Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The U.S. Department of Health and Human Services and the U.S. Department of Agriculture publish dietary guidelines for Americans every 5 years based on the most current evidence in nutrition science. These recommendations aim to promote health, prevent chronic disease and help individuals maintain a healthy weight. These guidelines also influence federal nutrition policies and product development. ( https://health.gov/dietaryguidelines/purpose.asp ). It is critical to remember, however, that these intake recommendations are targeted towards healthy Americans and do not include specific disease states, which often require different guidelines.
Ascorbate exists in both reduced (ascorbic acid [AA]) and oxidized (dehydro- l -ascorbic acid [DHAA]) forms. The physiologically important form is AA, which acts as a cofactor in a variety of metabolic pathways. AA keeps metal ions like iron and copper in their reduced forms, scavenges free radicals, synthesizes collagen and other connective tissue proteins, and plays a role in the regulation of CFTR-mediated chloride secretion in epithelial cells (see Chapter 101 ). Vitamin C deficiency leads to a variety of clinical abnormalities, including scurvy, poor wound healing, vasomotor instability, and some connective tissue disorders.
Primates, including humans, and guinea pigs, in contrast to most mammals, lack the enzyme l -gulonolactone oxidase and thus cannot synthesize vitamin C; as a result, they must obtain vitamin C from dietary sources. Rich dietary sources of vitamin C include fruits (citrus, cantaloupe, mango, strawberries, watermelon) and vegetables (cabbage, broccoli, cauliflower, potatoes, tomatoes). The recommended daily allowance (RDA) varies depending on age, gender and co-morbidities, but ranges between 90 and 120 mg/day for most adults.
Unlike a number of other water-soluble vitamins (see biotin, folate, niacin, pantothenic acid, pyridoxine, and riboflavin), where 2 sources are available to the host (dietary and production by normal colonic microbiota), vitamin C is only available through dietary consumption. Intestinal absorption of AA occurs via a concentrative, carrier-mediated, Na + -dependent mechanism that is localized to the apical brush border membrane (BBM) domain of polarized enterocytes ( Fig. 103.1 ). Once absorbed, AA leaves the absorptive cells across the basolateral membrane (BLM) via another carrier-mediated mechanism. Intestinal absorption of dietary DHAA occurs via a Na + -independent carrier-mediated process that is competitively inhibited by glucose because of structural similarities between these compounds. Internalized DHAA is subsequently metabolized to AA by the enzyme DHAA-reductase.
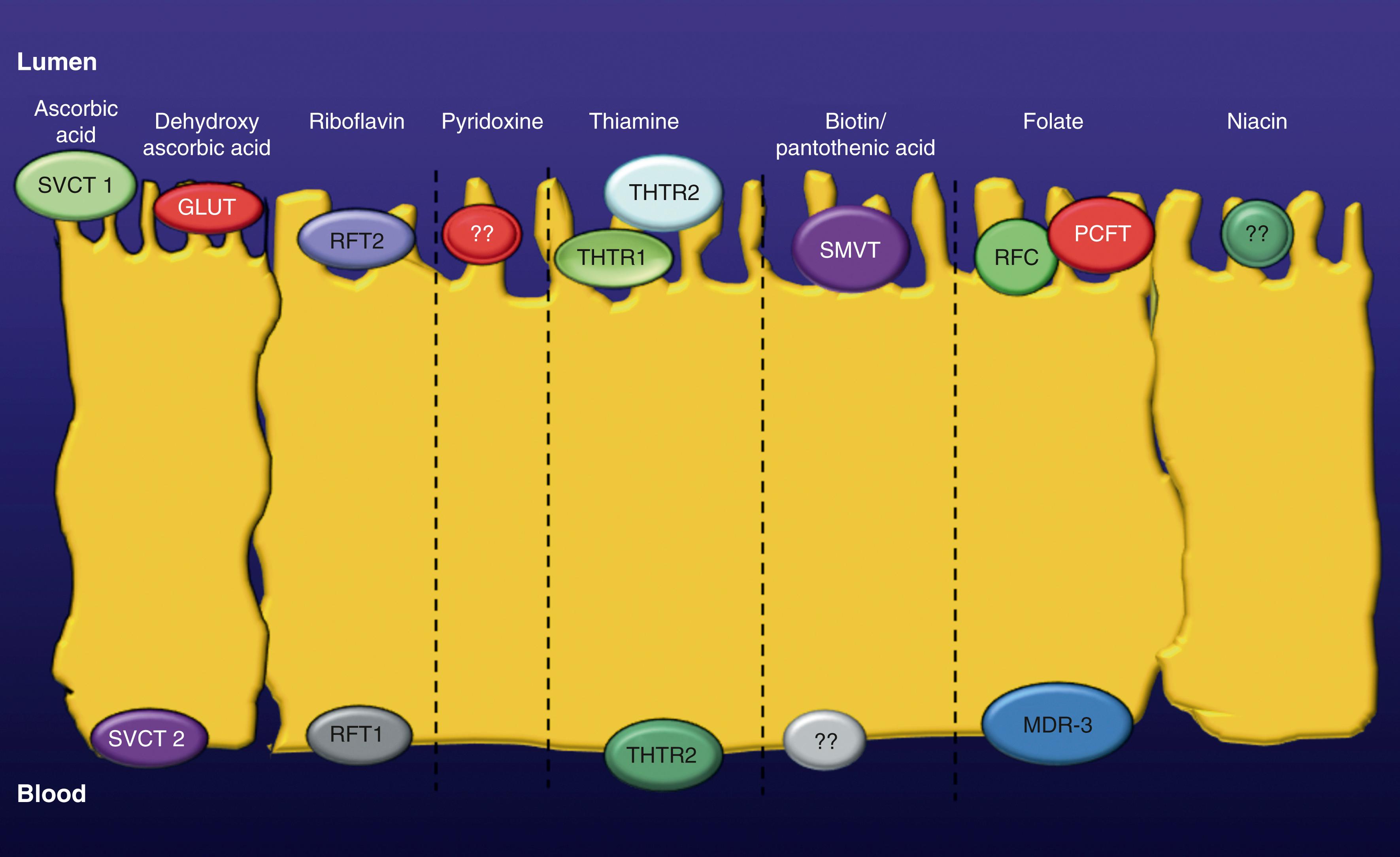
The 2 AA transport systems that have been identified in humans and other mammals consist of the Na + -dependent vitamin C transporter-1 (SVCT-1; product of the SLC23A1 gene) and the Na + -dependent vitamin C transporter-2 (SVCT-2; product of the SLC23A2 gene), both of which are expressed in the small intestine. Neither SVCT-1 nor SVCT-2 can transport DHAA; however, both transporters can act as Na + uniporters in the absence of AA, allowing Na + to enter cells. DHAA is absorbed via the glucose transporters GLUT1, GLUT3, and GLUT4, which are not capable of transporting AA.
