypotension if administered quickly. Hypertonic saline is an alternate option to decrease ICP and can be administered in concentrations of 2% to 23%. Hypertonic saline at a concentration of 3% or more should be administered via a central venous catheter as extravasation can lead to local tissue irritation. For adult patients, administration of 1 to 2 mL/kg of 3% sodium chloride over 5 minutes can be considered. Additional drug can be administered to obtain a target serum sodium concentration of 145 to 155 mEq/L and a serum osmolarity of less than 320 mOsm/L if the initial dose fails to reduce ICP. Serum sodium concentrations greater than 160 mEq/L can lead to renal injury, pulmonary edema, cardiac dysfunction, and seizures. As such, serum sodium should be checked frequently until target serum sodium and osmolarity are obtained and then at least every 6 hours for the duration of the infusion.
Loop diuretics, such as furosemide, have been used to decrease ICP, although their efficacy is significantly less than that of mannitol or hypertonic saline. Since loop diuretics exert their effect on brain water volume by inducing systemic hypovolemia, their use to treat intracranial hypertension is falling out of favor.
Corticosteroids, such as dexamethasone or methylprednisolone, are effective in lowering ICP caused by the development of localized vasogenic cerebral edema from loss of blood-brain barrier integrity. This is due in part to a steroid-induced upregulation of the expression of proteins responsible for the integrity of the tight junctions between endothelial cells constituting a major component of the blood-brain barrier. Patients with brain tumors often exhibit improved neurologic status and disappearance of headache within 12 to 36 hours after initiation of corticosteroid therapy. Corticosteroids are also effective in treating increased ICP in patients with pseudotumor cerebri (benign intracranial hypertension). On the other hand, corticosteroids are not effective in reducing ICP due to nonvasogenic edema–related causes. Corticosteroids can increase blood glucose concentration, which may adversely affect outcome if ongoing cerebral ischemia is present. Corticosteroids are associated with poor outcome when used to treat intracranial hypertension following traumatic brain injury.
Propofol is effective in treating increased ICP by inducing a decrease in both CMRO 2 and CBF. Patients receiving prolonged propofol infusions, particularly pediatric patients, should be monitored for drug-associated high-anion-gap metabolic acidosis (aka propofol infusion syndrome), which can also herald multiorgan dysfunction or injury and can be fatal.
Increased ICP is typically a sign of an underlying intracranial pathologic process. Therefore one should seek the cause of increased ICP in addition to instituting treatment. Causes of increased ICP are many. Tumors can lead to increased ICP either (1) directly because of their size, (2) indirectly by causing edema in normal surrounding brain tissue, or (3) by causing obstruction of CSF flow, as is commonly seen with tumors involving the third ventricle. Intracranial hematomas can cause increased ICP in a manner similar to mass lesions. Blood in the CSF, as is seen in subarachnoid hemorrhage, may lead to obstruction of CSF reabsorption at the arachnoid villi and granulations and may further exacerbate increased ICP. Infection, such as meningitis or encephalitis, can lead to edema or obstruction of CSF reabsorption. Some causes of intracranial hypertension not discussed elsewhere in this chapter are described in the following sections.
Stenosis of major CSF flow channels may impede CSF flow and can lead to increased ICP. Aqueductal stenosis, one of the more common causes of obstructive hydrocephalus, results from congenital narrowing of the cerebral aqueduct that connects the third and fourth ventricles. Obstructive hydrocephalus can present during infancy when the narrowing is severe. Lesser obstruction results in slowly progressive hydrocephalus, which may not be evident until adulthood. Diagnosis is made by brain imaging, and treatment includes ventricular shunting of endoscopic fenestration of the floor of the third ventricle allowing CSF to flow into the basal cisterna avoiding the need to flow through the cerebral aqueduct.
