Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
acyl-CoA:retinol acyltransferase
β-carotene 15,15′-oxygenase
β-carotene 9′10′-oxygenase 2
CEL knockout
carboxyl ester lipase
chylomicrons
cellular retinol-binding protein
diacylglycerol acyltransferase
knock out
lecithin:retinol acyltransferase
lutein
lycopene
oleic acid
pancreatic lipase-related protein
pancreatic triglyceride lipase
retinoic acid
retinoic acid receptors
retinyl esters
retinyl ester hydrolase
retinal pigment epithelium
retinoid X receptors
taurocholate
triglycerides
very low-density lipoproteins
wild type
zeaxanthin
α-carotene
β-carotene
Vitamin A deficiency affects more than 100 million children throughout the world. Thus, knowledge about the mechanisms of absorption of vitamin A can lead to better approaches for enhancing its absorption and could be helpful in ameliorating some of the deficiencies. The major sources of vitamin A in human diet are the provitamin A carotenoids in fruits and vegetables and retinyl esters found in foods of animal origin. In humans, carotenoids are either cleaved to generate retinol or absorbed intact. In contrast, retinyl esters are completely hydrolyzed in the intestinal lumen and free retinol is taken up by enterocytes. The intestinal absorption and metabolism of dietary carotenoids has been the subject of recent reviews, as have the biochemical and molecular mechanisms involved in the digestion and absorption of vitamin A.
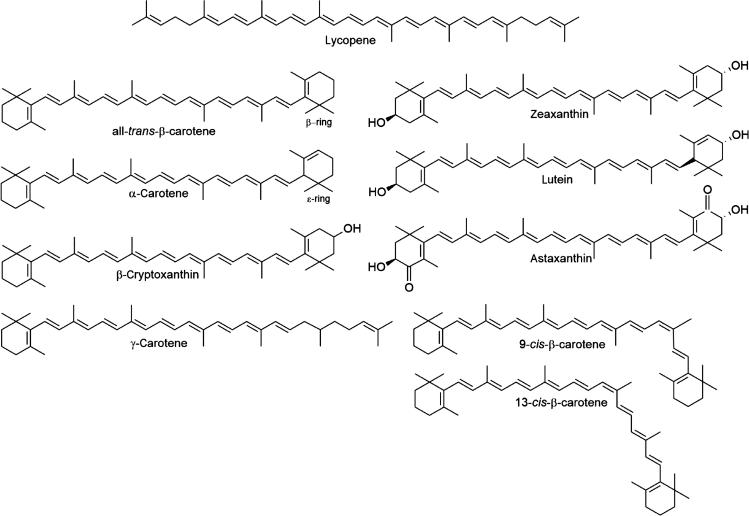
Carotenoids are synthesized in plants and in certain microorganisms such as some bacteria, algae, and fungi. They are a group of pigments that are widespread in nature and responsible for the yellow/orange/red/purple colors of many fruits, flowers, birds, insects, and marine animals. Over 600 carotenoids have been isolated from natural sources, but only ~ 60 of them are detected in the human diet and ~ 20 of them in human blood and tissues. β-Carotene (β-C), α-carotene (α-C), lycopene (LYC), lutein (LUT), and β-cryptoxanthin are the five most prominent carotenoids present in the human body.
All carotenoids are derived from the basic linear polyisoprenoid structure of LYC that contains 40 carbon atoms and an extended system of 13 conjugated double bonds. Carotenoids derive from this parent structure by cyclization (i.e., formation of β- or ε- ionone rings) at one (i.e., γ-carotene) or two ends (i.e., β-C and α-C) of the polyene chain and by dehydrogenation and/or oxidation. The structures of several major carotenoids are shown in Fig. 50.1 . The carotenoid group is divided into the carotenes, hydrocarbon carotenoids with unsubstituted rings, and xanthophylls, carotenoids with at least one oxygen atom. They exist mostly in the all- trans configuration, but they can be subject to a cis isomerization at any double bond of their polyene chain, resulting in a large number of mono- and poly- cis isomers.
Capability for the de novo synthesis of compounds with vitamin A activity is limited to plants and microorganisms. Thus, higher animals must obtain vitamin A from the diet, either as the preformed vitamin or as a provitamin carotenoid such as β-C. In the intestinal mucosa β-C is converted to retinal by β-C 15,15′ oxygenase 1 (BCO1) and the retinal is then reduced to retinol by a retinal reductase. In the human intestine about half the dietary provitamin A carotenoids are converted to retinol and about half are absorbed intact although the extent of conversion varies widely among individuals. The major dietary forms of preformed vitamin A are long-chain fatty acid esters of retinol. These esters must be hydrolyzed prior to intestinal absorption. Hydrolysis of the esters is catalyzed both by enzymes secreted by the pancreas into the intestinal lumen and by those associated directly with intestinal cells.
