Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
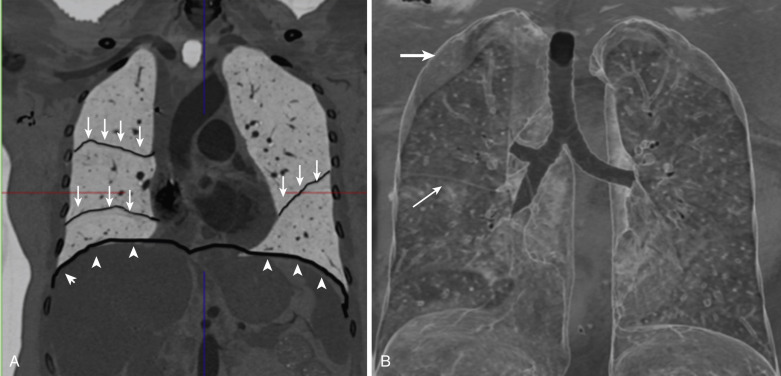
The pleura is a thin, two-layered serous membrane that lines the lungs and thoracic cavity. The visceral pleura lines the lungs and invaginates into a double layer to form the major and minor fissures, and the parietal pleura lines the chest wall, mediastinum, and diaphragmatic surface (sparing the hila). The pleural space is a potential space between the parietal and visceral pleurae. Under physiologic conditions, the pleural space of each hemithorax contains approximately 8 mL of acellular, clear fluid that lubricates and thus facilitates movement of the lungs in respiration. Normally, there is no communication between the pleural spaces of the right and left hemithoraces. The visceral and parietal pleura have different nodal drainage patterns in malignant states. External to the parietal pleura is a variable amount of extrapleural fat that separates the visceral pleura and the endothoracic fascia that covers the surface of the intercostal muscles and ribs ( Fig. 39-1 ).
The diaphragm is a complex muscle that separates the thorax from the abdomen. It is the primary muscle of respiration. The four major embryologic structures that develop into the diaphragm are the paired pleuroperitoneal folds, the esophageal mesentery, the transverse septum, and the muscular body wall. The diaphragm attaches to numerous sites on the body wall. The two crura are attached posteriorly to the upper lumbar spine and are connected by the median arcuate ligament. Anteriorly and laterally, the diaphragm is attached to the sternum, xiphoid process, lower six ribs, and costal cartilages. The three openings in the diaphragm that allow passage of structures from thorax to abdomen include the inferior vena caval hiatus at the level of T8, the esophageal hiatus at T10 that contains the esophagus and vagus nerve, and the aortic hiatus at T12, which contains the aorta, thoracic duct, and azygos and hemiazygous veins.
In general, when thoracic or pleural disease is suspected, chest radiography is the initial imaging study of choice. In normal patients, the pleura is seen on chest radiographs only where the visceral pleura has infolded to produce a double layer at the major or oblique fissures of both hemithoraces and the minor or horizontal fissure of the right hemithorax. Accessory fissures are variably present, the most common of which are the azygos fissure of the right upper lobe, the inferior accessory fissure of either lower lobe, the superior accessory fissure of either lower lobe, and the left minor fissure. In cases of pneumothorax, a thin “visceral pleural line” demarcates the superior or lateral edge of the collapsed lung. Fissures are visible only where they are tangential to the x-ray beam, and they may be “incomplete” in normal variants where they do not extend all the way to the hilum or chest wall.
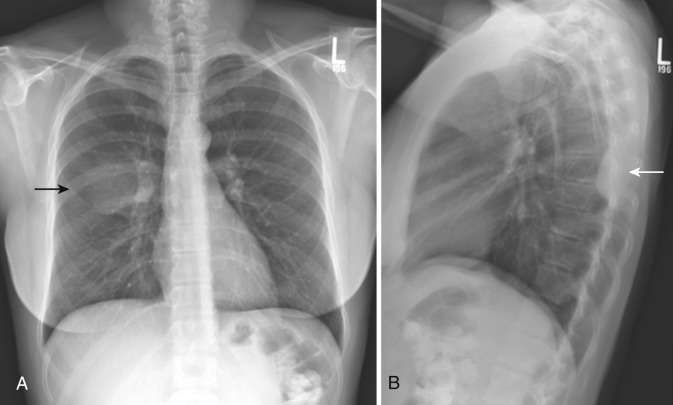
The presence of the incomplete border sign is a clue that a mass is extrapulmonary in the pleura or chest wall. The inner border of the radiopaque mass is tangential to the x-ray beam and is well defined by the adjacent lucent lung. The outer margin is ill defined because it is seen en face or partially en face ( Fig. 39-2 ).
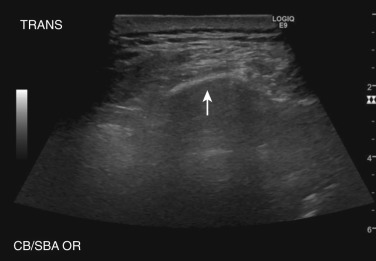
Ultrasound is frequently used to distinguish pleural thickening from effusion, characterize and quantify effusion, and guide procedures such as thoracentesis. As in other applications, ultrasound is inexpensive and portable, making it useful in a wide variety of venues including outpatient, inpatient, procedural, and critical care settings. The normal visceral pleura is seen as a bright specular reflector between the chest wall and the air-filled lung. Horizontal repetitions of the pleural line, or reverberation artifacts called A-lines, are seen deep to the visceral pleural line. Small inhomogeneities in the pleura produce “comet tails” or moving reverberation artifacts that extend deep from the pleural surface during normal respiratory motion. Lung sliding, or a shimmering movement at the pleural stripe–chest wall interface, is seen during normal respiratory movement and disappears along with comet tails in cases of pneumothorax.
Like the liver, the lung has a dual arterial supply and thus can be imaged using contrast-enhanced sonography (CES). Processes that have a pulmonary arterial vascular supply show short time to enhancement and marked parenchymal enhancement (e.g., pneumonia), whereas lesions supplied by the bronchial arteries (e.g., malignancy) show delayed time to enhancement and reduced enhancement during the arterial and parenchymal phases. In pulmonary embolism, there is decreased pulmonary arterial vascularity.