Intestinal inflammation and prolonged infectious states are associated with decreased vitamin C absorption in both in-vitro and in-vivo models. Data suggest that pro-inflammatory states with elevated serum and intestinal mucosal levels of TNF-α inhibit AA transport and intestinal uptake. Observational data suggest that 35% of people can be deficient in vitamin C after Roux-en-Y gastric bypass for weight loss, but this is not a consistent finding when malabsorptive weight loss procedures are evaluated prospectively.
Confocal imaging using live human intestinal epithelial cells and human SVCT-1 fused to yellow fluorescent protein (hSVCT1-YFP) has shown that the hSVCT-1 protein is expressed at the apical membrane domain of these cells. The second transporter protein SVCT-2 appears to be expressed at the BLM domain of the polarized intestinal epithelial cells. The molecular determinants that influence targeting of the human Na + -dependent multivitamin transporter (hSMVT) protein to the BBM of intestinal epithelial cells are located in the cytoplasmic tail of the SMVT polypeptide. Mobility of these structures depends on the temperature and existence of an intact intracellular microtubule network. Studies involving co-localization, in-vitro knockdown, and in-vivo knockout (KO) approaches have also shown that Rab8a (a small recycling GTPase protein) is important for physiologic function and BBM delivery of hSVCT-1 in intestinal epithelial cells.
A variety of extracellular and intracellular factors and conditions regulate intestinal uptake of ascorbate; thus changes in extracellular ascorbate levels result in intestinal adaptation whereby AA supplementation leads to down-regulation in intestinal AA absorption associated with a decreased expression of hSVCT-1 mRNA. Other studies have used senescence marker protein-30/gluconolactonase KO mice, which are incapable of synthesizing AA in vivo to demonstrate that feeding a vitamin C-deficient diet leads to induction of SVCT-1 mRNA expression in the intestine.
Biotin is a carboxyl carrier and cofactor for 5 carboxylase enzymes, which are involved in essential pathways of intermediate metabolism (e.g., fatty acid synthesis, β-oxidation, gluconeogenesis, catabolism of odd-carbon fatty acids and branched-chain amino acids). It also plays a role in regulating gene expression, intracellular cyclic guanosine monophosphate levels, immune function, and cell proliferation. Biotin deficiency leads to growth retardation, neurologic disorders, and dermatologic abnormalities. Animal studies have shown that biotin deficiency during pregnancy may lead to growth retardation, congenital malformations, and death. Deficiency and low levels of biotin have been reported in several conditions, including inborn errors of biotin metabolism, inflammatory bowel diseases, long-term therapy with anticonvulsant drugs, long-term parenteral nutrition, and chronic alcohol use disorder.
Biotin is obtained from dietary sources and is also produced by colonic microbiota. Biotin is found in many foods, although at a lower concentration than other water-soluble vitamins. Good sources of biotin include egg yolks, liver, nuts, legumes, and vegetables such as cauliflower. Precise data on biotin requirements in humans are not available, but the recommended safe and adequate daily oral intake of biotin in adults is estimated to be between 30 and 35 μg/day.
Dietary biotin exists in free and protein-bound forms; the latter cannot be absorbed and must first undergo digestion by intestinal proteases and peptidases to biocytin (biotinyl- l -lysine) and biotin-short peptides, which are then converted to free biotin by the enzyme biotinidase. Human biotinidase has been cloned, and a number of clinical mutations have been identified in patients with biotinidase deficiency. Those affected by this autosomal recessive disorder display seizures, vision problems, alopecia, developmental delay, and hearing loss. These individuals cannot use dietary protein-bound biotin owing to the fact that they are unable to convert biocytin and biotin-short peptides to absorbable free biotin. Biotinidase deficiency also precludes the recycling of endogenous biocytin and biotin-short peptides, which arise from catabolism of cellular protein-bound biotin to free biotin. Pharmacologic supplementation with high doses of free biotin improves clinical outcomes in patients affected by biotinidase deficiency.
Free biotin is negatively charged at physiologic pH and is absorbed in the intestine via a carrier-mediated Na + -dependent process, which is most active in the proximal small intestine. Functional, immunologic, and confocal imaging studies have localized the Na + -dependent carrier-mediated system to the apical BBM domain of the polarized intestinal epithelial cells ( Fig. 103.2 ). Biotin exits enterocytes across the BLM via a Na + independent carrier-mediated process. Of note, the Na + -dependent process through which biotin is absorbed also transports 2 other functionally unrelated micronutrients: pantothenic acid and lipoate, which is a potent intracellular and extracellular antioxidant. This uptake system is therefore referred to as the SMVT.
