Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
This work was supported by National Institutes of Health grant CA190710 and Welch Endowed Chair in Biochemistry, Grant No. BI-0028, at Texas Tech University Health Sciences Center.
Digestion and absorption of dietary nutrients constitute the primary physiologic function of the GI tract; this includes not only the 3 major nutrients, namely carbohydrates, proteins, and fat, but also the micronutrients, (i.e., vitamins [water-soluble and lipid-soluble], electrolytes, minerals). Some of these dietary constituents are obligatorily dependent on digestion as a prerequisite for absorption whereas others do not have this requirement. The need for digestion is dictated by the substrate selectivity of the transport processes involved in absorption. For example, there are no transport mechanisms in mammalian intestine for absorption of disaccharides and polysaccharides; only monosaccharides can be absorbed. However, dietary carbohydrates primarily consist of polysaccharides and disaccharides; until and unless these are broken down by digestion to monosaccharides, absorption cannot occur. The same is true with proteins. Transport systems in the intestine accept only free amino acids (AAs) or small peptides consisting of 2 or 3 AAs as substrates; larger peptides are excluded. This necessitates the digestion of dietary proteins to AAs, and di- and tri-peptides prior to absorption. In a similar manner, dietary fat, which consists primarily of triglycerides, are broken down to monoglycerides as a prerequisite for absorption. In contrast, many of the water-soluble and lipid-soluble vitamins in normal diet are absorbed as such without the need for prior digestion, but even here, there are exceptions. Some of the vitamins in the diet are covalently bound to proteins (e.g., biotin, vitamin B 12 ) and unless these vitamins are released in free form by digestion, absorption cannot occur.
Digestion is mostly an enzymatic process mediated by several classes of enzymes, which includes carbohydrases, proteases and peptidases, and lipases, phospholipases, and esterases. However, in some cases, for example, the digestion of dietary fat, the breakdown process is facilitated by physical and mechanical events, such as forceful mixing and detergent (bile salt)-assisted dispersion to promote accessibility of the enzymes to their substrates. Salivary and gastric secretions contain some of the digestive enzymes, but the most important among these enzymes come from pancreatic secretion. Bile salts enter the intestine via bile from the liver. The enzymes present in these secretions are responsible for luminal digestion of dietary carbohydrates, proteins, and fat, which occurs in the intraluminal fluid of the GI tract. In addition to these enzymes in various secretions, there are others that are associated with the apical membrane of the absorptive cells of the small intestine (enterocytes) that also participate in the digestive process. These are integral membrane proteins with their catalytic site exposed to the luminal surface of the apical membrane such that they have access to their substrates in the intestinal lumen to catalyze the digestive process. As this process occurs on the surface of the apical membrane, it is called “membrane digestion” to differentiate it from the “luminal digestion” that is mediated in the intraluminal fluid of the GI tract by the enzymes present in gastric and pancreatic secretions.
Even though some digestion occurs in the mouth and stomach prior to the entry of dietary constituents into the intestinal tract, the bulk of digestion and almost all absorption take place in the small intestine. Enterocytes, which constitute the absorptive cells of the small intestine, are polarized with a part of their plasma membrane facing the intestinal lumen and the rest facing the portal circulation. Accordingly, the portion of the membrane facing the lumen is called apical membrane or brush-border membrane (BBM) because of its unique morphology resembling a brush; the rest of the plasma membrane is known as the basolateral membrane (BLM), consisting of the basal membrane and the 2 lateral membranes of the cell. Despite the fact that both the BBM and the BLM make up the plasma membrane of the enterocytes, they differ markedly in protein composition. Various enzymes and transporters are trafficked and recruited to these 2 membranes differentially to enable the digestive process to occur solely on the luminal side and the absorption of the dietary nutrients to occur vectorially from the lumen into blood or lymph. In addition to this functional polarization of the enterocytes that is conducive for optimal digestion and absorption of dietary nutrients, the structure of the small intestine at the macro and micro level also contributes to this process. The mucosal surface of the small intestine is arranged in large folds, which are valvular flaps projecting into the intestinal lumen; these are called Kerckring folds or plicae circulares. These folds are further arranged in the form of villi, the external surface of which is lined by differentiated epithelial cells of various functional types (enterocytes, enteroendocrine cells, enterochromaffin cells, Paneth cells, Goblet cells, and stem cells); enterocytes form the majority of these cells and are located preferentially from the middle to the tip of the villi. Paneth cells and stem cells are present exclusively in the crypts of the villi whereas the enteroendocrine cells, enterochromaffin cells, and Goblet cells are distributed sporadically in the upper two thirds of the villi. The apical membrane of the enterocytes is arranged in a brush-like structure, often referred to as microvilli, the purpose of which is to increase the surface area of the apical membrane; together, the Kerckring folds, villi, and microvilli increase the surface area several fold. In humans, the surface area of the small intestine is approximately 250 square meters (the size of a tennis court!). This unique structure enhances the capability of the small intestine for maximal digestion and absorption of dietary nutrients.
The core of each villus contains blood vessels, lymphatic vessels, and immune cells. The terminal branches of the mesenteric artery bring oxygenated blood to the villi, the oxygen is extracted at the capillary level, and the draining venules ultimately join together to form the portal vein. All nutrients that are water-soluble (AAs, small peptides, monosaccharides, water-soluble vitamins, and electrolytes/minerals) are absorbed into the portal circulation, which feeds into the liver before joining the systemic circulation. Thus, the nutrients entering the portal blood are made available first to the liver for extraction and whatever remains is then made available to other organs. In contrast, the lipid-soluble nutrients (constituents of dietary fat and also fat-soluble vitamins) are absorbed into lymphatic vessels and thus enter the thoracic duct, which then empties into the left subclavian vein. Hence, lipid-soluble nutrients absorbed in the small intestine do not go to the liver first; instead, they enter the systemic circulation with no preferential exposure to the liver.
Most nutrients are absorbed very efficiently. In adults on a normal diet, less than 5% of the dietary carbohydrates, fats, and proteins is excreted in the feces. In neonates and premature infants, however, this process is significantly less efficient. The fraction of carbohydrates that is not digested and absorbed in the small intestine and thus enters the colon, is digested and fermented by bacteria to generate short-chain fatty acids (SCFAs), which are then absorbed for utilization in normal cellular metabolism in the colon and other organs. Similarly, dietary proteins that enter the colon are also digested and metabolized by bacteria; the resulting AAs as well as amino-acid-derived metabolites are absorbed in the colon. The SCFAs and other metabolites generated by colonic bacteria not only contribute to the nutrition of the colonic epithelial cells but also affect the physiology and function of cell types and organs distanced from the intestinal tract. These bacterial products are the principal mediators whereby the intestinal microbiome influences the function of the liver (gut-liver axis), pancreas (gut-pancreas axis), and brain (gut-brain axis). Colonic bacteria also modify the bile acids secreted by the liver. Even though ∼95% of bile acids that enter the small intestine is absorbed in the ileum as a part of the enterohepatic circulation, the ∼5% of the bile acids that enter the colon are chemically modified by bacteria (see Chapter 64 ). First, the conjugated bile acids (i.e., the glycine-linked and the taurine-linked bile acids) are de-conjugated; second, the hydroxyl group at position 7 is removed to convert cholic acid into deoxycholic acid, and chenodeoxycholic acid into lithocholic acid. These bacterially modified bile acids enter the portal circulation by diffusion and are taken up by the liver for subsequent secretion into bile. Thus, normal bile contains cholic acid, chenodeoxycholic acid, deoxycholic acid, and lithocholic acid, but only the first 2 are synthesized by the liver whereas the other 2 are produced by colonic bacteria via chemical modification of the first two. The 2 bile acids produced by the liver (cholic acid and chenodeoxycholic acid) are called primary bile acids, whereas the other 2 generated in the colon (deoxycholic acid and lithocholic acid) are called secondary bile acids. Therefore, even though we generally highlight the role of the small intestine as the major site of digestion and absorption of dietary carbohydrate, protein, and fat, the role of the colon and the intestinal bacteria in this process should not be overlooked.