In imaging the chest wall and pleura, higher-frequency linear array transducers (e.g., 7.5 MHz) provide the greatest spatial resolution; however, in larger patients or in patients with large-volume pleural disease, lower-frequency transducers are able to provide greater penetration. Variable-frequency 3.5- to 5-MHz sector transducers with small footprints are most versatile because they fit between the ribs and may be used to guide interventional procedures while still providing excellent image quality ( Fig. 39-3 ).
Following chest radiography findings that raise suspicion for pleural disease requiring further evaluation, computed tomography (CT) is usually the study choice owing to its wide availability, relatively low cost, efficiency, and ability to image the upper abdomen and lung parenchyma. Spatial resolution of CT is superior to that of magnetic resonance imaging (MRI). Unlike ultrasound and MRI, CT exposes the patient to ionizing radiation, which may be a relative drawback in young patients, especially those who are expected to incur high cumulative radiation doses from serial imaging.
Multidetector CT (MDCT) replaces the linear array of detector elements in a conventional CT scanner with a two-dimensional (2D) array, allowing acquisition of data from multiple sections simultaneously; this faster acquisition decreases respiratory motion artifact and improves image quality.
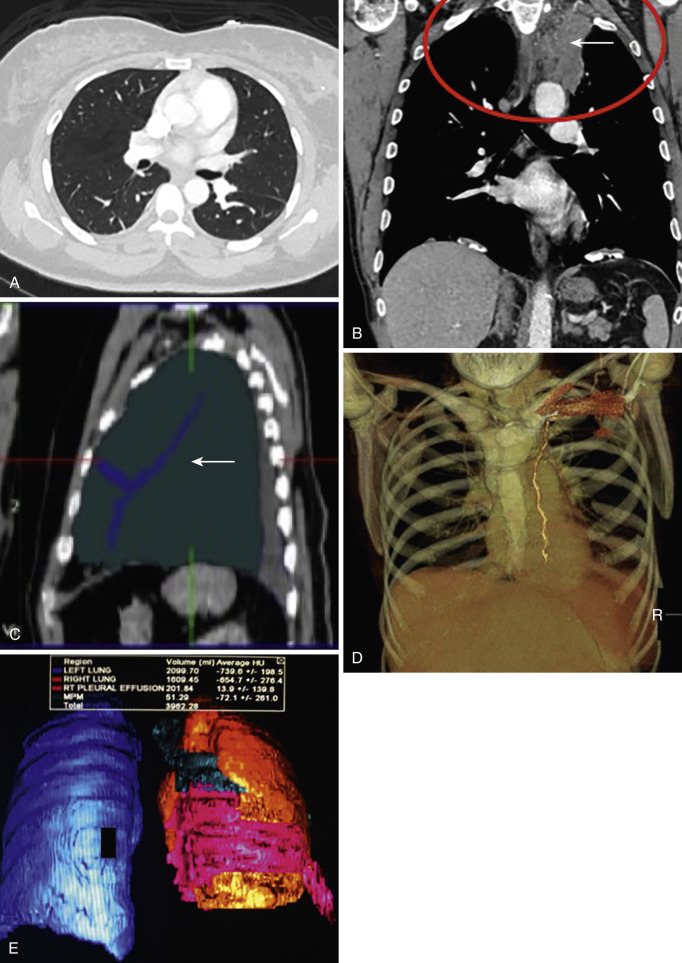
In evaluation of pleural pathology, coronal and sagittal reformations from thin-section (3 mm) axial images provide valuable information. MDCT acquisitions allow for application of advanced three-dimensional (3D) postprocessing techniques, which may be valuable for applications such as assessment of violation of critical structures by tumor, volumetric tumor quantification using serial segmentation, and surgical planning. Maximum intensity projection (MIP), which displays the highest-attenuation voxels in a given selection, are helpful in evaluating high-density structures such as contrast-enhanced vessels and stents, whereas minimum intensity projections (MiniP) that display the lowest-attenuation voxels are useful for assessing air-filled structures such as the lung parenchyma and airways ( Fig. 39-4 ).
Intravenous (IV) contrast is vital in assessing pleural pathology other than pleural plaques and pneumothoraces. For example, without IV contrast, it is difficult to distinguish pleural malignancy or pleura thickened by empyema from adjacent consolidated lung or pleural effusion. Imaging during the late venous or tissue parenchymal phase (20-60 seconds after peripheral venous injection) is ideal. Dynamic contrast-enhanced (DCE; perfusion) CT to assess blood flow dynamics and capillary permeability may be useful in some oncologic evaluations.
Mediastinal window settings are optimal for evaluation for pleural and chest wall disease. It is important to evaluate the images in lung windows to assess for associated pulmonary pathology, and bone windows are essential for detecting rib destruction. A sharp convolution kernel reconstruction algorithm should be used for lung and bone evaluation. High-resolution CT for evaluation of the lung parenchyma in the context of asbestos-related pleural disease has been largely replaced by MDCT.
MRI provides superior contrast resolution to CT and may be used for more refined characterization of tissues. It is the modality of choice for evaluating invasion of the chest wall, diaphragm, heart, vessels, airways, and other critical structures by tumor or infection. MRI is not useful to evaluate the air-filled lung parenchyma, and acquisition times are substantially longer than those for CT. Medically unstable patients or those with implanted devices such as pacemakers may not be able to undergo MRI. Unlike CT and positron emission tomography (PET), MRI does not expose the patient to ionizing radiation, and MRI may be useful in patients with allergies to iodinated contrast. In the thorax, limitations of MRI include respiratory and cardiac motion artifacts and susceptibility artifact from gas-containing structures, though many tools that mitigate these factors (e.g., cardiac gating) are available.
Large field-of-view scout images are obtained using a body coil, and specialized coils are selected for further imaging. Pulse sequences, imaging planes, and parameters such as slice thickness and interslice gap are specified according to the anatomic range being imaged and the study objectives. T1-weighted images are known for their excellent anatomic resolution and ability to resolve between the pleural space and extrapleural fat. T2-weighted images allow differentiation of pleural thickening and nodularity from pleural fluid and help differentiate tumor from adjacent muscle. Post-gadolinium T1-weighted images with fat saturation increase tissue contrast and are often helpful in identifying the borders between lesions and normal structures. Short tau inversion recovery (STIR) images are sensitive for bone involvement by tumor or infection, denoted by fluid signal in place of normal dark to intermediate marrow signal and breach of the normal dark cortical line.