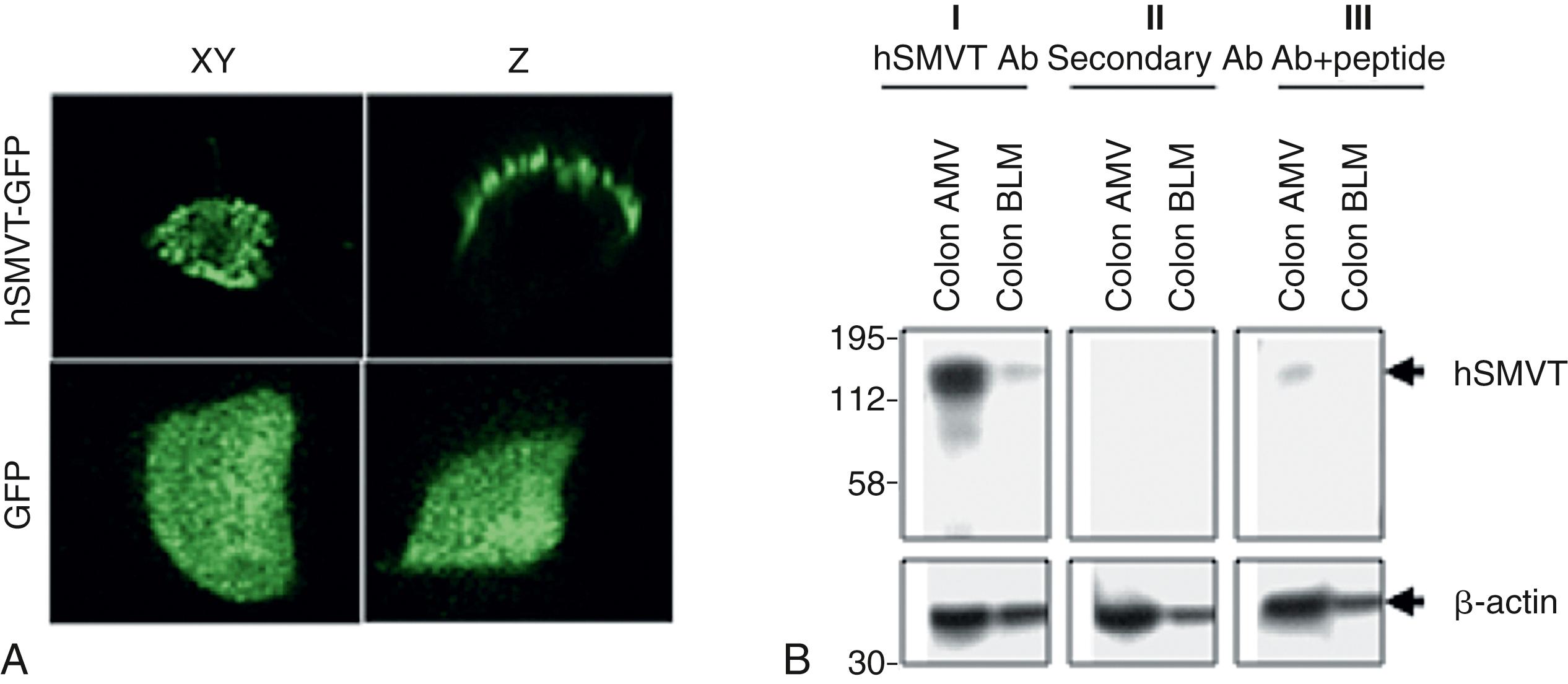
The normal colonic microbiota generates biotin predominantly in the free form, which is readily available for absorption through colonic epithelium via the SMVT. The combination of this efficient carrier-mediated process for biotin uptake in the colon and the significant duration that luminal contents remain in that region suggest that microbiota-generated biotin contributes to host biotin, and especially to the local needs of colonocytes.
The SMVT system is expressed throughout the intestinal tract. It has been cloned in several species, including humans, and its functionality has been characterized following expression in a number of cellular systems. SMVT encodes a protein of 635 amino acids that is predicted to have 12 transmembrane domains (TMD) and is N-glycosylated at positions Asn 138 and Asn 489. Glycosylation appears to be important for the function of hSMVT. In addition, a number of potential phosphorylation sites were predicted in the SMVT polypeptide, of which Thr286 has been experimentally shown to be involved in mediating the protein kinase C (PKC)-mediated regulation of intestinal biotin uptake.