As the digestion of dietary carbohydrate, protein, and fat is a complex process involving multiple organs, the activity and function of these organs have to be coordinated and integrated to optimize the process. The intestinal tract has to be ready when the dietary components arrive in the form of chyme from the stomach; this includes the priming of the intestine with secretions from the pancreas and liver, secretions that provide not only the enzymes and bile salts necessary for the digestive process, but also bicarbonate to neutralize the acidic chyme from the stomach. Salivary and gastric secretion is initiated with the cephalic phase, triggered by the sight, smell, and even thought of food; this phase is mediated by the autonomic nervous system. The presence of nutrients in the GI tract then provides additional signals for neuroendocrine mechanisms to control digestion and food intake (satiety and appetite). The parasympathetic innervation of the GI tract and pancreas, provided by the vagus nerve, is the principal component in this regulatory process, with the involvement of both afferent (sensory) and efferent (motor) pathways (see Chapter 99 ). The stomach and the upper intestinal tract possess dense parasympathetic innervation, which decreases gradually downstream along the intestinal tract. The mechanoreceptors present in vagal afferent fibers are activated by gastric distension, sending signals to the brain with regard to meal size. Chemo-sensitive vagal afferent fibers respond to many of the GI hormones (CCK, glucagon-like polypeptide-1 [GLP-1], peptide tyrosine tyrosine, [peptide YY PYY], ghrelin, and serotonin) via their respective cell-surface receptors. As dietary components as well as bacterial metabolites influence the secretion of many of these hormones from the enteroendocrine cells (e.g., glucagon-like polypeptide-1, peptide tyrosine tyrosine) and enterochromaffin cells (serotonin) of the small intestine and colon, signaling via the vagus nerve provides 1 of the pathways for the gut-brain axis.
Even though salivary secretion contains digestive enzymes (e.g., α-amylase), it has no significant role in the digestive process because the food is chewed and swallowed rapidly and the contents enter the stomach where the acidic pH of gastric secretion fails to provide optimal conditions for the activity of these salivary enzymes. The stomach also secretes enzymes such as pepsin and lipase; however, these enzymes possess an optimal pH in the 4 to 5 range, which is appropriate for the acidic conditions of the luminal fluid in the stomach. When the chyme enters the duodenum, it introduces partially digested dietary carbohydrate, protein, and fat and also high concentrations of proton (acid pH) to the mucosal surface. The duodenum contains specific enteroendocrine cells, which respond to these components in chyme and secrete hormones that affect the secretory and contractile functions of stomach, pancreas, bile duct, gallbladder, and sphincter of Oddi. Secretin is released from duodenal and jejunal S cells (a subtype of enteroendocrine cell) in response to acidic pH. This hormone acts on parietal cells in the stomach to reduce acid production and acts on ductal cells in the pancreas and biliary tract to stimulate bicarbonate secretion. With these actions, secretin reduces the acid load from the stomach and also delivers bicarbonate to the duodenum via the bile and pancreatic ducts to neutralize gastric acid. The net result is to bring the chyme in the duodenum to neutral pH so that the pancreatic digestive enzymes, all of which work optimally only in the neutral pH range, are able to perform their task in the intestinal lumen. CCK is released from I cells, another subtype of enteroendocrine cell in the duodenum, in response to partially digested proteins and fat. This hormone has multiple actions. First, it acts on the pancreatic acinar cells to promote the release of digestive enzymes. This effect of CCK on the pancreas is not direct because the acinar cells in humans do not possess cell-surface receptors for CCK. The mechanism involves activation of the vagus nerve through a CCK receptor, with subsequent release of acetylcholine, which then acts on the acinar cells via muscarinic receptor signaling. This is supported by the findings that atropine, an antagonist of the muscarinic receptor, blocks the effect of CCK on pancreatic secretion; this is a paracrine action of CCK on sensory vagal afferent fibers projecting from the upper small intestine into the pancreas. In addition, CCK might have other modes of action on pancreatic secretion via activation of vagal efferent fibers coming down from the higher brain centers and projecting into the pancreas ; this mechanism also involves atropine-sensitive muscarinic receptor signaling. Second, CCK initiates gallbladder contraction to promote the release of bile and, at the same time, relaxes the sphincter of Oddi to allow the entry of bile from the bile duct into the duodenum; this function is critical for fat digestion. Although hepatocytes secrete bile continuously (∼∼500 mL/day), bile is not released continuously into the duodenum because the sphincter of Oddi remains mostly closed in between meals; as a result, bile is stored and concentrated in the gallbladder. When CCK is released in response to entry of the chyme into the duodenum, it simultaneously brings about contraction of the gallbladder and relaxation of the sphincter of Oddi, thus releasing the concentrated bile into the duodenum.
GI hormones, whose release into the circulation is triggered by digestive products of dietary proteins and fat, also play a role in satiety and appetite control. The hormones CCK, PYY, and GLP-1 serve as satiety signals to the brain to inhibit food intake. While CCK is produced by duodenal I cells, PYY and GLP-1 are produced by another subtype of enteroendocrine cells (L cells) present predominantly in the ileum and colon. There is a logic behind the differential distribution of the CCK-producing I cells in the upper small intestine and the PYY/GLP-1-producing L cells in the lower small intestine and in the colon, considering the signals that trigger the release of these hormones. CCK is released in response to partially digested proteins and fat; these signals are present at high concentrations only in the upper small intestine. Accordingly, the CCK-positive I cells are located primarily in the duodenum and upper jejunum. The stimulatory signals for the release of PYY and GLP-1 are tryptophan, long-chain fatty acids (LCFAs) and their derivatives, SCFAs, and secondary bile acids. These signaling compounds arise either as the end products of digestion or as the products of bacterial fermentation/metabolism, and hence are expected to be found predominantly in the lower small intestine and in the colon. G-protein-coupled receptors mediate the effects of these compounds on L cells to promote the secretion of GLP-1 and PYY: GPR142 for tryptophan, GPR40 and GPR120 for LCFAs GPR119 for derivatives of the monounsaturated fatty acid oleic acid, GPR41 (also known as free fatty acid receptor 3 or FFAR3) and GPR43 (also known as free fatty acid receptor 2 or FFAR2) for SCFAs, and TGR5 for secondary bile acids. The fatty acid translocase CD36, a G-protein-independent cell-surface protein, is also involved in the effects of LCFAs on L cells. The colon contains a high density of L cells and the number of these cells significantly increases toward the colon. Therefore, it is physiologically relevant that the function of these cells is highly influenced by bacteria and bacterial metabolites. As GLP-1 and PYY elicit their functions not only on the pancreas but also on the brain, this provides a mechanistic insight into the gut-pancreas and gut-brain axes.
All 3 hormones, CCK, GLP-1, and PYY, secretion of which is stimulated by digestive products of dietary nutrients and bacterial fermentation/metabolism, suppress appetite. In fact, the only GI hormone that is known to induce appetite and promote food intake is ghrelin. The stomach is the principal source of ghrelin, secretion of which is induced by starvation and fasting and suppressed by feeding and obesity. In contrast to the regulatory mechanisms involved in the secretion of CCK, GLP-1, and PYY, the stimulatory signals for ghrelin secretion do not come from the lumen of the GI tract, but instead appear to originate from the blood. This is evidenced from the morphology of ghrelin-producing cells in the stomach; these cells are not open to the gastric lumen and thus are not exposed to luminal contents, but are in contact with blood. Circulating levels of glucose, which reflect the energy balance under most physiologic conditions, could be one of the signals that control ghrelin secretion. Excess glucose in blood has an inhibitory effect on ghrelin secretion, thus explaining the negative correlation between the fed state and the circulating levels of this hormone.
Many of the nutrients in the intestinal lumen derived from diet are water-soluble and hydrophilic; as such, these molecules cannot traverse the hydrophobic lipid bilayer of the BBM and the BLM of the absorptive cells of the intestinal tract. Specific transport proteins that are integral to the BBM and BLM function in the absorption of such nutrients across the intestinal and colonic epithelial cells. Most of these transport proteins and their genes have been identified at the molecular level. All of them belong to a superfamily of proteins known as SLCs (solute carriers), which are organized in different gene families based on homology in the primary structure of these proteins (see Chapter 101 ). For example, the transporter responsible for the first step in the absorption of glucose is known as the sodium-coupled glucose transporter 1 (SGLT1), which according to the Human Genome Organization Nomenclature describing the SLCs is identified as SLC5A1 (i.e., it is the first member of the SLC gene family 5A). As the classification is based on protein structure rather than on protein function, the transportable substrates for the members within a given gene family do not often belong to a similar group (e.g., AAs or sugars) or possess similar physico-chemical features (e.g., anions or cations or zwitterions). For example, members of the gene family 5A transport diverse substrates such as glucose (SLC5A1), iodide (SLC5A5), choline (SLC5A7), or SCFAs (SLC5A8).