Following the hydrolysis of dietary retinyl esters, the free retinol is then taken up by the mucosal cell. The free retinol, resulting either from hydrolysis of dietary retinyl esters or by conversion of dietary provitamin A carotenoids, is reesterified with long-chain, mainly saturated, fatty acids by the enzyme lecithin:retinol acyltransferase (LRAT) when physiological doses of vitamin A are ingested. The resulting retinyl esters are incorporated with other neutral lipid esters (i.e., triacylglycerols and cholesteryl esters) and intact carotenoids into chylomicrons and absorbed via the lymphatics. Thus, co-ingestion of lipid to insure robust chylomicron formation is required for the efficient intestinal absorption of dietary carotenoids and vitamin A. In the vascular compartment, much of the chylomicron triacylglycerol is hydrolyzed by lipoprotein lipase in extrahepatic tissues resulting in the production of a “chylomicron remnant” that contains most of the newly absorbed retinyl esters. In the rat, the chylomicron remnants are rapidly and almost quantitatively taken up by the liver, and there is evidence that the retinyl esters are rapidly hydrolyzed and reesterified during this process.
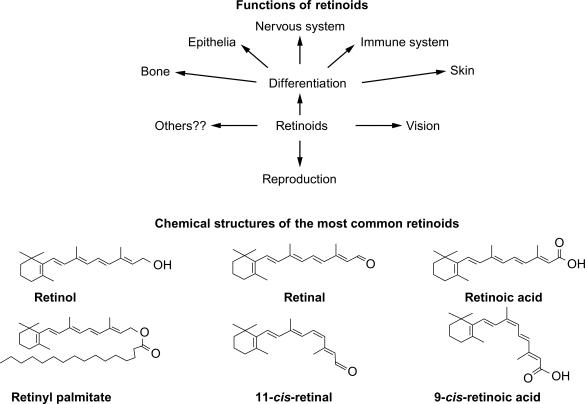
Under conditions of adequate vitamin A nutriture, the liver is the main site of vitamin A storage, with over 95% of the total neutral retinoid being present as retinyl esters, predominately retinyl palmitate, and stearate. Although chylomicron remnants (and the retinyl esters they contain) are initially taken up exclusively by the hepatocytes in liver, the retinyl esters are then transferred largely to the perisinusoidal stellate cells. In vitamin A-adequate rats, the stellate cells account for approximately 80% of the total retinyl ester store with the remainder being in hepatocytes. In both cell types, the retinyl esters are stored in cytoplasmic lipid droplets along with other neutral lipids. Prior to mobilization from the liver the retinyl esters are hydrolyzed, and free retinol is complexed to serum retinol-binding protein for secretion from the liver. Unesterified retinol is delivered to peripheral tissues via RBP4 (serum retinol-binding protein), where it is converted to its two major biologically active metabolites, 11- cis -retinal and all- trans -retinoic acid (RA). The former is the chromophore for the visual pigment rhodopsin, and thus is essential in the visual cycle. The RAs (all- trans and 9- cis ) are the ligands for two families of hormone-dependent transcription factors, the retinoic acid receptors (RARs) and retinoid X receptors (RXRs), which regulate the transcription of over 500 genes. Thus, vitamin A is essential for vision and for cell differentiation and development in vertebrates including humans. Fig. 50.2 shows the structures of the major dietary retinoids and their metabolites and outlines their functions.
As mentioned above, vitamin A activity in the diet derives from two sources: preformed vitamin A as retinyl esters in foods of animal origin and provitamin A carotenoids, such as β-C, α-C, and β-cryptoxanthin, found in plant–derived foods. Stoichiometric conversion of 1 mol of β-C (with 2 β-ionone rings) gives rise to 2 mol of retinol (via retinal), whereas conversion of a mole of either β-cryptoxanthin or α-C (each with only a single β-ionone ring) gives rise to a single mole of retinol. β-C is the most potent vitamin A precursor of all provitamin A carotenoids. In order to exhibit a provitamin A activity, the carotenoid molecule must have at least one unsubstituted β-ionone ring and the correct number and position of methyl groups in the polyene chain. In practice, α-C, β-cryptoxanthin, and γ-carotene show 30%–50% of provitamin A activity and 9- cis and 13- cis isomers of β-C less than 10%, compared with all- trans β-C.
Foods in the US diet with the highest concentrations of preformed vitamin A are avian and mammalian livers, meats, instant powdered breakfast drinks, ready-to-eat cereals, and margarines. Other than liver and meats, the other sources derive their high retinyl ester contents from fortification. The highest concentrations of vitamin A as provitamin A carotenoids are found in carrots, sweet potatoes, pumpkin, kale, spinach, collards, and squash. A retinol activity equivalent (RAE) is equal to 1 μg retinol or 12 μg β-C, or 24 μg of α-C or β-cryptoxanthin. Dietary survey data shows that the major contributors to the intake of preformed vitamin A are milk, margarine, eggs, beef liver, and ready-to-eat cereals, while the major sources of provitamin A carotenoids are carrots, cantelopes, sweet potatoes, and spinach. The mean intake of vitamin A in the United States was about 600 μg RAE/day from food and 70%–75% of this was as preformed vitamin A (retinol). In contrast, most populations in the developing world get most of their vitamin A from provitamin A carotenoids in fruits and vegetables.