Benign intracranial hypertension (pseudotumor cerebri) is a syndrome characterized by ICP higher than 20 mm Hg, normal CSF composition, normal sensorium, and absence of local intracranial lesions. This disorder typically occurs in obese women and is observed more commonly in patients with various systemic diseases, including polycystic ovary syndrome, systemic lupus erythematosus, Addison disease, hypoparathyroidism, and hypervitaminosis A. Imaging indicates a normal or even small cerebral ventricular system. Headaches and bilateral visual disturbances typically occur, and symptoms may be exaggerated during pregnancy. No identifiable cause of increased ICP is found in most patients, and the pathogenesis is still unknown. The prognosis is usually excellent. Treatment includes acetazolamide to decrease CSF formation, removal of CSF from the lumbar intrathecal space, ventricular shunting of CSF, or optic nerve fenestration. Anesthesia management for patients without shunts involves avoiding exacerbation of intracranial hypertension and ensuring an adequate CPP. Hypoxia and hypercarbia must be rigorously avoided. Spinal anesthesia may be used in select parturients with mildly increased ICP so long as the patient has no significant neurologic deficits or alterations in consciousness. In patients with elevated ICP, epidural analgesia should be avoided in most cases as the volume of drug required may exacerbate elevated ICP. In the presence of a lumboperitoneal shunt there is a theoretical possibility that local anesthetic solution injected into the subarachnoid space could escape into the peritoneal cavity, decreasing anesthesia density. In parturients with a functioning ventriculoperitonal shunt, spinal and epidural analgesia and anesthesia can be used safely as long as the shunt is working correctly. Occasionally, the intraperitoneal drainage catheter can become compressed by the gravis uterus resulting in inadequate shunting and elevated ICP causing signs and symptoms such as headaches, visual changes, papilledema, or alterations in consciousness.
Normal pressure hydrocephalus usually presents as the triad of dementia, gait changes, and urinary incontinence that develops over a period of weeks to months. The mechanism is thought to be related to compensated but impaired CSF absorption from a previous insult, such as subarachnoid hemorrhage, meningitis, or head trauma. In most cases, however, the cause is never identified. Lumbar puncture usually reveals normal or low CSF pressure, yet CT or MRI will often demonstrate large ventricles. Treatment typically involves drainage of CSF via ventriculoperitoneal shunting.
Patients with spontaneous intracranial hypotension (SIH) often present with an abrupt-onset orthostatic headache when assuming the upright position. Symptoms frequently increase during the second half of the day and may be associated with tinnitus, muffled hearing, or other cranial nerve deficits. The headache is relieved by assuming a horizontal position. SIH is caused by leaking of CSF from the spine though meningeal diverticula, dural tears, or CSF-venous fistulas. MRI of the brain can be normal, but findings suggestive of SIH include meningeal enhancement, engorgement of venous sinuses, or herniation of the cerebellar tonsil though the foramen magnum. A CSF opening pressure of less than 6 cm H 2 O may or may not be present in patients with SIH as symptoms are thought to be related to low CSF volume rather than low CSF pressure. CT myelography is the gold standard to identify spinal CSF leaks. Dynamic imaging studies using CT or fluoroscopy can also be used with or without digital subtraction, which can be helpful in localizing leaks. Treatment of SIH can include supportive measures, epidural blood patch, and surgery. Surgical procedures to repair the site of leak can be performed. Of note, patients may experience rebound intracranial hypertension following surgical repair and may require therapeutic lumbar puncture.
Intracranial tumors may be classified as primary (those arising from the brain and its coverings) or metastatic. Primary brain tumors, also called gliomas, can originate from virtually any cell type within the central nervous system. The classification of gliomas depends on histologic cell type, but these tumors are frequently subclassified based on specific oncogenic mutations that can influence choice of treatment and prognosis. Supratentorial tumors are more common in adults and often present with headache, seizures, or new neurologic deficits, whereas infratentorial tumors are more common in children and often present with obstructive hydrocephalus and ataxia.
Treatment may consist of surgical resection or debulking, chemotherapy, or radiation. Gamma knife irradiation differs from traditional radiation therapy in that multiple radiation sources are used, and because the tumor is addressed from multiple angles, radiation to the tumor can be maximized while the radiation dose to any single area of surrounding brain tissue can be diminished. Such treatment can also be accomplished with the use of x-ray and particle-based modalities such as proton beam. Emerging therapies include immunotherapy and oncolytic virotherapy, the latter employing viruses specifically programmed to kill neoplastic cells.