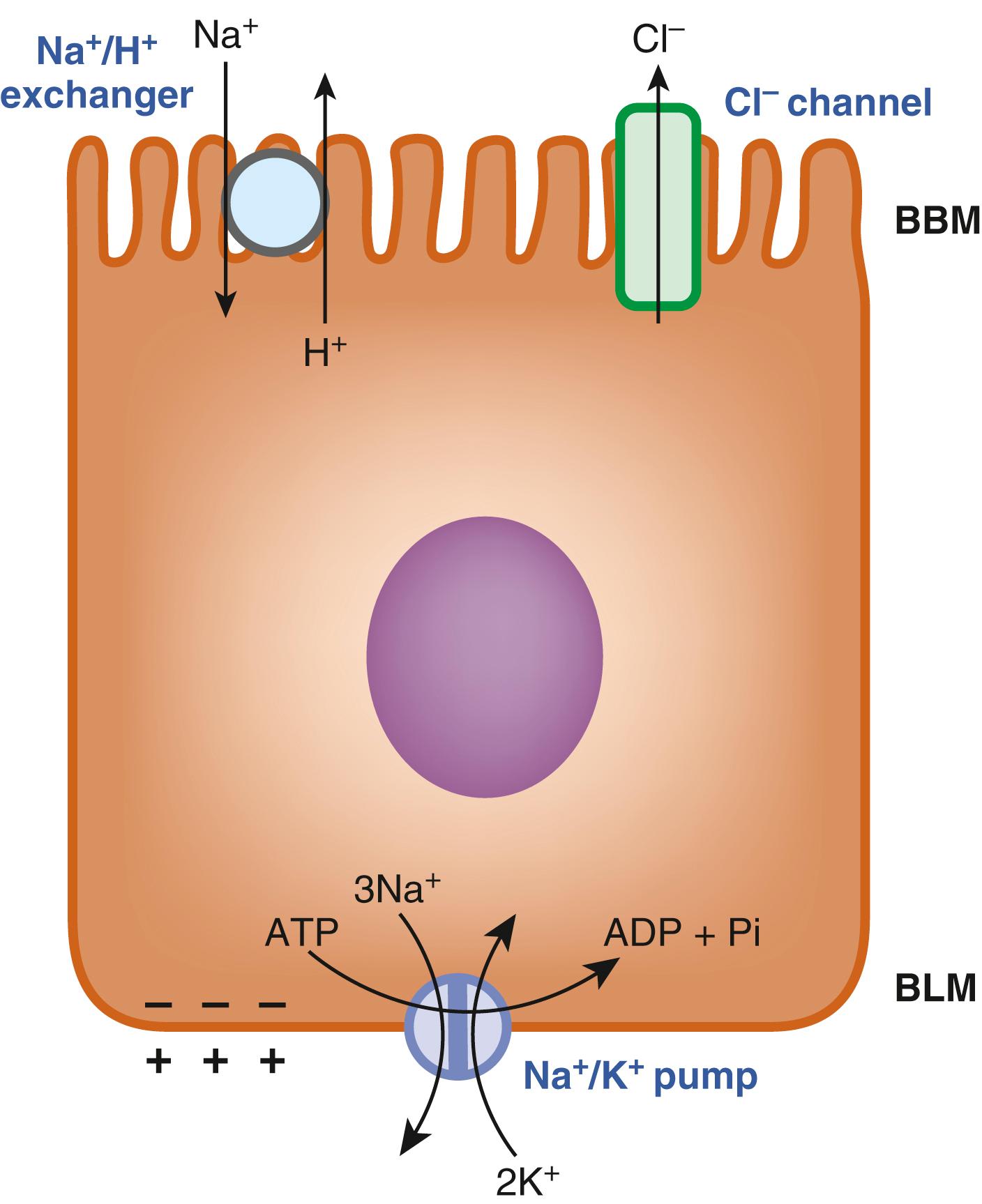
The nutrient transporters in the intestinal tract are grouped into 2 classes: active transporters and passive transporters. Active transporters are capable of accumulating their substrates in cells against a concentration gradient whereas passive transporters are only capable of transferring their substrates down a concentration gradient. Accordingly, active transporters require some form of driving force to support their uphill transport function; there is no involvement of any driving force for passive transporters. Five different driving forces operate in the absorptive cells of the intestinal tract to provide energy for various active transporters involved in nutrient absorption; these are (1) an inwardly directed Na + gradient; (2) an inwardly directed H + gradient; (3) an inwardly directed Cl − gradient; (4) an outwardly directed K + gradient; and (5) the membrane potential. These driving forces are generated via multiple mechanisms, all of which rely on the Na + /K + pump ( Fig. 102.1 ). As mentioned earlier, the absorptive cells of the intestinal tract are polarized. The Na + /K + pump is expressed only on the BLM of these cells; it transports Na + out of the cells and K + into the cells with a Na + :K + stoichiometry of 3:2, and the energy for this pump comes from ATP hydrolysis. The action of the Na + /K + pump in the intestinal and colonic epithelial cells creates a condition in which the intracellular concentration of Na + is lower than the extracellular concentration while the intracellular concentration of K + is higher than the extracellular concentration. The end result is an inwardly directed Na + gradient and an outwardly directed K + gradient across the BBM and BLM. In addition, the 3:2 stoichiometry for Na + and K + also generates an inside-negative membrane potential across these membranes. The Na + /H + exchanger, located in the BBM, then uses the inwardly directed Na + gradient to generate an inwardly directly H + gradient because the influx of Na + down its electrochemical gradient is coupled to the efflux of H + against its electrochemical gradient with a Na + :H + stoichiometry of 1:1. There are several isoforms of the Na + /H + exchanger; the isoform in the BBM is primarily NHE3 (SLC9A3). Even though the pH of the bulk fluid in the intestinal lumen is in the neutral range, the pH on the external surface of the BBM is relatively acidic (pH in the range of 5.5 to 6.0), and known as “microclimate acid pH,” the H + efflux mediated by the Na + /H + exchanger is responsible for this acidic pH. The chloride channel present in the BBM releases Cl − from the cells into the lumen in response to the inside-negative membrane potential, which generates the inwardly directed Cl − gradient across the membrane. This Cl − channel, mostly represented by CFTR, is expressed primarily in the BBM of the enterocytes present in the villus crypts. The various driving forces thus generated are then utilized by nutrient transporters in a differential manner to support their active transport function across the BBM, and in some instances, also across the BLM. In the BBM, the transfer of glucose and galactose is coupled to the electrochemical Na + gradient; the transfer of Fe 2+ , folate, and di- and tripeptides is coupled to the electrochemical H + gradient; the transfer of glutamate and aspartate involves the transmembrane gradients of Na + , K + , and H + as well as the membrane potential. Most AAs are transported with the involvement of the electrochemical Na + or H + gradient, although there are some important exceptions.