Carotenoids are hydrophobic molecules and are thus located in lipophilic sites of the cells. To move through an aqueous environment, carotenoids can form complexes with proteins. Specific carotenoid-protein complexes have been reported mainly in plants and in invertebrates (e.g., cyanobacteria, crustaceans, silkworm). In vertebrates, data on the existence of carotenoproteins are limited. Although no intracellular β-C-binding protein was found in bovine liver and intestine, specific xanthophyll-binding proteins were reported in the human retina and macula. As an alternative mechanism for their water solubilization, carotenoids could use small cytosolic carrier vesicles. In nature, carotenoids can be also present in very fine physical dispersions (or crystalline aggregates) in aqueous media; oranges, tomatoes, and carrots are well-known examples of sources that contain such aggregates. These different physicochemical characteristics, i.e., chemical structure, positioning in biological membranes, and interaction with proteins, may account for the differences observed among individual carotenoids in their absorption and metabolism as well as their biological activities.
Retinoids are also lipophilic molecules that require solubilization. Unesterified retinol exerts detergent-like properties on cellular membranes and is usually sequestered in cells by a variety of retinol-binding proteins which are discussed in more detail below. The very nonpolar retinyl esters are usually found in cells in stabilized lipid droplets and emulsions. The digestion of retinyl esters and the conversion of carotenoids to retinoids require catalysis by enzymes that utilize these water-insoluble substrates. In some cases, the enzymes that metabolize retinoids or carotenoids are themselves hydrophobic and membrane bound.
There is little detailed information on the physical forms or “phases” that retinyl esters and carotenoids adopt in the intestinal lumen. Much more detailed information on these issues is available for other major dietary lipids such as triglycerides, phospholipids, and cholesterol. In those cases, the major phases found in the intestinal lumen are micoemulsions, micelles, mixed micelles, and unilamellar liquid crystalline vesicles. Nonetheless, it is clear from studies both in experimental animals and humans that the co-ingestion of dietary fat markedly enhances the intestinal absorption of dietary vitamin A and carotenoids. The presence of dietary fat in the intestine can stimulate retinyl ester digestion and provitamin A conversion by (1) stimulating pancreatic enzyme secretion, (2) stimulating the secretion of bile salts, which serve to form mixed micelles of lipids, and (3) providing products of lipid digestion (i.e., lysophospholipids, monoglycerides, and free fatty acids), which themselves can serves as components of micelles. Finally, fat ingestion promotes vitamin A and carotenoid absorption by providing the lipid components for intestinal chylomicron assembly, a process discussed in more detail below.
Almost five decades ago Erlanson and Borgström reported the partial separation of two different pancreatic retinyl ester hydrolase (REH) activities in the rat. These are now recognized to be carboxylester lipase (CEL) and pancreatic triglyceride lipase (PTL) .
Pancreatic carboxylester lipase catalyzes the hydrolysis of cholesteryl esters, triglycerides, and lysophospholipids. It was thought to hydrolyze retinyl esters also in the intestine. CEL knockout (CEL KO) mice were generated to study the functions of CEL. Although neither CEL KO nor wild-type (WT) mice absorbed non-hydrolyzable cholesteryl ether, CEL KO mice absorbed about half the amount of cholesterol provided as cholesteryl ester compared with WT mice. These data indicated that hydrolysis of cholesteryl esters is necessary prior to absorption, and that CEL plays an important role in cholesterol absorption. In contrast to the results for cholesteryl ester, CEL KO mice absorbed the same amount of retinol, when provided as retinyl ester, as did WT mice. On the other hand, neither mouse absorbed the non-hydrolyzable retinyl hexadecyl ether. These data suggested that retinyl ester hydrolysis was required for absorption and that CEL was not the responsible enzyme. The triglyceride absorption was also comparable between CEL KO and WT mice indicating that the absence of CEL does not affect triglyceride hydrolysis. Therefore, if intestinal retinyl ester absorption is unaffected in CEL KO mice, one or more other retinyl ester hydrolytic enzymes must be present in the gut lumen or on the enterocyte surface.
Studies were then conducted to identify the non-CEL pancreatic REH activity that appeared to be present in CEL KO mice, as well as to investigate this activity in WT mice and in rats. Several lines of evidence suggest that this activity is due to PTL. Although these data strongly suggest that PTL is a major REH activity in rat and mouse intestinal lumen, other enzymes synthesized and secreted by pancreas probably play a role in the lumenal hydrolysis of retinyl esters. For example, studies have suggested that pancreatic lipase-related protein 2 (PLRP2) may play a role. PLRP2 is 65% identical to PTL and hydrolyzes phospholipids and shows activity toward triglycerides in the classical PTL assay. It has been demonstrated that purified PLRP-2 (but not the related PLRP-1) can catalyze the hydrolysis of retinyl palmitate in vitro, but the activity was about one-third that of PTL. Thus, more than one enzyme may be responsible for the complete hydrolysis of retinyl esters in the intestinal lumen.
In addition to pancreatic REH activities, an REH activity intrinsically located in the brush border membrane of the absorptive enterocytes was reported in rat and human intestines. This enzyme activity was due to an intestinal phospholipase B (PLB). It is likely that PTL, PLRP2, and PLB all contribute to retinyl ester digestion.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here