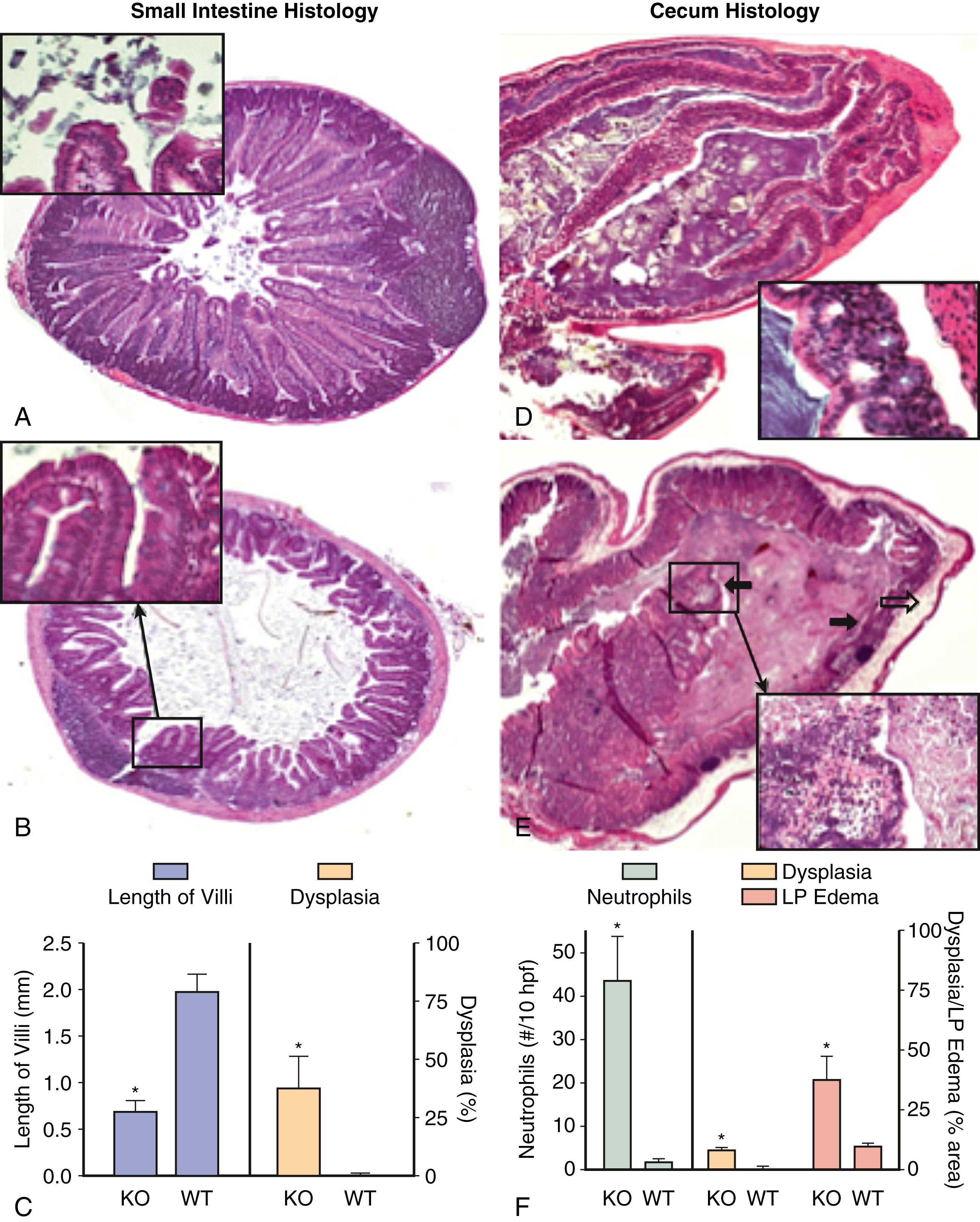
It has been postulated that there is an alternative biotin uptake system that functions in cells outside of the GI tract, (e.g., peripheral blood mononuclear cells, keratinocytes); however, experiments in an intestinal-specific (conditional) SMVT KO mouse model suggest that the SMVT is the only biotin uptake system that operates in the intestine. In these studies, complete inhibition of intestinal biotin and pantothenic acid uptake was observed in the KO mice compared with their sex-matched wild-type littermates. Interesting phenotypes were also observed in these KO animals. First, two thirds of the animals died before reaching the age of 2.5 months owing to acute peritonitis. Second, all KO mice, which had decreased biotin levels compared with same-sex control littermates, showed severe growth retardation and decreased bone density and length. Third, the KO mice had evidence of shortened and dysplastic villi in the small intestine and chronic active inflammation with dysplasia in the colon ( Fig. 103.3 ). The mechanisms mediating the intestinal inflammation seen in the KO mice are unclear, but may be related to the role played by biotin in maintaining normal innate and adaptive immune functions. For example, biotin is essential for activity of intestinal natural killer cells, which play an important role in maintaining intestinal epithelial homeostasis and promoting antipathogen response. Activity of these cells and biotin levels are both decreased in patients with Crohn disease (see Chapter 115 ).
The SMVT system is exclusively expressed at the apical membrane domain of polarized enterocytes, and the molecular determinants that dictate the targeting of the hSMVT protein to the apical membrane are located in the cytoplasmic tail of the SMVT polypeptide. Distinct trafficking vesicles are involved in the intracellular movement of the hSMVT protein, and this process requires an intact microtubule network.
Studies have identified a PDZ-containing protein, namely PDZD11, that interacts with hSMVT at a sequence in the cytoplasmic tail of the latter ( Fig. 103.4 ); this interaction influences the function and cell biology of hSMVT. Co-expression of PDZD11 with hSMVT leads to an increase in biotin uptake, whereas knocking down PDZD11 leads to an inhibition in uptake.

A variety of extracellular and intracellular factors regulate the intestinal uptake of biotin. For example, the SLC5A6 gene encodes SMVT in human enterocytes. and intestinal uptake is adaptively regulated by extracellular biotin availability. Biotin deficiency leads to a specific and significant up-regulation in intestinal carrier-mediated biotin uptake through increased transcriptional activity of the SLC5A6 gene, which induces expression of the hSMVT protein and mRNA. Studies have identified the region in the SLC5A6 promoter that responds to biotin deficiency as a 103-bp stretch that contains the cis-element, gut-enriched, Kruppel-like factor. Mutational analysis studies have established the role of the gut-enriched, Kruppel-like factor site in mediating the upregulatory response seen in biotin deficiency.
In early life, animal studies have shown that the preferential site of biotin uptake during the suckling period is the ileum and with maturation it transitions to the jejunum. This alteration is associated with parallel changes in the transcriptional activity of the SLC5A6 gene and expression of the SMVT protein and mRNA.