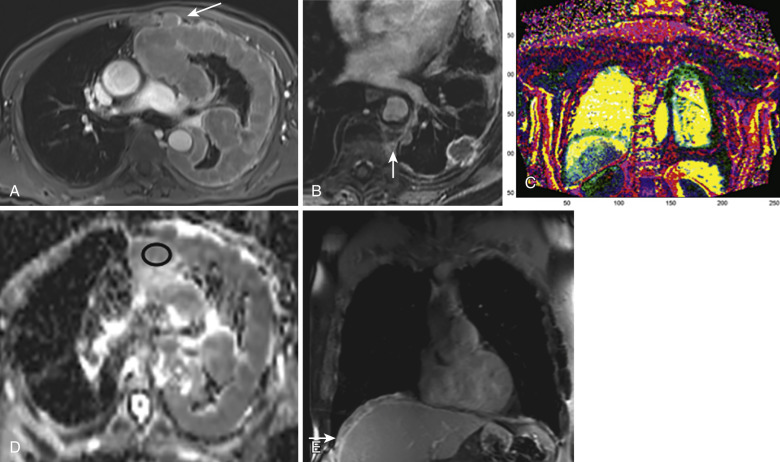
Parallel MRI enables assessment of tumor mobility and local lung motion. This, and sequences such as steady-state free precession (SSFP) that allow for cine imaging with high temporal resolution, may reveal subtle invasion of mediastinal structures and the chest wall. Additional sequences that have utility in selected cases of tumor assessment include diffusion-weighted imaging (DWI) and DCE (perfusion) MRI, which are subsequently explained in depth ( Fig. 39-5 ).
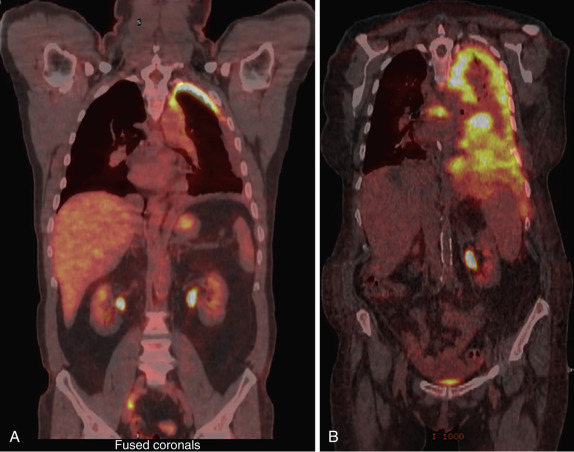
PET with the radiotracer F-fluorodeoxyglucose (FDG) complements CT and MRI in evaluation of pleural and chest wall malignancies. PET allows for evaluation of metabolic activity of tumor, which concentrates FDG to a greater degree than normal tissue. This type of assessment is useful in the context of pretreatment planning, staging, and follow-up. Furthermore, different tumor subtypes have shown differences in FDG avidity—for example, more indolent epithelioid mesothelioma tends to be less metabolically active than more aggressive sarcomatoid mesothelioma. Poor spatial resolution of PET is largely compensated for by co-registration with CT (PET-CT). Hybrid PET-CT systems that are capable of performing PET and CT in a single session have come into widespread use.
Integrated PET-CT is superior to PET and CT alone in the diagnosis and staging of thoracic malignancies. Importantly, PET-CT is superior in identifying patients with occult metastases and unresectable tumors. PET-CT is also very useful for distinguishing malignancy from atelectasis, infection, and other benign processes ( Fig. 39-6 ).
Apical opacities, or apical caps, are a common nonspecific finding that may be idiopathic or seen in various disease states including infection, inflammatory conditions, reaction to radiotherapy, neoplasms such as Pancoast tumor, mediastinal hemorrhage, mediastinal lipomatosis, and peripheral upper lobe collapse.
On chest radiographs, idiopathic asymptomatic apical pleural caps are irregular homogeneous densities less than 5 mm in thickness over the lung apex. The undersurface is usually sharply marginated. Apical pleural caps are as commonly unilateral as bilateral and are increasingly common with aging; they are found in as many as 16% of patients over age 45. When they are bilateral, apical pleural caps are usually asymmetric. Apical opacities related to tuberculosis tend to be thicker than idiopathic scars, measuring up to 2.5 cm in thickness. CT has shown these to be composed of thickened extrapleural fat.
On CT, apical pleural caps may appear more irregular than on radiographs and are occasionally difficult to distinguish from spiculated lung cancers. Compared with tumors, however, idiopathic caps have been shown to be smaller in craniocaudal than axial dimensions. Superior sulcus or Pancoast tumor should be considered in the setting of local pain or underlying bone destruction.
Pneumothorax is defined as gas within the pleural space, which may or may not be under tension. Pneumothorax occurs spontaneously in otherwise healthy individuals and in trauma and iatrogenic injury (e.g., from central line placements, biopsies, and mechanical ventilation). Predisposing conditions include emphysema, pulmonary fibrosis, cystic fibrosis, cavitary pneumonia, and cystic interstitial lung diseases such as Langerhans cell histiocytosis and lymphangiomyomatosis.
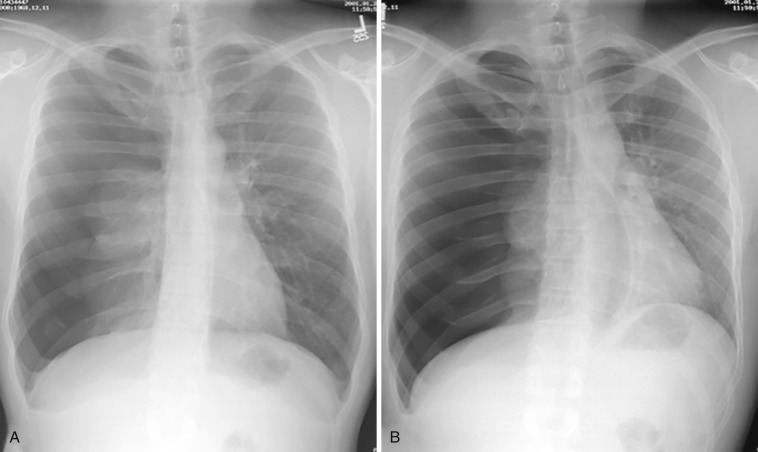
Chest radiography is the first-line imaging test in patients with suspected pneumothorax. The classic chest radiography finding of pneumothorax is a thin visceral pleural line beyond which normal lung markings do not extend. In supine patients, gas may collect at the lung bases, including the costophrenic angles (deep sulcus sign), or adjacent to the heart in the cardiophrenic recess. At times, it may be difficult to differentiate bullae, the medial border of the scapula, skin folds, or objects external to the patient from pneumothorax, but knowledge of these potential confounders is helpful in radiographic interpretation. Expiratory and decubitus radiographs accentuate small pneumothoraces ( Fig. 39-7 ).
