Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The liver has quite accurately been called the custodian of the milieu intérieur, and is vulnerable to a variety of metabolic, vascular, toxic, infectious, and neoplastic insults. Diffuse liver disease can be diagnostically challenging because of nonspecific and overlapping clinical signs and symptoms or even asymptomatic disease. The ability to assess liver and spleen size and the presence of ascites is limited on physical examination. Liver function tests also lack diagnostic specificity, are affected by non-hepatic factors, and in general poorly correlate with physiologic hepatic function.
Imaging plays a critical role in the diagnosis and treatment of diffuse liver disease by detecting its presence, determining its distribution and severity, and identifying associated complications, such as cirrhosis, portal hypertension, and malignant disease. Dramatic advances, particularly in magnetic resonance imaging (MRI), have led to novel techniques to noninvasively diagnose and to characterize patterns of diffuse hepatic disease.
The excessive accumulation of intracellular fat within hepatocytes, known as hepatic steatosis, occurs as a metabolic complication of a variety of hepatic exposures ( Table 56.1 ). Common causes include alcohol abuse and viral hepatitis (discussed separately), obesity and metabolic disorders, toxins, HIV infection, genetic lipodystrophies, and chemotherapy. This abnormal intracellular accumulation of fat within hepatocytes is now recognized as an important causative factor in liver injury. Although it is initially reversible, the process may also progress and can result in cirrhosis and increased risk for hepatocellular carcinoma (HCC).
| TOXIN OR MEDICATION RELATED |
| Alcohol |
| Chemotherapy |
| Carbon tetrachloride |
| Corticosteroids |
| Phosphorus |
| Tetracycline |
| Reye’s syndrome |
| INFECTIOUS |
| Viral hepatitis |
| Tuberculosis |
| METABOLIC |
| Obesity |
| Malnutrition |
| Total parenteral nutrition |
| Small bowel bypass surgery |
| Glycogen storage disease |
| Hyperlipidemia |
| CHRONIC DISEASE |
| Congestive heart failure |
| Inflammatory bowel disease |
| Cystic fibrosis |
| Obstructive sleep apnea |
| OTHER |
| Pregnancy |
| Trauma |
Nonalcoholic fatty liver disease (NAFLD) is a disease spectrum extending from simple steatosis to the more severe form with inflammation and fibrosis known as nonalcoholic steatohepatitis (NASH), the result of hepatic metabolic disturbance in the absence of alcohol abuse. Steatosis is the hallmark feature of NAFLD. NASH is established histopathologically by the presence of steatosis with evidence of hepatitis (centrilobular inflammation, hepatocyte ballooning degeneration, and spotty necrosis) and variable degrees of fibrosis (stages 0–3). Advanced or stage 4 fibrosis is indicative of cirrhosis. Severity of NASH is based on grading of inflammation and different stages of fibrosis. As a result of the current epidemic of obesity and metabolic syndrome, NAFLD is reported to occur in 20 to 80 million Americans and is the most common chronic liver disease in the United States. Recent research suggests that NAFLD is an independent risk factor for cardiovascular disease and contributes to the development of diabetes by impairment of insulin regulation within the liver.
The development of HCC is common in steatohepatitis; up to 7% of patients with NAFLD-related cirrhosis develop HCC within 10 years. The population of patients with chronic liver disease is growing, particularly from NAFLD, and it is anticipated to become an increasingly significant burden on the health care system as well as the number one cause of cirrhosis and HCC.
Several major patterns of fat deposition can be visualized at cross-sectional imaging: diffuse deposition; focal deposition and focal sparing; multifocal deposition; perivascular deposition; and subcapsular deposition. Diffuse deposition is the most common pattern and is associated with homogeneous involvement and hepatomegaly. Focal deposition or diffuse deposition with focal sparing is a less common pattern that characteristically occurs adjacent to the falciform ligament, fissure for the ligamentum venosum, porta hepatis, and gallbladder fossa. This probably relates to variant arterial supply or venous drainage as described before. A true mass can be differentiated from focal regions of fat deposition and focal sparing because these occur in characteristic locations and typically have a geographic shape (but may appear nodular), poorly delineated margins, and, importantly, absence of mass effect on blood vessels.
Multifocal deposition of fat may simulate true masses such as metastases. In these patients, differentiation can be made by measurement of the tissue density on computed tomography (CT) or specific sequences performed with MRI to evaluate for the presence of fat. With perivascular fat deposition, halos of fat surround the hepatic or portal veins. This fat is tram-like or tubular in appearance when the course is in the imaging plane and ringlike or round for vessels with a course perpendicular to the imaging plane. Subcapsular fat deposition can be seen in patients with renal failure and insulin-dependent diabetes in which insulin is added to the peritoneal dialysate. The dialysate exposes the subcapsular hepatocytes to a higher concentration of insulin than in the remainder of the liver and promotes the esterification of free fatty acids into triglycerides. This in turn results in subcapsular deposition of fat.
Ultrasound is the most common modality used to evaluate the liver and is often the first imaging examination performed. It is low in cost, fast, and readily available. The positive predictive value for the presence of steatosis is low, however, at 62% to 77%. It is also operator dependent and difficult in obese patients, who represent a large population of those patients requiring assessment.