Astrocytes are the most prevalent glial cells in the central nervous system and give rise to many types of infratentorial and supratentorial tumors. Well-differentiated (low-grade) gliomas are the least aggressive class of astrocyte-derived tumors. They often are found in young adults and generally present as new-onset seizures. Imaging generally shows minimal enhancement with contrast. Surgical or radiation treatment of low-grade gliomas usually results in symptom-free long-term survival.
Pilocytic astrocytomas usually affect children and young adults. They often arise in the cerebellum (cerebellar astrocytoma), cerebral hemispheres, hypothalamus, or optic pathways (optic glioma). The tumor usually appears as a contrast-enhancing, well-demarcated lesion with minimal to no surrounding edema. Because of its benign pathologic characteristics, prognosis following surgical resection is generally very good. However, the location of the lesion, such as within the brainstem, may preclude resection.
Anaplastic astrocytomas are poorly differentiated, usually appear as a contrast-enhancing lesion on imaging because of disruption of the blood-brain barrier, and generally evolve into glioblastoma multiforme. Treatment involves resection, radiation, or chemotherapy. Prognosis is intermediate between that for low-grade gliomas and glioblastoma multiforme.
Glioblastoma multiforme accounts for 30% of all primary brain tumors in adults. Imaging usually reveals a ring-enhancing lesion reflecting central necrosis and surrounding edema. Because of microscopic infiltration of normal brain by tumor cells, resection alone is usually inadequate. Instead, treatment generally consists of surgical debulking combined with chemotherapy and radiation and is aimed at palliation, not cure. Despite treatment, life expectancy may be measured in weeks.
Oligodendrogliomas arise from myelin-producing cells within the central nervous system and account for only 6% of primary intracranial tumors. Classically, seizures predate the appearance of tumor on imaging, often by many years. Calcifications within the tumor are common and are visualized on CT imaging. The tumor usually consists of a mixture of both oligodendrocytic and astrocytic cells. Treatment and prognosis depend on the pathologic features. Initial treatment involves resection, since early in the course the tumor typically consists of primarily oligodendrocytic cells, which are radioresistant.
Arising from cells lining the ventricles and central canal of the spinal cord, ependymomas commonly present in childhood and young adulthood. Their most common location is the floor of the fourth ventricle. Symptoms include obstructive hydrocephalus, headache, nausea, vomiting, and ataxia. Treatment consists of resection and radiation. Tumor infiltration into surrounding tissues may preclude complete resection. Prognosis depends on the completeness of resection.
Primitive neuroectodermal tumor represents a diverse class of tumors, including retinoblastoma, medulloblastoma, pineoblastoma, and neuroblastoma, all believed to arise from primitive neuroectodermal cells. Medulloblastoma is the most common pediatric primary malignant brain tumor and may disseminate throughout the central nervous system via the CSF. The presentation of medulloblastoma is similar to that of ependymoma. Treatment usually involves a combination of resection, radiation, and possibly intrathecal instillation of chemotherapeutic drugs. Prognosis is very good in children if treatment leads to disappearance of both tumor on MRI and tumor cells within the CSF. Prognosis is less optimistic if there is evidence of tumor dissemination within the central nervous system.
Meningiomas are usually extraaxial (arising outside of the brain proper), slow-growing, well-circumscribed benign tumors arising from arachnoid cap cells, not the dura mater. Because of their slow growth, they can be very large at the time of diagnosis. They can occur anywhere arachnoid cap cells exist, but are most common near the sagittal sinus, falx cerebri, and cerebral convexity. Tumors are usually apparent on plain radiographs and CT scans as a result of the presence of calcifications. On MRI and conventional angiography, these tumors are often seen to receive their blood supply from the external carotid artery. Surgical resection is the mainstay of treatment. Prognosis is usually excellent. However, some tumors may be recurrent and require additional resection. Malignant meningiomas are rare.