Ultrasound is a useful adjunct to chest radiography, particularly in critically ill patients who may require evaluation at the bedside. Ultrasound has been shown to be more sensitive for pneumothorax than chest radiography in postbiopsy patients. Typical signs of pneumothorax on ultrasound include loss of the normal comet tail and lung sliding signs. False positives on ultrasound may be seen in patients with impaired lung function. It is not possible to quantify the volume of pneumothorax using ultrasound, and comprehensive assessment of the entire thorax, as is possible with radiographs and cross-sectional imaging, is not feasible.
CT detects pneumothoraces that are below the limit of detection of chest radiography and is helpful to distinguish pneumothorax from bullous disease. Furthermore, CT may be used to assess the degree of loculation in complicated pleural collections, guide drainage of pneumothoraces, and assess positioning of drainage catheters. Multiplanar reformations and thin-section technique are often useful. IV contrast is not necessary for evaluation of pneumothorax.
Given susceptibility artifact produced by gas, MRI has no significant role in the evaluation of pneumothoraces.
The normal volume of fluid in each pleural space is approximately 8 mL. Pleural fluid volumes increase in myriad pathologic processes. Broadly, pleural effusions are divided into transudates and exudates. Transudates are caused by imbalanced hydrostatic or oncotic forces between lymphovascular structures and the pleural space, whereas exudates are caused by altered permeability of the lymphovascular structures themselves. Table 39-1 lists a selection of conditions that cause exudative and transudative effusions.
| Transudate | Exudate | Either Exudate or Transudate |
|---|---|---|
| Congestive heart failure | Pneumonia | Asbestos exposure |
| Cirrhosis/hypoalbuminemia | Empyema | Intraabdominal abscess |
| Nephrotic syndrome | Malignancy (may or may not contain malignant cells) | |
| Cardiac tamponade | Autoimmune serositis | |
| Pregnancy | Pulmonary embolism | |
| Vasculitis | ||
| Dressler syndrome (often bloody) | ||
| Medications | ||
| Uremia (sometimes bloody) |
Biochemical pleural fluid evaluation (e.g., using Light's criteria; Box 39-1 ) helps differentiate exudate from transudate. When diagnostic thoracentesis is performed for a pleural fluid collection of unknown etiology, typical basic laboratory evaluation includes Gram stain, culture, cell count and differential, lactate dehydrogenase (LDH), glucose, pH, specific gravity, and protein. Fluid should be sent for cytologic evaluation if malignancy is in the differential diagnosis. Cases that may call for specialized lab testing include chylothorax, pancreatitis-related effusion, esophageal rupture, and tuberculous infection.
Pleural fluid protein–to–serum protein ratio > 0.5
Pleural fluid LDH–to–serum LDH ratio > 0.6
Pleural fluid LDH > 2/3 times the upper limit of normal for serum
Malignant effusions and empyema will be discussed in greater depth in subsequent sections.
In patients with suspected pleural effusion, chest radiography is the first-line test and ultrasound is a useful adjunct. Many cases can be managed using radiography and ultrasound alone. CT is useful in cases requiring comprehensive assessment of the entire pleura and extrapleural structures. The roles of various modalities in the diagnosis and treatment of pleural effusion are summarized in Table 39-2 .
| Radiography |
|
| Ultrasound |
|
| CT |
|
| MRI |
|
Typical radiographic findings include unilateral or bilateral blunting of the costophrenic or cardiophrenic sulci with a meniscus sign, diaphragmatic silhouetting, fluid in the fissures, and mediastinal shift in cases of large effusion. Detection of pleural fluid on radiographs requires at least 175 mL on upright frontal radiographs, 75 mL on lateral views (in the posterior costophrenic sulcus), and as little as 10 mL on lateral decubitus views, which are most sensitive. Identification of hydropneumothorax or empyema (loculated effusion) is often possible with radiography ( Fig. 39-8 ). Whether the effusion is unilateral (more likely exudative) or bilateral (more likely transudative) may be helpful in determining the character of an effusion in a given clinical context, though there is considerable overlap; biochemical analysis of pleural fluid is usually necessary for definitive assessment.
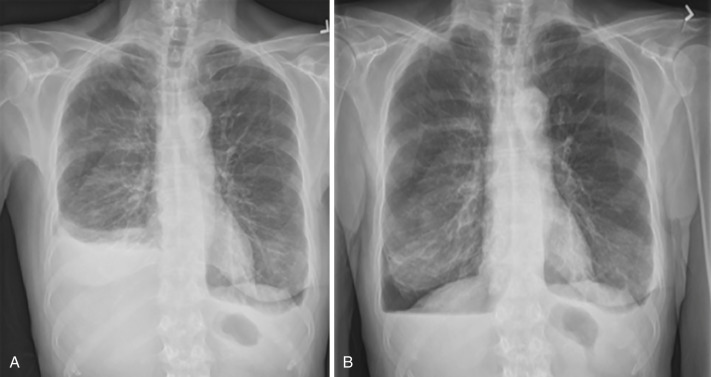
After thoracentesis, radiography is routinely used to evaluate for complications such as pneumothorax. After evacuation of large pleural effusions, postthoracentesis pneumothorax ex vacuo, or “trapped lung,” may occur and should not be confused with iatrogenic pneumothorax caused by pulmonary puncture. The appearance of pneumothorax ex vacuo is caused by air in the pleural space, with lack of reexpansion of the chronically atelectatic or fibrotic lung ( Fig. 39-9 ).
Ultrasound is more accurate than radiography for estimating pleural fluid volume (as little as 5 mL may be detected) and more sensitive than CT in identifying septations. Importantly, ultrasound is useful in guiding interventions such as diagnostic and therapeutic thoracentesis, particularly in cases of small or loculated effusion, and pleural mass biopsy. Sonography has also been used to guide chest tube drainage and thoracoscopy in patients with parapneumonic effusion and empyema.