Finally, intestinal biotin absorption is under the regulation of an intracellular PKC-mediated pathway that appears to affect activity and/or number of carriers, thereby influencing the V max of the uptake process. The PKC appears to act through Thr286 of the hSMVT polypeptide; mutation of this phosphorylation site leads to a significant reduction in the PKC-mediated effect on hSMVT function.
Lower plasma biotin levels are seen in people with chronic alcohol use disorder. Animal models that are chronically fed alcohol have shown that this results, at least in part, from inhibition of intestinal biotin absorption. This decreased biotin uptake is associated with a significant reduction in expressed SMVT protein, mRNA, and heterogenous nuclear RNA levels, as well as a decrease in the activity of the SLC5A6 . Chronic alcohol feeding also caused a significant inhibition of biotin uptake in the colon, which would limit the absorption of bacterially generated biotin. Similar findings were observed in studies using human intestinal epithelial cells that were chronically exposed to alcohol. Chronic alcohol use also inhibits renal biotin reabsorption via similar transcriptional mechanisms. Other drugs have been found to decrease intestinal biotin uptake, such as the anticonvulsant medications carbamazepine and primidone.
Cobalamin (Cbl, vitamin B 12 ) refers to cyanocobalamin and all other forms of vitamin B 12 that have biologic activity. Cbl in its active coenzyme forms (i.e., 5′-deoxy-adenosyl-Cbl [Ado-Cbl, the most abundant natural form of Cbl] and methyl-Cbl [Met-Cbl]) plays an essential role in the catabolism of fatty acids in mitochondria and in the conversion of homocysteine to methionine (and, hence, the production of S-adenosyl-methionine, the active methyl group donor) in the cytoplasm. Cbl deficiency leads to many conditions, including megaloblastic anemia, and, when prolonged, may lead to irreversible neurologic damage. Cbl deficiency is common and occurs in patients with restrictive diets, pernicious anemia (PA), atrophic gastritis, celiac disease, Crohn disease, and after significant bowel resection or surgery. In older adults, Cbl deficiency and suboptimal levels of Cbl are often caused by intestinal malabsorption. Cbl deficiency in patients who are capable of absorbing the vitamin can be supplemented with physiologic doses, whereas patients unable to absorb Cbl require lifelong therapeutic injections or high doses of the oral or nasal form of the vitamin. Mega doses of hydroxy-Cbl (OH-Cbl) are used to treat cyanide poisoning because cyanide displaces the hydroxyl group from OH-Cbl, leading to the formation of CN-Cbl, which is excreted in urine.