Pituitary adenomas usually arise from cells of the anterior pituitary gland. They may occur along with tumors of the parathyroid glands and pancreatic islet cells as part of multiple endocrine neoplasia type I. These tumors are usually divided into functional (i.e., hormone-secreting) and nonfunctional types. The former usually present as an endocrinologic disturbance related to the hormone secreted by the tumor. Functional tumors are usually smaller (<1 cm in diameter) at the time of diagnosis; hence they are often called microadenomas. Macroadenomas are usually nonfunctional, present with symptoms related to their mass (i.e., headache or visual changes resulting from compression of the optic chiasm), and are larger at the time of diagnosis, usually greater than 1 cm in diameter. Panhypopituitarism may be caused by either tumor type because of compression of normally functioning pituitary gland tissue. Pituitary tumors may also present as pituitary apoplexy, which is characterized by the abrupt onset of headache, visual changes, ophthalmoplegia, and altered mental status secondary to hemorrhage, necrosis, or infarction within the tumor. These tumors can also invade the cavernous sinus or internal carotid artery or compress various cranial nerves, causing an array of symptoms. Treatment depends on tumor type. Prolactinomas are often initially treated medically with bromocriptine. Surgical resection via the transsphenoidal approach or open craniotomy can be curative for most pituitary tumors.
Corticosteroids, such as dexamethasone for nausea and vomiting prophylaxis, should not be administered during pituitary tumor resection. Dexamethasone is a potent suppressor of the hypothalamic-pituitary-adrenal axis. Often, serum cortisol is assessed on the day following surgery to screen for postoperative hypopituitarism, and dexamethasone use may result in a false diagnosis of hypopituitarism.
The term acoustic neuroma is a misnomer as the tumor is usually a benign schwannoma involving the vestibular (not auditory) component of cranial nerve VIII within the internal auditory canal. However, bilateral tumors may occur as part of neurofibromatosis type 2. Common presenting symptoms include hearing loss, tinnitus, and disequilibrium. Larger tumors, which grow out of the internal auditory canal and into the cerebellopontine angle, may cause symptoms related to compression of a cranial nerve, especially the facial nerve, or compression of the brainstem. Treatment usually consists of surgical resection with or without radiation therapy. Surgery generally involves intraoperative cranial nerve monitoring with electromyography or brainstem auditory evoked potentials as resection carries a high risk for cranial nerve injury. Prognosis is usually very good; however, recurrence of tumor is not uncommon.
Central nervous system lymphoma is a rare tumor that can arise as a primary brain tumor or via metastatic spread from a systemic lymphoma. Primary central nervous system lymphoma can occur anywhere within the brain but is most common in supratentorial locations. Immunocompromised patients are at increased risk for central nervous system lymphoma as are those with autoimmune diseases. Diagnosis is made by imaging as well as biopsy. During biopsy, it may be reasonable to wait to administer corticosteroids, such as dexamethasone, until after pathologic specimens have been obtained since these tumors may be very sensitive to steroids. Indeed, steroid-associated tumor lysis before a biopsy is performed may result in failure to obtain an adequate sample to make the diagnosis. The mainstay of treatment is chemotherapy (including intraventricularly delivered drugs) and whole-brain radiation. Prognosis is poor despite treatment.
Metastatic brain tumors originate most often from primary sites in the lung or breast. Malignant melanoma, renal cell cancer, and carcinoma of the colon are also likely to spread to the brain. Metastatic brain tumor is the likely diagnosis when more than one intracranial lesion is present. Because of abnormal angiogenesis in metastatic lesions, these tumors tend to bleed more during resection than other central nervous system tumors.
Management of anesthesia during tumor resection procedures can be challenging since patients may be of any age and a variety of operative positioning issues may arise. Furthermore, some procedures may be conducted with electrophysiologic monitoring, which may have implications for anesthetic drug choices and the use of muscle relaxants. Some procedures may even be performed in awake patients to facilitate resection of a mass located near an eloquent region of brain, such as the motor cortex. Major goals during anesthesia include (1) maintaining adequate cerebral perfusion and oxygenation of normal brain, (2) optimizing operative conditions to facilitate resection, (3) ensuring a rapid emergence from anesthesia at the conclusion of the procedure to facilitate neurologic assessment, and (4) accommodating intraoperative electrophysiologic monitoring if needed.