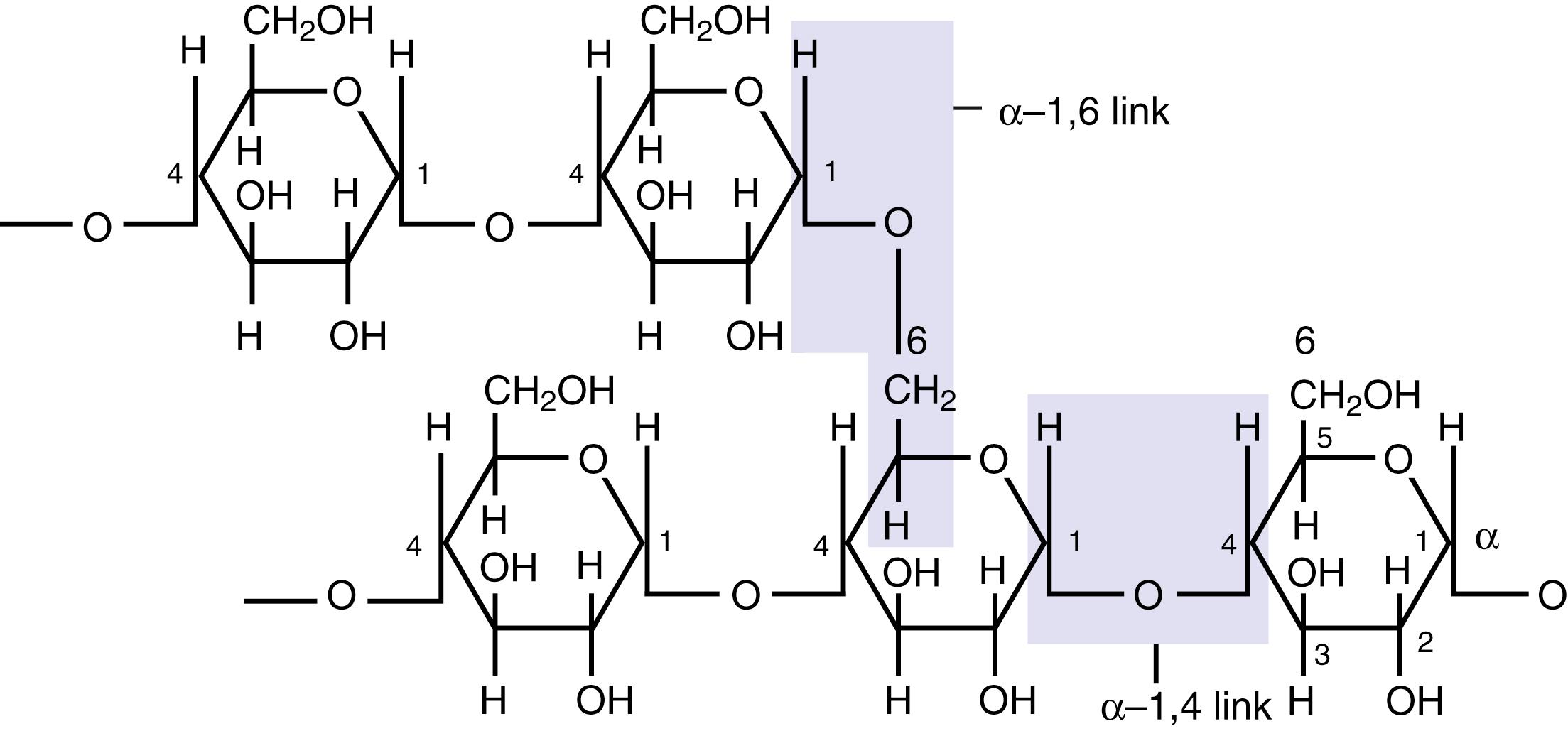
The total amount of carbohydrate in a normal diet is 220 to 330 g/day for men and 180 to 230 g/day for women. Dietary carbohydrate exists in different molecular forms: polysaccharides, disaccharides, and monosaccharides. Starch from plant products and glycogen from meat are polysaccharides. Even though both are homopolymers consisting of only glucose, starch and glycogen differ in structure. Starch exists in 2 forms, namely amylose and amylopectin. Amylose is a linear polysaccharide in which glucose residues are linked solely by α-1,4 glycosidic bonds. In contrast, amylopectin is a branched molecule in which the branch points consist of α-1,6 glycosidic bonds while the linear portions of the branches are made up of α-1,4 bond as in amylose ( Fig. 102.2 ) Glycogen, in contrast, exists only as a branched molecule similar to amylopectin, except that it has more branches than amylopectin. Together, starch and glycogen make up approximately 50% of the carbohydrate content in a normal diet. The next quantitatively important carbohydrates are the disaccharides sucrose and lactose, which account for 30% to 40% of dietary carbohydrates. Sucrose is the commonly used sugar and is made up of glucose (α-form) and fructose (β-form), linked via their anomeric carbon atoms (carbon 1 in glucose and carbon 2 in fructose). Lactose is the milk sugar and is made up of galactose (β-form) and glucose (α- or β-form), linked via carbon 1 of galactose and carbon 4 of glucose. The monosaccharide fructose makes up the remainder of dietary carbohydrate (∼10%) and is found in fruit juices, honey, and soft drinks (high-fructose corn syrup). Mushrooms contain the disaccharide trehalose, which consists of 2 α-glucose residues bonded by their anomeric carbon atoms.
In addition to the aforementioned carbohydrates, diet also contains carbohydrates in the form of fiber, which is neither digestible nor absorbable by the human intestine. Fiber includes cellulose, hemicellulose, gums, pectins, and chitin, all derived from plant sources. Cellulose is a linear polysaccharide consisting of glucose linked together via β-1,4 linkage; hemicellulose is also a polysaccharide but consisting of several types of sugars and sugar derivatives; gums too are made up of a variety of sugars; pectins are heteropolysaccharides and are rich in galacturonic acid; chitin is a polysaccharide consisting of the glucose derivative N-acetylglucosamine. These indigestible carbohydrates, however, still provide significant health benefits by various mechanisms: (1) they increase the bulkiness of the luminal contents in the intestinal tract, thereby influencing transit time; (2) they affect the rate at which other components of the diet are digested and absorbed; (3) they pass through the small intestine undigested and when they reach the colon, bacteria are able to digest and ferment them to generate SCFAs, which are then absorbed for metabolic utilization in colonocytes or enter the portal circulation to be presented to the liver and then to other organs. These bacterial metabolites also elicit a multitude of biologic actions on colonic epithelial cells, enteroendocrine cells of the colon, and immune cells in the lamina propria via different mechanisms including the involvement of specific cell-surface G protein-coupled receptors.
Glycemic index provides an assessment of the carbohydrate content of a food in terms of its effect on postprandial glucose levels in the blood. The glycemic index is measured as the area under the curve for blood glucose above the basal level over a period of 2 hours after ingestion of the food containing a fixed amount of carbohydrate (e.g., 50 g) compared with ingestion of the same amount of glucose (reference standard). The glycemic index for the reference is taken as 100. Carbohydrate-containing foods with a glycemic index of 55 or less are considered “good,” and those with a glycemic index of 70 or more are considered “bad.” The ability to control blood glucose levels after a carbohydrate meal to prevent persistent hyperglycemia and hence reduce the risk of potential complications of diabetes is better achieved with foods having a low glycemic index. Various intrinsic and extrinsic factors dictate the glycemic index of a given food; these include the physico-chemical properties of the carbohydrates (e.g., the relative content of amylose versus amylopectin), nature of the food (e.g., whole grain versus flour, cooked versus raw), ripeness of the fruits, and the presence or absence of fiber. In general, carbohydrates in foods with a low glycemic index are digested and absorbed more slowly than carbohydrates in foods with a high glycemic index.
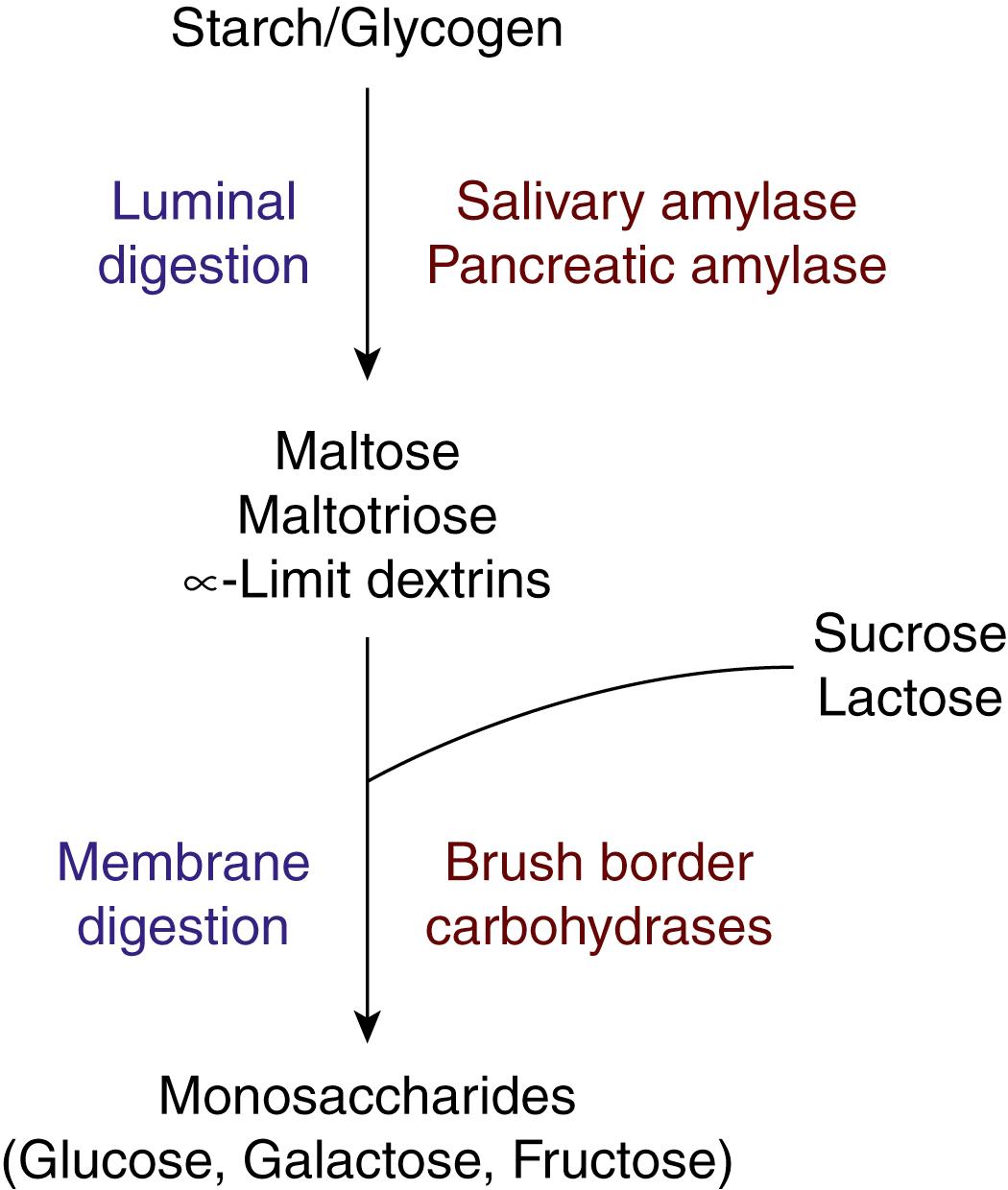
Dietary carbohydrates are digested and absorbed predominantly in the upper small intestine. Except for dietary fiber, very little of the carbohydrates escapes the small intestine and enters the colon. Saliva contains α-amylase that is capable of digesting starch and glycogen by hydrolyzing the α-1,4 linkages, but it has little physiologic significance because of the rapid entry of ingested food into the stomach where the acidic pH inactivates the enzyme. Carbohydrate digestion in the small intestine occurs in the lumen (luminal digestion) and on the BBM of the enterocytes (membrane digestion). The net result of luminal digestion and membrane digestion is to generate monosaccharides (glucose, galactose, and fructose) from the ingested polysaccharides and disaccharides, which are then absorbed across the enterocyte via selective transporters to enter the portal blood.