CT is more sensitive than radiography for detection of small pleural effusions. The capability of CT to evaluate the entire pleural surface, lung parenchyma, mediastinum, and chest wall makes it the ideal modality for gauging the extent and nature of pleural fluid collections. Small simple effusions are seen as meniscoid low-attenuation collections that layer dependently, whereas loculated effusion is most often seen as lenticular fluid-attenuation masses along the dependent posterior pleural surface. CT readily diagnoses loculated effusion in the major or minor fissure, which produces a pseudotumor appearance on plain radiographs. At times it may be difficult to differentiate pleural effusion from ascites using CT in a supine patient. Table 39-3 lists some signs that may be helpful in making this distinction.
| “Displaced crus” sign | Anterior displacement of the diaphragmatic crus by pleural fluid adjacent to the vertebral bodies, not seen in ascites |
| “Interface” sign | Hazy rather than sharp interface between pleural fluid and the abdominal viscera created by diaphragmatic interposition; ascites has a sharp interface with the liver or spleen owing to lack of diaphragmatic interposition. |
| “Diaphragm” sign | Pleural fluid lies peripheral to the diaphragm rather than ascites, which lies medially. |
| Sparing of the “bare area” of the liver | Ascites cannot interpose between the posterior right lobe of the liver and the abdominal wall because this portion is attached directly to the abdominal wall; fluid posterior to the right lobe of the liver must be in the pleural space. |
Attenuation values (Hounsfield units [HU]) are sometimes helpful in determining the content of a pleural effusion. Acute hemothorax is seen as a high-attenuation effusion. Whether attenuation value is reliable in differentiating exudate from transudate remains controversial. In their study of 100 patients, Abramowitz et al. concluded that attenuation values of pleural fluid do not reliably differentiate exudate from transudate. Additional features such as fluid loculation, pleural thickening, and pleural nodules were more commonly present with exudative effusion, but these findings were not statistically significant. Yildiz et al. asserted that this study's conclusions may have been confounded by relatively low numbers of organizing consolidated effusions and inadequate statistical power. A study of 106 patients by Cullu et al. reached a differing conclusion, that exudates had a significantly higher median attenuation value (12.5 HU) than transudates (5 HU). Though there was significant overlap between exudates and transudates, it was concluded that a discriminatory value of 15 HU or greater is useful for identifying exudates. Aquino et al. determined that parietal pleural thickening is 96% specific for exudate in the setting of pleural effusion and that exudate without parietal pleural thickening is most common in malignancy and uncomplicated parapneumonic effusion. Outside the realm of exudative versus transudative effusion, CT is capable of identifying blood (hemothorax), enhancing septations or nodules (infection, malignancy), and gas (instrumentation, esophageal rupture, bronchopleural fistula [BPF], infection). The presence of small locules of gas in the pleural space is expected after pleural intervention but is specific for infection in other contexts.
MRI plays a limited role in the evaluation of pleural effusions, especially in benign disease, but may add value in cases where rib or chest wall invasion by the underlying process is suspected. Subacute or chronic hemothorax has high signal intensity on T1- and T2-weighted images. In chronic hemorrhagic effusion, there may be a concentric ring sign caused by peripheral hemosiderin (dark on T1- and T2-weighted images) and central methemoglobin (bright on T1- and T2-weighted images). Though chylothorax usually appears as a simple pleural effusion on all modalities, there have been reports of high signal intensity on T1-weighted MRI.
MRI is sensitive for pleural septations and may be useful when cross-sectional imaging is needed but iodinated contrast is contraindicated. Simple fluid demonstrates low signal intensity on T1-weighted imaging and high intensity on T2-weighted imaging. Triple-echo pulse sequences have been used to differentiate complex exudate (containing malignant cells or infection, highest signal) from simple exudate (no malignant cells or infection, high signal) and transudate (low signal). In their evaluation of 57 patients with pleural effusion using DWI, Baysal et al. found that the mean apparent diffusion coefficient (ADC) value of exudative effusions was lower than that of transudative effusions, with an optimum cutoff value of 3.38 × 10 −3 mm 2 /sec, yielding 91% sensitivity and 89% specificity. In their study of 58 pleural effusions, Inan et al. reached similar conclusions. With current technology, however, routine use of MRI for this application is not feasible owing to factors including expense and technical limitations in scanning tachycardic, dyspneic, and critically ill patients.
Pleurodesis is therapeutic sealing of the pleural space by inciting an organizing inflammatory reaction that causes the visceral and parietal pleura to adhere to one another. The usual indications are recurrent pneumothorax and recurrent malignant effusion. Talc has been found to have higher success rates and a lower rate of systemic complications than the chemical agents bleomycin and tetracycline (doxycycline is the most commonly used agent in the tetracycline class). The agent is applied as an aerosolized powder at thoracoscopy or thoracotomy (poudrage) or at the bedside as slurry instilled via a chest tube. The major complications of talc pleurodesis are acute pneumonitis, acute respiratory distress syndrome (ARDS), and pulmonary edema.
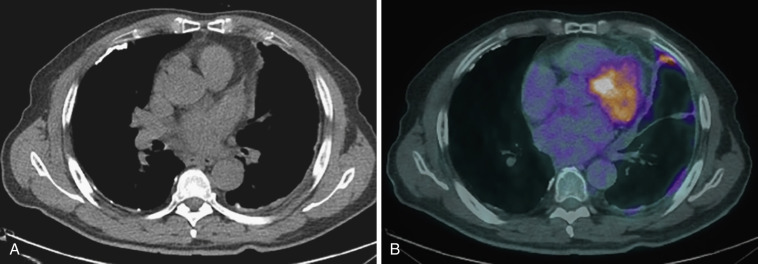
Following pleurodesis, the organizing phase is characterized on radiographs as pleural thickening and loculation. Over half of patients have visible pleural thickening on long-term follow-up imaging. Following talc pleurodesis, CT usually shows pleural thickening and nodularity, with some residual loculated effusion and dependently located high-attenuation foci along both the visceral and parietal pleura that may mimic pleural calcification. The high-attenuation foci may also be located in the fissures. Areas correlating with these high-attenuation foci may have markedly increased FDG uptake on PET imaging that can simulate malignancy and persist for many years ( Fig. 39-10 ).