Cbl is found in animal-derived foods in which the vitamin was produced by intestinal bacteria, then absorbed and accumulated in the animal’s tissues. Rich sources of Cbl include meat, poultry, eggs, and dairy products. Food of plant origin is virtually devoid of Cbl; however, some nutritional yeast products contain vitamin B 12, as do fortified cereals. The RDA of Cbl is 2.4, 2.6, and 2.8 μg, respectively, for adults, during pregnancy, and during lactation.
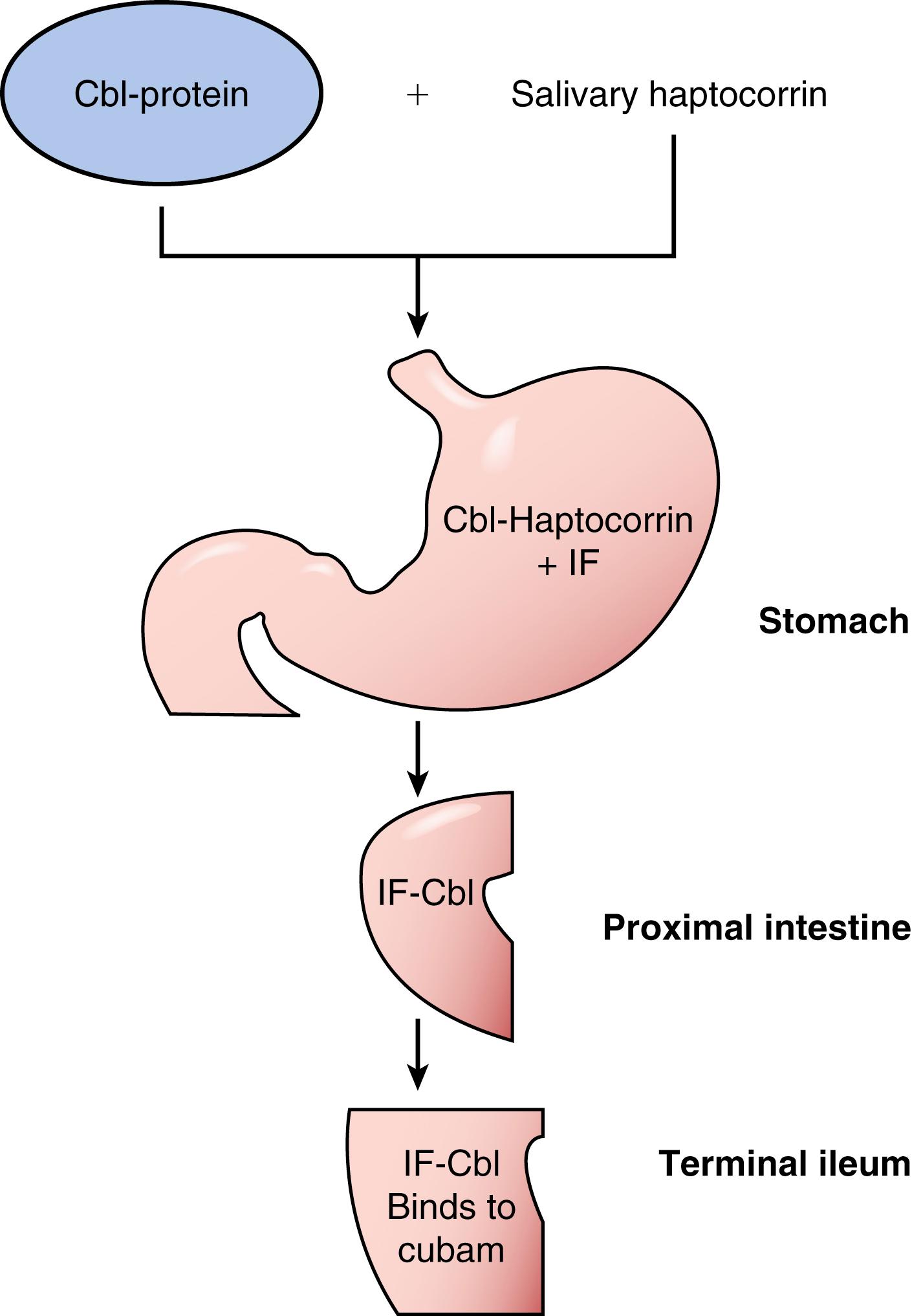
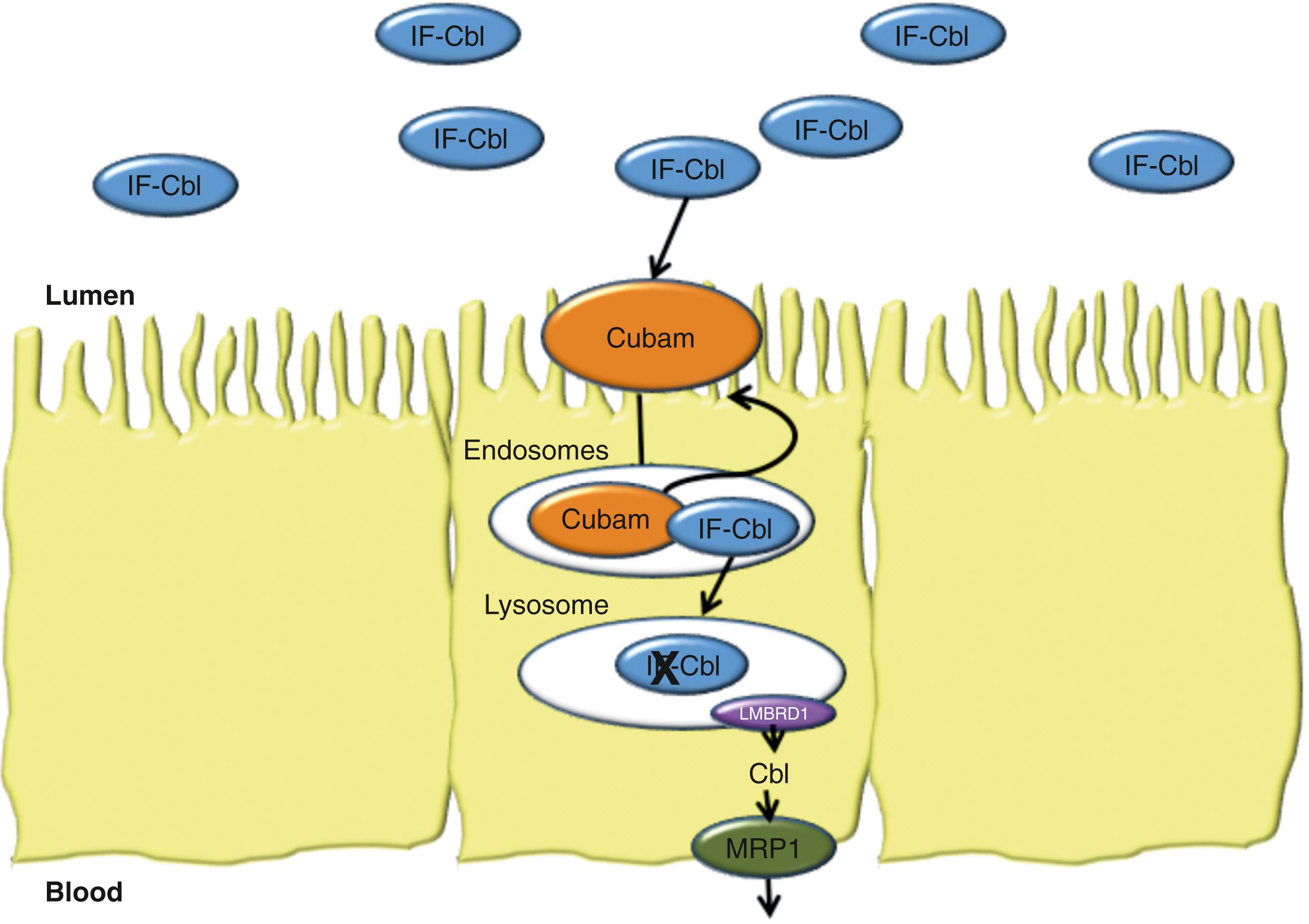
Dietary Cbl is bound to proteins, which is hydrolyzed to free Cbl during food preparation, mastication and by the proteolytic action of pepsin at the low pH of the gastric lumen ( Fig. 103.5 ). In the stomach, free Cbl and other inactive derivatives of the vitamin, called cobamides, bind to haptocorrin (rapid binder, HC), which is a glycoprotein synthesized predominantly by the salivary glands. The HC is resistant to digestion by gastric acid and pepsin and protects the Cbl molecule as it passes through the stomach. Cbl is released from HC in the upper small intestine by pancreatic trypsin and chymotrypsin, after which Cbl binds to intrinsic factor (IF) with high affinity (dissociation constant >10 −12 mol/L). IF is a glycoprotein that is synthesized by gastric parietal cells and is resistant to the effect of the digestive enzymes present in the upper GI tract. Cbl binds preferentially to IF over inactive cobalamins, thereby insuring selective absorption of the bioactive molecule. In the terminal ileum, the IF-bound Cbl (IF-Cbl) binds to cubam, a specific receptor located at the apical BBM domain of ileal enterocytes, which facilitates endocytosis of the entire complex ( Fig. 103.6 ). Following internalization, the cubam-IF-Cbl complex goes to endosomes where cubam is cleaved and recycled to the apical BBM. The IF-Cbl complex moves to the lysosomes where IF is degraded and the free Cbl is transported out of the lysosomes, likely via the lysosomal membrane protein LMBRD1. Cbl is then exported across the BLM via a process that involves the multidrug-resistance–associated protein-1 (see Fig. 103.6 ). Circulatory transcobalamin II carries Cbl to the liver for storage and use. Bacterial production of cobalamin analogues (cobamides), which deprives the host of usable B 12, and competitive interference with binding of IF-Cbl to cubam by these analogues are part of the explanation for vitamin B12 deficiency in SIBO (see Chapter 105) .