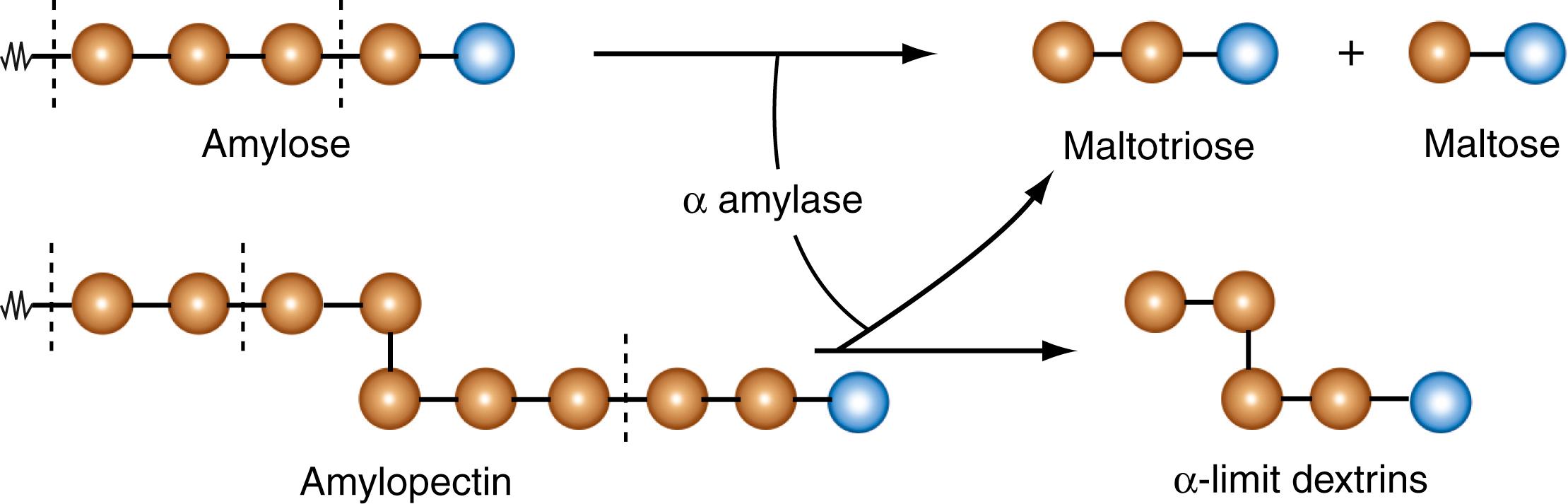
Pancreatic α-amylase is the principal enzyme responsible for the luminal digestion of dietary carbohydrates in the intestine. This enzyme acts solely on starch and glycogen, without any effect on dietary disaccharides such as sucrose and lactose. Similar to salivary α-amylase, pancreatic α-amylase also has a neutral pH for its optimal activity. Because the pH of the gastric chyme is neutralized with bicarbonate that is present in biliary and pancreatic secretions, the pH of the luminal fluid is appropriate for the activity of pancreatic amylase. Again, similar to salivary amylase, pancreatic amylase also has specificity toward the α-1,4 linkages in starch and glycogen. Both enzymes are endoglycosidases, meaning that they hydrolyze only the internal glycosidic linkages; the α-1,4 bonds located at the ends of the linear amylose or at the ends of the branches in amylopectin and glycogen are not hydrolyzed by these amylases ( Figs. 102.2 and 102.3 ). Consequently, amylose yields maltose and maltotriose (2 or 3 glucose residues bonded by α-1,4 linkages, respectively). Amylopectin and glycogen have branch points with α-1,6 linkages, and amylases do not act on these bonds. Therefore, the linear portions of the branches in these 2 polysaccharides yield maltose and maltotriose, while the regions containing the branches yield α-limit dextrins (polymers with an average of 5 to 8 glucose residues with 1 or more branch points) (see Fig. 102.3 ). As such, maltose, maltotriose, and α-limit dextrins are the products of the action of salivary and pancreatic amylases on dietary starch and glycogen. There is no release of free glucose by the action of amylases.
Salivary amylase and pancreatic amylase are coded by separate genes in humans, both of which are located on chromosome 1. As one would expect from the identical enzymatic activities of both proteins, the primary structures of salivary amylase and pancreatic amylase show high similarity with 94% homology in AA sequence.
The products of the luminal digestion of starch and glycogen by salivary and pancreatic amylases, along with the disaccharides sucrose and lactose present in diet form the substrates for membrane digestion, which occurs on the external surface of the BBM of the intestinal absorptive cells ( Fig. 102.4 ). At least 4 enzymes are involved in membrane digestion: maltase-glucoamylase, sucrase-isomaltase, lactase, and trehalase; all of them are integral proteins in the BBM with their catalytic sites exposed to the luminal surface of the membrane so that their respective intraluminal substrates have access to the active site. Maltase-glucoamylase hydrolyzes maltose and malto-oligosaccharides to generate free glucose. Sucrase-isomaltase is a bifunctional enzyme with 2 catalytic sites (i.e., sucrase and isomaltase) that reside in different parts of the same protein. However, though the enzyme is initially synthesized as a single polypeptide that gets inserted into the BBM, it is subsequently cleaved by pancreatic proteases into 2 subunits, one with sucrase activity and the other with isomaltase activity. The sucrase component of the enzyme is responsible for the digestion of sucrose into glucose and fructose, and also for the digestion of maltose into glucose. The isomaltase component of the enzyme is selective for the α-1,6 glycosidic bond present in α-limit dextrins. As the α-1,6 glycosidic bond is present only at branch points in α-limit dextrins, its hydrolysis by isomaltase results in debranching of α-limit dextrins after which maltase-glucoamylase and sucrase act on the resultant maltose and other linear malto-oligosaccharides to generate free glucose. Lactase acts on the milk sugar lactose to release glucose and galactose. Trehalase breaks down trehalose to generate glucose. Interestingly, mRNAs are found for all of these brush-border carbohydrases in the epithelial cells in the crypts, as well as in the upper parts of the villus, indicating that transcription of the respective genes occurs throughout the villi us, but the enzyme proteins are found mostly in differentiated epithelial cells of the upper villi us. As the luminal contents containing the products of salivary and pancreatic amylases (maltose, maltotriose, and α-limit dextrins) and other dietary disaccharides have access only to the upper parts of the villi and do not generally reach the crypts, the presence of the enzymes mostly in the differentiated epithelial cells makes physiologic sense. With regard to the longitudinal distribution of these brush-border enzymes, they are found at much higher levels in the jejunum than in the ileum. Collectively, the BBM-associated carbohydrases bring about the digestion of dietary carbohydrates to completion, releasing monomeric units of the polysaccharides and disaccharides present in the diet (see Fig. 102.4 ). The resultant monosaccharides (i.e., glucose, galactose, and fructose) are subsequently absorbed into enterocytes and then into portal blood.