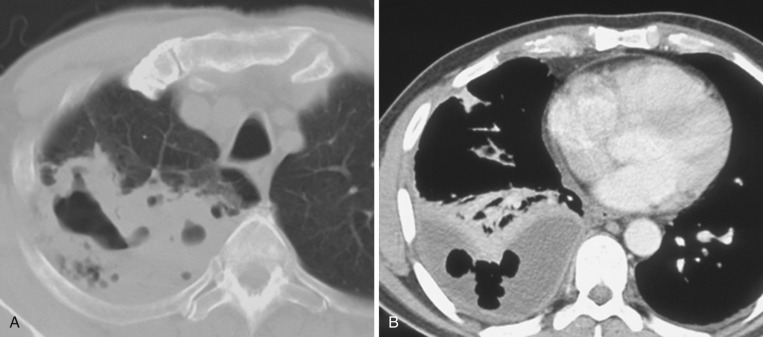
Aggressive antibiotic therapy is necessary in empyema. Surgical decortication may be helpful in patients who do not respond to antibiotics or in cases of moderate or large empyema and may include a Clagett window procedure , in which a small thoracotomy is left open for ongoing irrigation with antibiotic solution and drainage. When infection has resolved and granulation tissue has formed, antibiotic solution is instilled into the pleural space and the window may be closed using muscular flaps from the latissimus dorsi ( Fig. 39-11 ).
Although approximately 40% of patients with pneumonia develop an associated parapneumonic effusion, only about 10% develop an empyema, which is defined as a purulent pleural collection. Pneumonia is the most common cause of empyema. Other causes include lung abscess, BPF, esophageal perforation, complications of surgery, and trauma. The evolution of empyema in three stages is summarized in Table 39-4 . If each stage is not treated adequately, there is progression to the next stage.
|
|
|
|
|
|
As previously discussed, radiography and ultrasound are the modalities of choice for initial evaluation of pleural effusion. On plain radiographs, lenticular biconvex shadows rather than meniscoid shape suggests loculation and empyema rather than layering effusion. Loculated effusions do not layer on supine or decubitus views.
Contrast-enhanced CT is a vital adjunct to pleural fluid analysis in managing patients with infected effusions or empyema and is highly accurate in differentiating empyema from lung parenchymal abscess abutting the pleura.
Differentiation of abscess from empyema is important because attempted drainage of parenchymal abscess may seed the sterile pleural space with bacteria, whereas drainage is a mainstay of therapy for empyema. CT features differentiating empyema from parenchymal abscess are summarized in Table 39-5 ( Fig. 39-12 ).
| Empyema | Parenchymal Abscess |
|---|---|
| Thin, uniform-thickness walls | Thick walls of nonuniform width |
| Smooth luminal and exterior margin | Irregular luminal and exterior margin |
| Split pleura sign present | Split pleura sign absent |
| Obtuse angle with chest wall | Acute angle with chest wall |
| Lenticular shape | Round shape |
| Compression rather than obliteration of lung structures | Abrupt vessel cutoff and presence of bronchi at interface with normal lung |
| Increased attenuation of extrapleural fat | Normal attenuation of extrapleural fat |
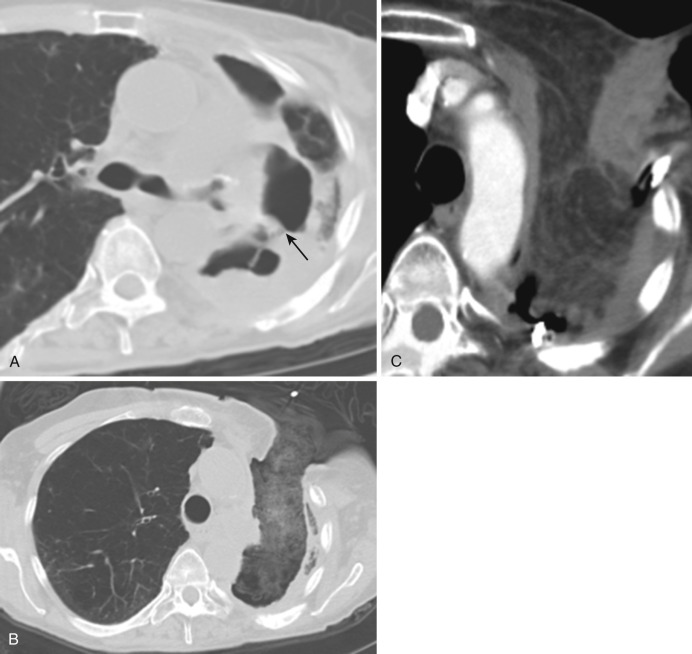
A classic contrast-enhanced CT sign of empyema is the split pleura sign, which describes thickened, enhancing visceral and parietal pleural layers separated by low-attenuation effusion. Pleural thickening and enhancement characterizes 86% to 100% of cases of empyema as opposed to only 60% of parapneumonic effusion. Increased attenuation of the extrapleural fat (>50 HU greater than subcutaneous fat) is an associated finding that may persist for a period of time following resolution of infection.
Ultrasound is more sensitive than CT in detecting septations and is often used to guide intervention; CT is capable of detecting separate loculations of pleural fluid (inferred by trapped gas pockets within the collection rather than by visualization of the septa themselves) in approximately 20% of cases. CT is useful to guide thoracentesis or drainage catheter placement when ultrasound is not feasible because of large patient body habitus or poor visualization of critical structures owing to air in the lung parenchyma or adjacent bony structures. Radiography and CT play complementary roles in assessing appropriate catheter positioning after placement and in cases where catheter drainage has ceased or the patient is not responding to therapy.
MRI is rarely needed for routine evaluation of empyema. Relative weaknesses as compared with CT are inferior spatial resolution and susceptibility to artifacts; however, MRI is sensitive for demonstrating septations within pleural collections and may be a useful alternative in patients with contrast allergies or in contexts where avoidance of ionizing radiation exposure is desired. Rarely malignancies such as non-Hodgkin's lymphoma, squamous cell carcinoma, sarcoma, and mesothelioma arise in chronic empyema cavities. MRI, because of to its excellent contrast resolution, is the modality of choice for differentiating neoplasm from fibrotic tissue.
BPF is defined as a direct communication between the bronchial tree and the pleural space that may occur spontaneously, as in cases of necrotizing pleural infection or malignancy, or as a result of trauma or iatrogenic injury. BPF is associated with high morbidity and mortality.
Central BPF is a connection between a central large bronchus and the pleural space, whereas peripheral BPF is a connection between a peripheral airway and the pleural space. The most common causes of central BPF are dehiscence of a bronchial stump after lung transplantation or pneumonectomy, and trauma. Peripheral BPF is caused by necrotizing pneumonia, empyema, trauma, ruptured bullae, and iatrogenic injury from radiation therapy or thoracentesis.