In humans, the IF gene is located on chromosome 11, and the protein has been cloned and characterized. IF is a 417-amino acid protein that may disintegrate into 2 moieties, a 30-kd N-terminal portion and a 20-kd C-terminal portion. Each of these moieties can recognize Cbl, but when they exist together in solution the joint moieties bind only to 1 Cbl molecule. A 20- to 50-amino acid sequence in the C-terminal portion of IF appears to be important for the high-affinity binding to Cbl. Also, the carbohydrate moiety on this glycoprotein plays a role in protecting IF against enzymatic degradation.
The IF receptor cubam is composed of 2 units: cubilin and amnionless. Cubilin is the unit that recognizes IF and is expressed in both ileal and renal proximal tubule epithelial cells. Cubulin consists of an N-terminal portion that does not span the membrane, followed by 8 epidermal growth factor repeats and 27 CUB domains. The N-terminal portion of cubilin is responsible for recognizing IF. Amnionless is a transmembrane protein that provides membrane anchorage and endocytic capacity via 2 sequence signals within its cytosolic domain (phenylalanine-X-asparagine-proline-X-phenylalanine FXNPXF). These signals promote internalization of the entire complex via binding to the clathrin-associated sorting protein disabled-2 (Dab2). Mutations in amnionless have been identified in patients with Imerslund-Gräsbeck disease, which is characterized by Cbl malabsorption and proteinuria with affected individuals clustered in Norway and some Mediterranean regions. Amnionless is also believed to play a role in delivering cubilin to the cell membrane.
Cbl absorption in the intestine is under limited regulation compared with other water-soluble vitamins. The process appears to be dependent on the capacity of cubam, the intestinal IF-Cbl receptor. After ingestion of 2 to 4 μg of vitamin B 12 , the intestine cannot absorb a second dose of the vitamin with equal efficiency until 4 to 6 hours later. In healthy adults, fluctuation in the normal level of IF appears to have little effect on Cbl absorption, because the daily output of IF (≈20 nmol) is markedly higher than the few nanomoles needed to absorb ingested Cbl. Additionally, thyroid hormone has been reported to have a role in regulating Cbl absorption by influencing the expression of cubilin.
PA is a well-recognized cause of Cbl malabsorption and deficiency. PA results from a lack of IF caused by autoimmune-mediated atrophic gastritis (see Chapter 52 ). PA is more common in older adults and those who suffer from other autoimmune disorders such as thyroid disease and type-1 diabetes. Other conditions associated with decreased levels of IF include patients who have undergone total gastrectomy, weight loss surgery, those with Helicobacter pylori infection, and those on long-term treatment with acid-reducing medications—usually PPIs.
Intestinal absorption of Cbl is also influenced by conditions that affect the cubam receptor. Inherited defects in the cubilin and amnionless units of this receptor are rare. Intestinal disorders like Crohn disease, especially when there has been ileal resection, are well-recognized causes of Cbl malabsorption.
Chronic or recurrent exposure to nitrous oxide (N 2 O), a commonly used inhaled anesthetic, may lead to oxidation of Cbl to an inactive form that can cause neurologic complications associated with Cbl deficiency, ranging from polyneuropathy to spinal cord degeneration.
A number of phenomena combine to make SIBO an important cause of vitamin B 12 (cobalamin) deficiency (see Chapter 105 ). These include consumption of cobalamin by anaerobes, bacterial production of cobalamin analogs called cobamides (with subsequent loss of the parent vitamin to the host), malabsorption of the vitamin at the ileal receptor as a result of competitive binding with cobamides, and, in instances of severe SIBO, mucosal injury that involves the cubulin-amnionless binding site.
Finally, chronic use of metformin in patients with diabetes reduces Cbl levels via malabsorption, owing to a reduction in the levels of haptocorrin.
Folate (vitamin B 9 ) refers to a group of 1-carbon derivatives of folic acid required for the synthesis of pyrimidine and purine nucleotides (precursors of DNA and RNA, respectively) and metabolism of several amino acids, including homocysteine and serine. Cellular deficiency of folate leads to impairment in 1-carbon metabolism, DNA synthesis and methylation, incorporation of uracil into DNA, and in the metabolism of several amino acids. Suboptimal levels of folate lead to several clinical abnormalities that include megaloblastic anemia, growth retardation, and neural tube defects in the developing embryo. Folate deficiency is highly prevalent and results from a variety of causes, including impairment of the intestinal folate uptake system (e.g., hereditary folate malabsorption syndrome), intestinal diseases (e.g., celiac disease, tropical sprue), drug interactions (e.g., sulfasalazine, trimethoprim, pyrimethamine, diphenylhydantoin), and chronic alcohol use.