Absorption of glucose, galactose, and fructose occurs predominantly in the jejunum, which suggests that as these monosaccharides are generated by the brush-border carbohydrases, they are immediately absorbed into the enterocytes. This explains the similar distribution pattern of the brush-border enzymes and the absorptive sites along the small intestine (jejunum > ileum). The entry mechanisms for these 3 monosaccharides across the BBM are different from the exit mechanism across the BLM ( Fig. 102.5 A ). Glucose and galactose are taken up by the enterocytes via an active transport process whereas fructose enters the cells by a passive, but facilitated mechanism. SGLT1, also known as SLC5A1, is responsible for active uptake of glucose as well as galactose from the intestinal lumen into the cells. This transporter accepts either glucose or galactose as the substrate but does not transport both monosaccharides at the same time in a given transport cycle. The driving force for this active transport process comes from the electrochemical Na + gradient present across the BBM. The Na + /K + pump in the BLM maintains intracellular Na + at low levels whereas the luminal contents have higher levels of Na + originating from biliary, pancreatic, and intestinal secretions and also from the diet. The uptake of each monosaccharide via SGLT1 is coupled with the simultaneous transport of 2 Na + . As glucose and galactose are neutral molecules, their cotransport with 2 Na + renders the transport process electrogenic, i.e., leading to depolarization of the membrane with a net transfer of 2 positive charges into the cell per transport cycle. Thus, the inwardly directed Na + gradient as well as the inside-negative membrane potential that are present across the BBM provide the driving force for the active entry of glucose and galactose from the lumen into the absorptive cells of the small intestine.
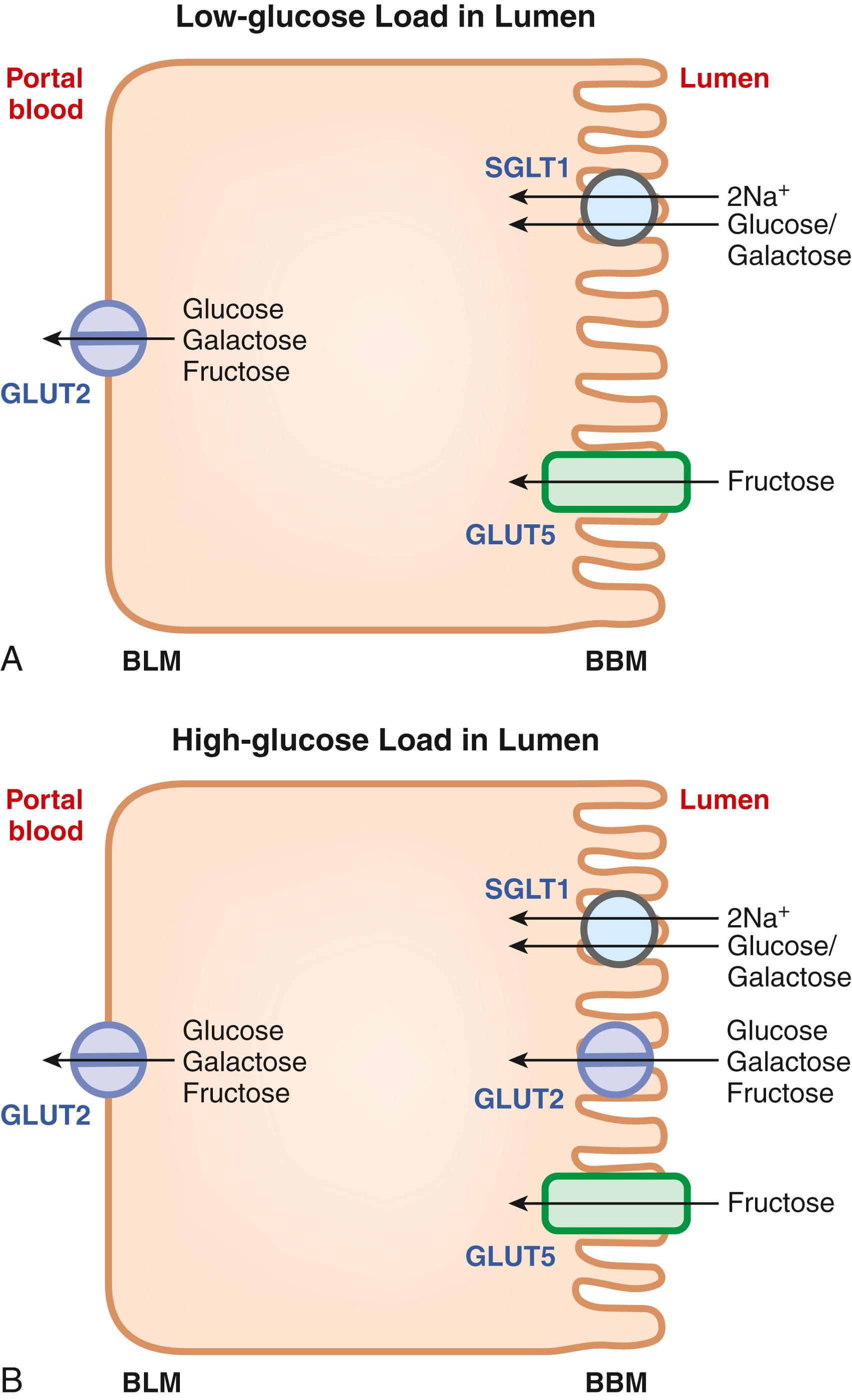
Fructose is not transported via SGLT1; its entry from the lumen into the intestinal absorptive cells occurs via the facilitative sugar transporter GLUT5, also known as SLC2A5. The transport process is energy-independent and has no involvement of Na + .
Once all 3 monosaccharides enter the enterocyte, they are exported out of the cells into the portal circulation across the BLM. This process occurs via GLUT2, also known as SLC2A2, a low-affinity facilitative sugar transporter. All 3 monosaccharides are substrates for GLUT2. The low affinity of this transporter is physiologically relevant because it dictates that the net release of glucose, galactose, and fructose from the cells occurs only down their concentration gradients when the intracellular concentrations of these sugars exceed those in the portal blood.
Even though the general scheme describing the role of various sugar transporters in the intestinal absorption of the 3 monosaccharides traditionally depicts GLUT2 as the transporter expressed exclusively in the BLM (see Fig. 102.5 A ), this transporter does traffic to the BBM when the intestinal lumen is faced with a high load of sugar, particularly glucose (see Fig. 102.5 B ). SGLT1-mediated glucose entry is the signal for this trafficking of GLUT2 to the BBM, which has physiologic importance not only in terms of glucose/galactose absorption but also fructose absorption. SGLT1 is a relatively high-affinity transporter for glucose and galactose, and, therefore, it is not efficient in absorbing glucose and galactose under high-sugar load conditions. In contrast, GLUT2 is a low-affinity transporter for all 3 monosaccharides, and therefore, its appearance in the BBM only when the concentrations of these monosaccharides are high in the intestinal lumen ensures maximal absorption. This phenomenon is also important for intestinal fructose absorption. SGLT1 plays no role in fructose transport whereas GLUT2 can transport fructose; therefore, the recruitment of GLUT2 to the BBM under high-sugar conditions suggests that intestinal absorption of fructose across the BBM involves not only GLUT5 but also GLUT2 when dietary intake of carbohydrate is high. In addition, the introduction of a high load of fructose to the small intestinal lumen itself increases the density of GLUT5 in the BBM.
Genetic deletion studies with all 3 transporters have confirmed their biologic functions. Deletion of Sglt1 in mice leads to glucose/galactose malabsorption without affecting fructose absorption; the trafficking of Glut2 to the BBM in response to high glucose load in the intestine is absent in Sglt1 -null mice, highlighting the essential role of Sglt1-mediated glucose entry as the signal for the trafficking of Glut2 to the BBM. Deletion of Glut5 leads to defective intestinal absorption of fructose without affecting glucose/galactose absorption. The biochemical phenotype of Glut2 -knockout mice was a little surprising and unexpected. As this transporter was thought to be the only mechanism for the exit of all 3 monosaccharides, defective absorption of glucose in the intestine was expected in Glut2 -knockout mice. Contrary to this expectation, however, no defect in the intestinal absorption of glucose was observed, suggesting the presence of other possible mechanisms for the exit of glucose from the cells (e.g., exocytosis). Deletion of Glut2 is much more lethal than deletion of Sglt1 and Glut5 , which is expected given the fact that this low-affinity transporter functions in the pancreas as a sensor of circulating levels of glucose to promote insulin secretion in proportion to changes in blood glucose levels. As such, the whole-body deletion of this transporter has a severe phenotype because of the inability of the β cells in the pancreas to secrete insulin in response to blood glucose, thus leading to hypoinsulinemia and hyperglycemia.