On radiography, increasing volume of pleural air (without a history of instrumentation), mediastinal shift, and loss of fluid in a previously filled or filling postpneumonectomy space are indirect signs of BPF. New gas within an empyema may also suggest the diagnosis. CT with IV contrast is the modality of choice in suspected BPF, given its ability to depict the vasculature and evaluate for the underlying cause. Indirect findings on CT are analogous to the previously described radiographic findings. Direct findings of BPF include the fistulous connection itself or extraluminal air bubbles adjacent to the bronchial stump. Multiplanar reformation and advanced postprocessing techniques (e.g., volume rendering, virtual bronchoscopy, MIP, MiniP imaging) are valuable in the evaluation of BPF. Demonstration of inhaled radiotracer (e.g., xenon) in the pleural space on ventilation scintigraphy may be useful in cases of occult BPF.
Treatment of central BPF includes broad-spectrum antibiotics, protection of the contralateral lung from spilled pleural fluid by appropriate patient positioning, drainage or surgical washout of the pleural space, and primary repair of the bronchial stump in postpneumonectomy patients (sometimes with reinforcement using omentum or other vascularized soft tissue). The goal of interventional therapy for peripheral BPF is pleural apposition, which is accomplished by thoracostomy, decortication, pleurodesis, and open drainage, or transbronchial occlusion using muscle flaps, fibrin sealants, coils, stents, and one-way valves.
Pleural calcifications may be seen in numerous conditions, including asbestos-related pleural disease, resolved hemothorax, and pyogenic or mycobacterial infection. Prior talc pleurodesis should also be considered in the differential diagnosis for high-density material in the pleural space ( Fig. 39-13 ).
Diffuse fibrous pleural thickening, likely a reaction to chronic inflammation of the visceral pleura that results in fusion of the parietal and visceral pleura, is seen in a number of disease states. It is especially common in diseases that cause exudative pleural effusion. Tuberculous or bacterial empyema, hemothorax (especially posttraumatic), talc pleurodesis, asbestos exposure, radiation therapy, and some medications are the most common causes. Pleural thickening related to asbestos exposure and malignancy is discussed subsequently.
On radiographs, pleural thickening may be indistinguishable from effusion. Typical findings include blunting of the costophrenic sulci, a several millimeter-thick soft tissue density stripe separating the lungs from the adjacent chest wall, thickened soft tissue internal to the ribs in patients with pneumothorax, pleural calcification, and asymmetric increase in low-attenuation extrapleural fat.
When radiographs raise suspicion for diffuse pleural thickening, CT is useful for distinguishing thickened pleural from prominent extrapleural fat and malignant disease. On CT, pleural thickening may be seen as a soft tissue density stripe at least 1 mm in thickness internal to the ribs, innermost intercostal muscles, and paravertebral regions. The appearance of the thickened pleura itself is nonspecific, and evaluation of patient exposure history (e.g., tuberculosis, asbestos) and assessment of the chest wall and lungs by CT is often necessary to narrow the differential diagnosis. Pleural thickening should be evaluated at several levels and outside the paraspinal region, where intercostal vessels and normal thickened fat may be difficult to distinguish from the pleura. Mimics of pleural thickening include the normal subcostalis and transverse thoracis muscles, and intercostal veins.
In patients without a history of asbestos exposure, ancillary findings in the chest wall and lungs may permit accurate diagnosis. For example, presence of rib fractures and normal lung parenchyma may suggest pleural thickening due to prior hemothorax, whereas postinfectious (including posttuberculous) pleural thickening may have volume loss, extrapleural fat thickening, or parenchymal scarring. After talc pleurodesis, high-attenuation talc between the thickened visceral and parietal pleura (usually in the posterior thorax) creates the talc sandwich sign.
Erdheim-Chester disease (ECD) is a rare idiopathic form of non–Langerhans cell histiocytosis that may cause diffuse pleural thickening. The most typical presentation is bone pain with symmetric osteosclerosis of the metadiaphyses of the long bones. Thoracic involvement is seen in about 15% to 30% of cases. In their study of 40 patients with thoracic manifestations of ECD, Brun et al. reported perivascular and mediastinal infiltration, pericardial effusion and thickening, ground-glass opacities and poorly defined pulmonary nodules, pulmonary cysts, pleural effusions, and pleural thickening.
Asbestos is a naturally occurring substance with fire-resistant properties that has been used in many industrial applications including building insulation, manufacturing, and shipbuilding. Inhalation of asbestos fibers has been associated with benign and malignant pleuropulmonary diseases such as formation of fibrous pleural effusions, pleural plaques, diffuse pleural thickening, round atelectasis, lung cancer, and malignant mesothelioma. Many pathologic entities related to asbestos exposure tend to manifest in a delayed fashion, with latency of up to 60 years.
Benign hemorrhagic exudative pleural effusions are the earliest manifestation of asbestos-related pleural disease, appearing within 10 years of exposure. Risk of pleural effusion appears to be dose dependent. Asbestos-related effusions may be asymptomatic. They tend to resolve over months but may persist or recur. Development of diffuse pleural thickening often follows.
Pleural plaques are discrete areas of fibrosis that arise more commonly from the parietal than visceral pleura, appearing 20 to 30 years after exposure. They are the most common manifestation of asbestos-related pleural disease and are usually asymptomatic.
Asbestos fibers are long and thin; therefore they are not cleared by macrophages or lymphatics. They tend to remain in the lower lung zones where ventilation is higher than in the upper lung zones. Thus, distribution of asbestos-related lung disease is predominantly basal. The anterior and lateral chest wall have greater excursion with respiration than the posterior chest wall, which is relatively fixed to the spine. Together, these attributes have led some to speculate that the reason asbestos-related pleural plaques form preferentially in the basal anterior and posterolateral chest is because of greater chest wall motion and hence more friction, leading to more irritation of the pleura by asbestos fibers.