The human intestine is exposed to dietary folate and bacterial folate, which is generated by the normal colonic microbiota. Folate is widely distributed in food, and rich sources include green vegetables, liver, beans, and lentils. Since 1998, cereal products in the US and several other countries have been supplemented with folate and also represent a good source of the vitamin. The RDA for folate is 400 μg, which is the same dose recommended for women of child-bearing age to reduce the incidence of neural tube defects.
Dietary folates exist in the free (i.e., folate monoglutamate) and predominant polyglutamate form. Conjugated folate polyglutamates cannot be absorbed because of their size and multiple negative charges. Prior to absorption, they must undergo hydrolysis to free folate by the enzyme folylpoly-γ-glutamate carboxypeptidase, which is also called folate hydrolase . There are 2 forms of this enzyme in intestinal absorptive cells: the first is expressed at the BBM domain of the cells, and the other is intracellular (localized in lysosomes). The BBM form of folate hydrolase is expressed mainly in the proximal part of the small intestine, and the intracellular form is expressed uniformly along the length of the small intestine. The membrane form of the enzyme has been cloned, and its activity appears to be adaptively upregulated in folate deficiency and during development.
Intestinal absorption of the negatively charged dietary folate (pKa values of the α and γ carboxyl groups of the folate molecule are 3.5 and 4.8, respectively) occurs in the proximal half of the small intestine and involves a specific pH- (but not Na + -) dependent carrier-mediated process (see Fig. 103.1 ). In animal studies, resection of the proximal small intestine leads to a marked induction of carrier-mediated folate uptake in the ileum. The intestinal folate uptake process has similar affinities for reduced (e.g., 5-methyltetrahydrofolate, 5-formyltetrahydrofolate), oxidized (e.g., folic acid), and substituted (e.g., methotrexate) folate derivatives. Absorbed folate leaves the enterocytes across the BLM via another carrier-mediated process.
A substantial portion of the folate synthesized by the normal microbiota exists in the absorbable free form, and the large intestine is capable of absorbing free folate via a highly efficient and specific carrier-mediated mechanism. Interferences with this mechanism may contribute to the development of localized folate deficiency and premalignant changes in colonic mucosa.
Three specific systems are known to transport folate in mammalian cells: the transmembrane reduced folate carrier (RFC, the product of the SLC19A1 gene); the transmembrane proton-coupled folate transporter (PCFT, the product of the SLC46A1 gene); and the membrane-anchored (via a glycosyl phosphatidyl-inositol linkage) folate receptor. The first 2 systems (RFC and PCFT) are both expressed and functional in the intestinal tract, but the third is neither expressed nor functional under normal physiologic conditions.
The human RFC (hRFC) operates optimally at a pH of 7.0 to 7.4. The hRFC polypeptide consists of 591 amino acids, and shares a high degree of sequence homology with the RFC system of other mammals (e.g., rodents, chimpanzees). RFC functions as an anion exchanger and moves the negatively-charged folate molecule against its concentration gradient in exchange for downhill movement of an anion. RFC mRNA is expressed along the length of the intestinal tract, with expression of the RFC protein at the apical membrane domain of the polarized small intestinal and colonic epithelia.
The human PCFT (hPCFT) system operates optimally at acidic pH (5.8 to 6.0). The hPCFT polypeptide consists of 459 amino acids and is predicted to form 12 TMD. The PCFT functions electrogenically as a folate − :H + symporter, transporting folate by the net movement of a positive charge (see Chapter 101 ). Folate is transported by this system against its concentration gradient using the energy generated by the downhill movement of protons. The inwardly directed proton gradient is provided by the intestinal surface acid microclimate, which has a pH of 5.8 to 6.0 at the proximal small intestine. The hPCFT mRNA is expressed mainly in the proximal small intestine, with less expression in the distal small intestine and the colon. Expression of hPCFT is restricted to the apical membrane domain of intestinal epithelial cells.
Both the PCFT and RFC systems contribute to overall intestinal assimilation of exogenous folates. PCFT appears to be the predominant folate uptake system in the proximal small intestine, where the pH at the luminal surface is approximately 5.8 to 6.0. This belief is supported by the identification of individuals with hereditary folate malabsorption syndrome, a condition caused by mutations in hPCFT. The hRFC system operates in the distal small intestine and colon, where the pH at the luminal surface is near neutral.
The folate transport system at the BLM domain of the intestinal absorptive epithelial cells is believed to be a member(s) of the MRPs.
In recent years, knowledge of the structural features of the hPCFT and hRFC systems that are important for their function has evolved. The conserved histidine residues located at positions 247 and 281 of the hPCFT polypeptide appear to be critical for function of the transporter. In addition, clinical mutations identified in hPCFT in patients with hereditary folate malabsorption syndrome have implicated a role for the amino acid residues located at positions 65, 66, 113, 147, 318, 376, and 425 of the polypeptide. These mutations were shown to lead to a spectrum of consequences that include an early stop codon and a frame shift (both of which lead to absence of hPCFT protein), or to a defective intracellular trafficking/membrane targeting of the protein and/or protein instability.
Amino acid residues located at positions 45, 46, 104, 105, 127, 130, 297, and 309 as well as the intracellular loop between transmembrane 6 and 7 of the polypeptide appear to be important for the function of RFC.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here