As the small intestine is capable of absorbing only monosaccharides, dietary polysaccharides and disaccharides must be digested completely prior to absorption. If the digestive process is faulty, either because of pancreatic insufficiency (i.e., decreased pancreatic amylase) or defects in brush-border carbohydrases, dietary carbohydrates cannot be digested. The undigested carbohydrates then reach the colon where they increase the osmotic pressure leading to secretion of water into the lumen, with resultant abdominal bloating and diarrhea (osmotic diarrhea). The resident bacteria in the colon hydrolyze these carbohydrates and ferment the released sugars. In the process, gas is produced, largely in the form of hydrogen, leading to flatulence and increased appearance of hydrogen in the expired air from lungs. This is the basis of the breath hydrogen test that is used to monitor defects in carbohydrate digestion in the intestine (see Chapter 105 ).
Lactose intolerance is the most common defect in the digestion of dietary carbohydrates and results from deficiency of the brush-border disaccharidase lactase. Contrary to common assumption, however, lactose intolerance is the normal phenomenon and it is lactose tolerance that results from genetic mutations. In all mammals including humans, milk was supposed to be a normal dietary component only during infancy. Accordingly, the intestinal enzyme lactase that hydrolyzes the milk disaccharide lactose to generate the absorbable monosaccharides glucose and galactose is expressed at high levels at birth and stays high until the weaning period. Subsequently, the expression of the enzyme decreases significantly to the much lower levels found in adults. This makes teleologic sense because if milk is not a normal dietary constituent in adults, why would the intestine need to express the enzyme? However, when domestication of ruminants as a source of milk started during civilization in certain populations of the world tens of thousands of years ago, milk became a normal component of diet, even in adults. Milk has high nutritional value not only for the infant but also for the adult. Milk albumin is a protein with a 100% nutritional value and is the gold standard against which the nutritional value of any other protein is evaluated. Milk is also rich in carbohydrate (lactose) and calcium. However, the normal phenomenon of decreased lactase expression in adults became a problem for those who consumed milk because of their inability to digest lactose and the resultant clinical manifestations (see Chapter 104 ). Some adults, however, were able to tolerate milk in their diet and these individuals were found to have mutations in the gene coding for lactase, which prevented the normal age-related decline in expression of the enzyme. Such mutations provided a biologic and probably survival advantage in those civilizations in which milk and other dairy products were normal components of the adult diet, most notably peoples of Northern European descent and certain African nomads; as such, lactose intolerance is not common in these populations. Thus, the “wild type” is characterized by lactose intolerance whereas the “mutant type” is characterized by the ability to tolerate milk without undesirable clinical symptoms. The clinical manifestations in lactose-intolerant subjects are solely associated with the presence of milk and other dairy products in the diet. These individuals have no problems digesting carbohydrates from other sources. Therefore, an increase in breath hydrogen in expired air from the lungs is seen in lactose-intolerant subjects only upon ingestion of lactose-containing foods; ingestion of starch, glycogen, or sucrose does not increase the levels of breath hydrogen nor does it produce any of the symptoms associated with lactose intolerance. Sometimes there is a misconception in the lay public that lactose intolerance is due to an allergy to milk; this is not true.
Congenital sucrase-isomaltase deficiency is a rare autosomal recessive disease that is associated with defective digestion of starch, glycogen, and sucrose. As this bifunctional enzyme possesses maltase, sucrase, and isomaltase activities, it is obligatory not only for the debranching of α-limit dextrins arising from the digestion of amylopectin and glycogen, but also for the hydrolysis of sucrose as well as maltose arising from the digestion of starch and glycogen. The clinical manifestations of the disease are again related to undigested carbohydrates reaching the colon, resulting in osmotic diarrhea, bacterial fermentation, and production of excess of gas.
Congenital trehalase deficiency is another rare disorder that is associated with inability to digest the disaccharide trehalose, which is present in mushrooms and certain prepared frozen foods, like ice cream, to which it is added because it lowers the freezing point. Patients with trehalase deficiency cannot digest trehalose and as a consequence suffer from abdominal bloating, flatulence and diarrhea after ingestion of trehalose-containing foods. This disease is not common in Caucasian Americans but is quite prevalent in Greenland Inuit natives, occurring in 10% to 15% of the population. In addition to the aforementioned genetically driven brush-border enzyme deficiencies, there are secondary causes of defects in carbohydrate digestion in the intestine, examples of which include celiac disease and ZES. Celiac disease is a genetic disease that results in severe intestinal inflammation upon ingestion of gluten-containing foods such as wheat, rye, and barley (see Chapter 107 ). The inflammation begins in upper small intestine as this is the part of the intestinal tract that is exposed first to dietary gluten. As digestion and absorption of dietary carbohydrates occur primarily in the upper small intestine, celiac disease leads to defects not only in the digestion of carbohydrates but also in their absorption. The principal reason for the malabsorption of carbohydrate in celiac disease is the inflammation-associated blunting of the intestinal villi, thus resulting in a markedly decreased density of absorptive enterocytes and, hence, a decreased surface area of the BBM. The BBM is the membrane that expresses all the carbohydrases (except for amylase) and also the transporters for the monosaccharides; therefore, celiac disease results in carbohydrate malabsorption. ZES is a disease caused by gastrinoma; the resultant increased production of the hormone gastrin promotes massive acid secretion from parietal cells of the stomach (see Chapter 34 ). Due to the massive acid load from the gastric chyme, the bulk fluid in the lumen of the upper small intestine remains acidic, which is not conducive for the enzyme activities of amylase and brush-border carbohydrases, thereby causing defective digestion of dietary carbohydrates.
Glucose-galactose malabsorption is the primary defect associated with the transport of monosaccharides in the small intestine. It is an autosomal recessive disorder affecting only the absorption of glucose and galactose; fructose absorption is normal. Based on the substrate selectivity of the sugar transporters in the enterocyte, it is obvious that the disease is related to defective function of SGLT1 (SLC5A1), which is a Na + -coupled active transporter for glucose and galactose, but not fructose. This transporter is expressed in the BBM of the absorptive cells of the small intestine. Mutations in the gene coding for the transporter form the molecular basis for the disease. The SLC5A1 gene is located on human chromosome 22q13.1; disease-causing mutations can be either homozygous or compound heterozygous, and are of different types. The nonsense, frame shift, and splice-site mutations all generate truncated proteins that possess no transport activity. The protein has 14 transmembrane domains, and the disease-causing mutations are found throughout the protein. Some of these mutations cause trafficking defects that render the transporter protein trapped in intracellular compartments and not able to reach the BBM, whereas others do not interfere with the protein trafficking but compromise the transport function. Clinical manifestations of the disease become very obvious early in life. Affected neonates suffer from severe diarrhea and dehydration as soon as milk is introduced as the major dietary source of carbohydrate. Lactose is digested normally in these patients, but the resultant glucose and galactose are not absorbed because of the defective SGLT1, with resultant osmotic diarrhea, massive fluid loss and dehydration. Symptoms occur with exposure to any type of dietary carbohydrate that contains glucose and/or galactose (starch, glycogen, sucrose or even partially hydrolyzed starch). If left untreated, affected patients may develop kidney stones because of chronic dehydration and may die from hypovolemic shock. The only treatment available for these patients is to provide fructose in their diet, which is absorbed via GLUT5 and does not involve SGLT1. For affected neonates, fructose can be given in the form of fruit juices. With fructose in the diet to meet the energy requirements, normal growth and neurological development can be preserved.