Preoperative evaluation of a patient with an intracranial tumor is directed toward identifying the presence of increased ICP, documenting preoperative neurologic deficits, and optimizing other systemic diseases. Patients with an intracranial pathologic process may be extremely sensitive to the central nervous system depressant effects of opioids and sedatives. Drug-induced hypoventilation can lead to accumulation of arterial carbon dioxide and further increase ICP. Likewise, drug-induced sedation can mask alterations in the level of consciousness that accompany intracranial hypertension. Preoperative sedation can also unmask subtle neurologic deficits that may not usually be apparent. This is thought to result from an increased sensitivity of injured neurons to the depressant effects of various anesthetic and sedative drugs. Considering all the potential adverse effects of preoperative medication, it is prudent to use premedication very sparingly and with continuous respiratory and neurologic monitoring.
Anesthesia induction is typically achieved with propofol as it produces a rapid, reliable onset of unconsciousness without increasing ICP. A nondepolarizing muscle relaxant can be used to facilitate tracheal intubation. Administration of succinylcholine may be associated with a modest transient increase in ICP and may be considered if otherwise indicated. Succinylcholine should be avoided in patients with preexisting significant motor deficits due to concern for hyperkalemia. Mechanical hyperventilation is initiated with the goal of decreasing Paco 2 to approximately 35 mm Hg. Adequate depth of anesthesia and profound skeletal muscle paralysis should be achieved before laryngoscopy to avoid the noxious stimulation or patient movement that can abruptly increase CBF, CBV, and ICP. Additional doses of intravenous anesthetic drugs, lidocaine, esmolol, or potent short-acting opioids may help blunt the response to laryngoscopy or other forms of intraoperative stimulation, such as placement of pinions or skin incision.
Abrupt, sustained increases in systemic blood pressure, particularly in areas of impaired cerebral autoregulation, may be accompanied by undesirable increases in CBF, CBV, and ICP and precipitate cerebral edema. Sustained hypotension must also be avoided to prevent brain ischemia. Positive end-expiratory pressure has a highly variable effect on ICP. Hence it should be used with caution, and attention must be paid to changes in ICP, MAP, and CPP as a result of this intervention. The efficacy of brain volume management can be assessed after craniotomy by direct visualization and communication with the surgeon.
Maintenance of anesthesia in patients undergoing surgical resection of supratentorial brain tumors is often achieved by combining drugs of various classes, including nitrous oxide, volatile anesthetics, opioids, dexmedetomidine, and propofol. Both nitrous oxide and potent volatile anesthetics have the potential to increase CBV and ICP as a result of direct cerebral vasodilation. However, low concentrations of volatile anesthetics (0.6–1.0 MAC) likely have minimal effect on cerebral volume. In patients with significantly elevated ICP, a cerebral vasoconstricting anesthetic, such as one that employs propofol as the hypnotic instead of inhaled drugs, can be used. Although modest cerebrovascular differences can be demonstrated with different combinations of drugs, there is no evidence that any particular combination is significantly different from another or superior in terms of effects on ICP and short-term patient outcome.
The use of nitrous oxide is controversial if there is any potential for venous air embolism, such as during procedures performed in the sitting position. Despite theoretical concerns, however, the actual incidence of venous air embolism in sitting patients is not influenced by nitrous oxide use. Once a venous air embolism has been detected, nitrous oxide use must be discontinued because of the concern that the embolus volume will expand and exacerbate the physiologic consequences of the embolus. Nitrous oxide should also be avoided if there is concern for preexisting air within the central nervous system, as may occur after prior craniotomy, spine surgery involving durotomy, basilar skull fracture, or percutaneous instrumentation (e.g., insertion of a ventricular shunt, pneumoencephalography), as nitrous oxide has the potential to expand these gas spaces leading in increased ICP or exacerbating preexisting intracranial hypertension.
Spontaneous movement by patients undergoing surgical resection of brain tumors must be prevented. Such movement could result in an increase in intracranial volume and ICP, increased surgical bleeding (making surgical exposure difficult), or direct injury to the head and brain from pinions or surgical instrumentation. Therefore, in addition to adequate depth of anesthesia, skeletal muscle paralysis is typically maintained during intracranial surgery.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here