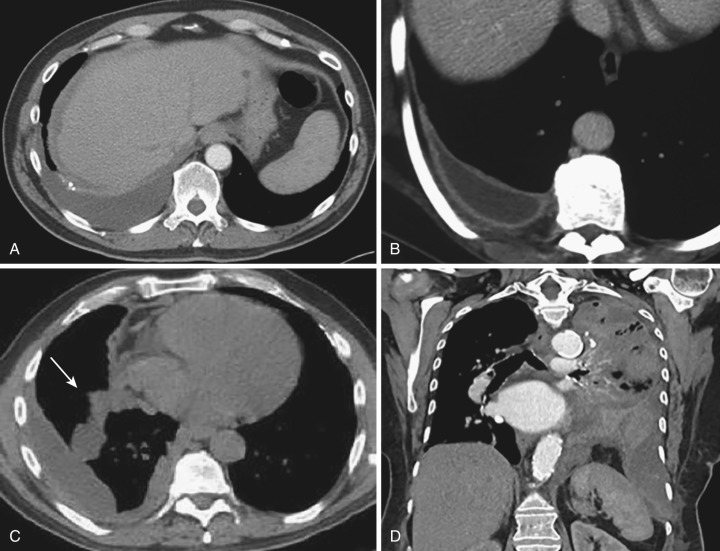
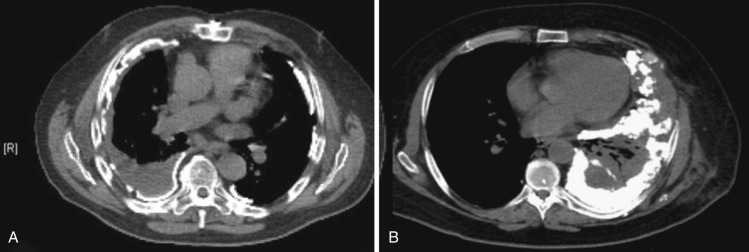
On radiographs, pleural plaques appear as opacities that demonstrate the incomplete border sign. They are most commonly identified at the posterolateral chest wall and against the mediastinum and diaphragm, where thick flat opacities are virtually pathognomonic for asbestos-related pleural plaques. The apices and costophrenic sulci tend to be spared, unlike in diffuse pleural thickening. Calcification, present in 10% to 15% of cases, increases conspicuity. The holly leaf sign describes the appearance of pleural plaques as irregular in shape with angular outer contours. CT is more sensitive than radiography and reveals additional anterior and paravertebral plaques that may not be detected on radiographs. CT is also helpful in distinguishing pleural disease from extrapleural fat. Plaques involving the visceral pleura are sometimes called hairy plaques because of their association with short interstitial linear opacities in the adjacent lung parenchyma that appear to radiate from the plaque ( Fig. 39-14 ).
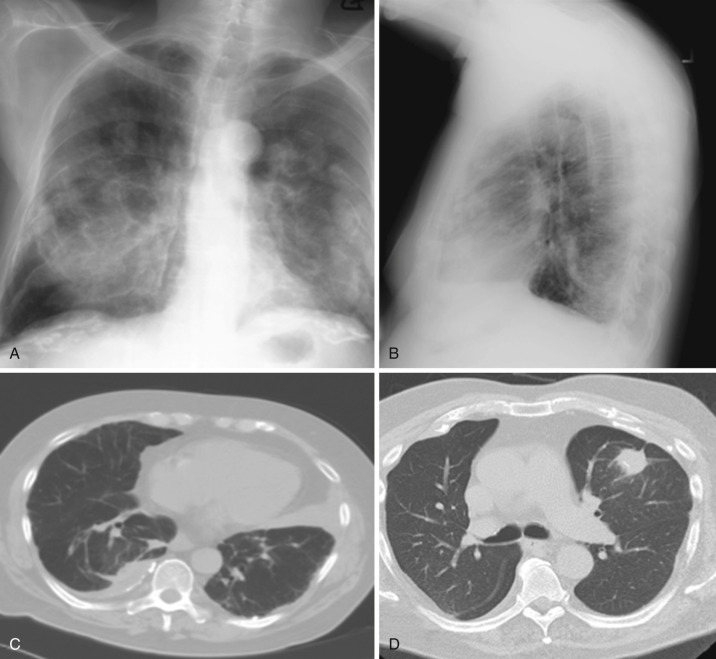
In patients with a history of asbestos exposure, diffuse pleural thickening is less common than focal pleural plaques, occurring in less than 10%. Incidence is, on average, 20 to 40 years after initial exposure. A dose-dependent relationship has been described. Presenting symptoms include progressive dyspnea and decreased exercise tolerance. Extensive pleural thickening is associated with restrictive physiology, which is reflected by pulmonary function testing in affected patients. Pulmonary function testing abnormality has been shown to correlate with the degree of pleural thickening.
For the diagnosis of diffuse pleural thickening, CT is more sensitive and specific than radiography. The CT appearance of diffuse pleural thickening in patients with a history of asbestos exposure has been described by McLoud et al. as smooth, uninterrupted pleural opacity extending over at least one quarter of the chest wall, with or without obliteration of the costophrenic angle. Calcification is more rare in diffuse pleural thickening than in focal pleural plaques, and diffuse pleural thickening more often involves the apices and costophrenic angles. Unlike diffuse pleural thickening, nonconfluent plaques do not extend over more than four interspaces, and diffuse pleural thickening may involve the interlobar fissures, whereas plaques typically do not. Reactive proliferation of extrapleural fat is a common associated finding. Ninety percent of patients with diffuse pleural thickening due to asbestos exposure have associated parenchymal abnormalities. Diffuse pleural thickening due to asbestos exposure is more often bilateral than pleural malignancy and lacks infiltration and nodularity.
Round atelectasis associated with asbestos-related disease, also known as asbestos pseudotumor, is thought to occur as a result of pleural inflammation and fibrosis that results in infolding into the lung parenchyma, which becomes trapped and atelectatic.
The classic radiographic appearance on chest radiographs is a masslike opacity with a broad-based interface with thickened pleura, associated with bronchovascular comet tails. The presence of volume loss rather than mass effect may favor this diagnosis over true masses such as lung cancer, the primary and most feared differential diagnosis.
Contrast-enhanced CT is helpful for further characterization and shows uniform enhancement of the atelectatic lung (though this is not a reliable feature for distinguishing atelectasis from lung cancer). Given the sometimes nonspecific appearance of round atelectasis and the primary differential consideration of lung cancer, biopsy or demonstration of stability or shrinkage on serial interval follow-up may be necessary to confirm the diagnosis.
On T1-weighted MRI, atelectasis may appear as a mass with signal characteristics similar to those of liver tissue. MRI may also demonstrate the relationship of the pseudomass with bronchovascular structures. Curvilinear hypointense lines are thought to represent thickened indentations of visceral pleura.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here