There are no genetic defects known in humans that involve fructose absorption. However, mutations in GLUT2, the transporter in the BLM and BBM of the intestinal absorptive cells for all 3 monosaccharides, have been identified in humans. Notable clinical manifestations in patients with mutations in GLUT2 include tubular nephropathy, fasting hypoglycemia, rickets, stunted growth, and hepatomegaly secondary to glycogen accumulation ; the disease resulting from GLUT2 defect is called Fanconi-Bickel syndrome and, interestingly, is not associated with any overt intestinal phenotype in terms of carbohydrate absorption. Many of the aspects of Fanconi-Bickel syndrome can be explained on the basis of expression of the transporter in the kidney, liver, and pancreas as well. As in the intestine, the transporter plays a role in the exit of glucose, galactose, and fructose from the renal tubular cells into blood. The same transporter also plays a role in the exit of glucose resulting from gluconeogenesis, which occurs in the liver and kidney during fasting; this is the likely explanation for the fasting hypoglycemia in patients with loss-of-function mutations in GLUT2. The transporter is also the glucose sensor in the β cells of the pancreas where it plays a role in glucose-induced insulin secretion. Based on this function, one would expect to see diabetes in patients with defective GLUT2, but this is not always the case. Some specific mutations in GLUT2 do lead to fasting hyperglycemia, which eventually transitions to type 2 diabetes. Some mutations, however, result in gain of function, which leads to stimulation of insulin secretion even in the absence of glucose. This observation indicates that the mutant transporter functions as a glucose receptor in β cells and that these mutations render the mutant transporter capable of eliciting the signaling pathways for insulin secretion even in the absence of glucose transport and metabolism. In fact, such mutants also promote differentiation of β cells. The lack of any defect in the intestinal absorption of sugars in patients with Fanconi-Bickel syndrome is also seen in Glut2 -knockout mice, suggesting the presence of other mechanisms for the exit of monosaccharides from the enterocytes.
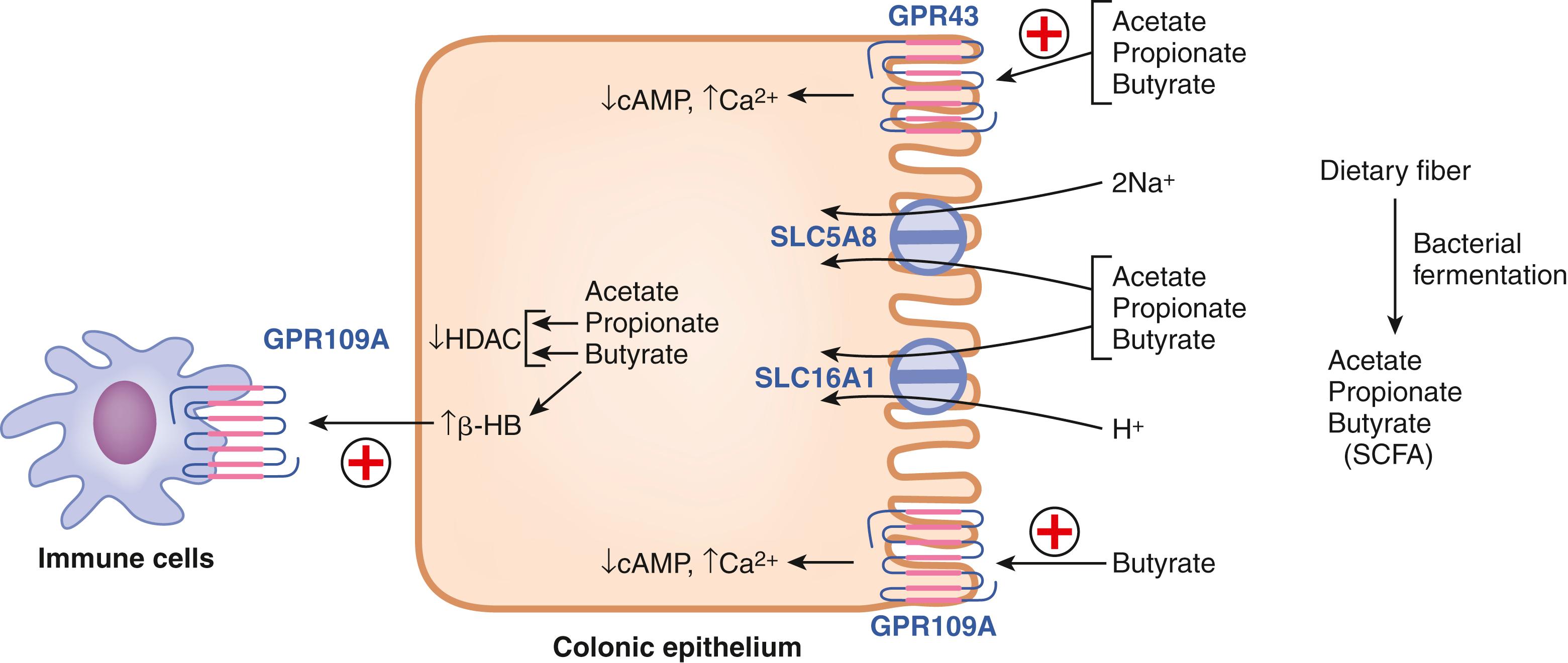
Dietary fiber consists of carbohydrates such as cellulose, hemicellulose, gums, pectins, and chitin, but these are not digested by any of the enzymes arising from mammalian tissues associated with the function of the intestinal tract. Consequently, these carbohydrates remain undigested in the small intestine and reach the large intestine where they are subjected to digestion and fermentation by colonic bacteria. The end products of this process comprise SCFAs containing mostly 2 to 4 carbon atoms (acetate, propionate, and butyrate) ( Fig. 102.6 ). These bacterial metabolites are effectively absorbed in the colon via H + -coupled and Na + -coupled monocarboxylate transporters, primarily MCT1 (SLC16A1) and SMCT1 (SLC5A8). SCFAs elicit a plethora of biologic functions locally in colon and also in other organs through mediators from immune cells and enteroendocrine cells. The most recognized function of SCFAs, especially butyrate, is their role as the preferred energy substrates for colonocytes. In addition, butyrate functions as an inhibitor of histone deacetylases and thus modulates the epigenetic profile and consequently the transcription of selective genes in the colon (e.g., the cell-cycle regulator p21, GI-selective transcription factor CDX2, intracellular signaling kinase p38); it also serves as the carbon source for the generation of the ketone body β-hydroxybutyrate by colonocytes (see Fig. 102.6 ). These bacterial metabolites also impact colonic function by serving as agonists for certain cell-surface G-protein-coupled receptors that are expressed on the luminal surface of the epithelial cells and enteroendocrine cells present in the colon and lower small intestine and also on certain specific immune cells present in the lamina propria (see Fig. 102.6 ). The receptor GPR109A is selectively activated by butyrate and β-hydroxybutyrate whereas GPR43 is activated by all 3 SCFAs. Intracellular signaling for both receptors includes a decreased cAMP and/or an increase in calcium. Butyrate also influences the biology of the gut-associated immune system. Inhibition of histone deacetylases caused by butyrate and propionate block the development of dendritic cells, which could be at least one of the mechanisms for immune tolerance that is necessary for the host-microbiome symbiosis in the lower intestinal tract. SCFAs function as effective tumor suppressors in the colon; the cell-surface receptors as well as transporters in the BBM play a role in this function. In particular, the butyrate receptor GPR109A and the Na + -coupled monocarboxylate transporter SLC5A8 have been shown to protect against colon cancer. Recent studies with Slc5a8 -null mice have uncovered an interesting connection between dietary fiber content and the tumor-suppressive function of the transporter. Because of the high-affinity transport of SCFAs, particularly butyrate, by the transporter, the ability of the transporter to protect against colon cancer becomes obvious only under conditions of low dietary fiber content when the luminal production of butyrate by bacterial fermentation becomes markedly reduced, thereby making the high-affinity and low-capacity transport of this SCFA by SLC5A8 quantitatively relevant.
Proteins in the diet serve as the source of essential as well as non-essential AAs for cellular metabolism. Deficiency of dietary protein intake will lead to negative nitrogen balance, primarily due to the non-availability of the essential AAs. Proteins provide approximately 10% to 15% of energy intake in an average Western diet, which amounts to about 70 to 100 g protein per day. In addition to the exogenous proteins present in the diet, the intestinal tract is also exposed to endogenous proteins, which arise from salivary, gastric, intestinal, pancreatic, and biliary secretions, and also from desquamated cells of the intestinal tract; collectively, this amounts to about 30 g protein per day. Proteins are digested and absorbed mostly in the small intestine with little or no proteins entering the large intestine under normal conditions.
Dietary proteins are either of plant or animal origin. The nutritional value of dietary proteins depends primarily on their composition of AAs, particularly the essential AAs. The body needs all essential AAs; even when just one of the essential AAs is deficient, it will lead to negative nitrogen balance. Milk proteins and egg proteins are considered as the standard for comparing the nutritional value of dietary proteins, with the nutritional value of these standard proteins taken as 100. In general, animal proteins have higher nutritional value than plant proteins. However, plant proteins from different dietary sources can be combined to increase their overall nutritional value, and the deficiency of a given essential AA in one particular plant protein can be complemented by another plant protein that is rich in that selective essential AA. The quality of dietary proteins is also determined to some extent by their digestibility. For example, a high content of proline generally compromises protein quality because of increased resistance to hydrolysis by proteases and peptidases in the intestinal tract.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here