On sonographic evaluation, in diffuse hepatic steatosis, there is increased hepatic echogenicity roughly proportional to the histopathologic grade of steatosis ( Fig. 56.1 ). Sonographic changes also tend to correlate with the severity of biochemical and clinical dysfunction. The key ultrasound findings are accentuation of the brightness of parenchymal echoes and an increased number of echogenic foci resulting from the proliferation of fat-nonfat interfaces. These small echogenic foci increase attenuation of the ultrasound beam, resulting in poor visualization of deep hepatic structures and of the hepatic venous system.
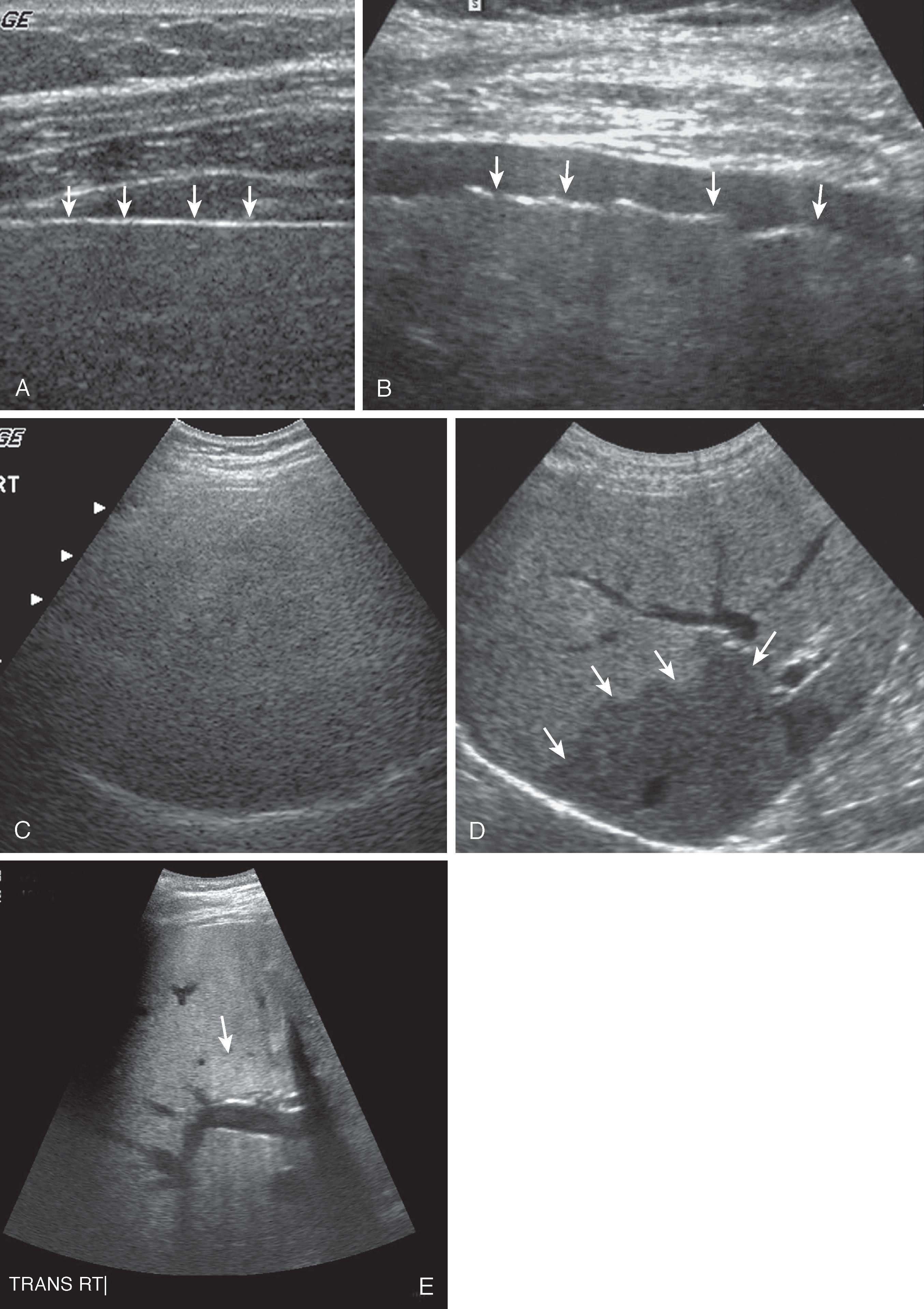
Normally, four to six portal and hepatic veins are seen per longitudinal section of the right lobe. With fatty infiltration, fewer vessels are seen, and the smaller vascular structures may be nonvisible. This dropout of vessels is due to physical vascular compression by parenchymal swelling, decreased sound penetration, and loss of contrast between echogenic vascular walls and abnormally increased echogenicity of the surrounding parenchyma.
Four sonographic patterns of focal fatty infiltration have been described: (1) hyperechoic nodule, (2) multiple confluent hyperechoic lesions, (3) hypoechoic skip nodules, and (4) irregular hyperechoic and hypoechoic areas. On occasion, a focal area of liver parenchyma may be spared from fatty metamorphosis and appear as an ovoid, spherical, or sheetlike hypoechoic mass in an otherwise echogenic liver. The characteristic location, lack of mass effect on surrounding vessels, straight linear interface between normal and fatty parenchyma, and increased echogenicity of the liver compared with the cortex of the right kidney are useful diagnostic criteria. Hepatic hemangiomas, most often sonographically hyperechoic, in the presence of fat may appear hypoechoic compared with the adjacent liver and simulate a suspicious mass.
Focal fatty infiltration in an otherwise normal-appearing liver may also produce a hyperechoic space-occupying mass. An angular or interdigitating geometric margin, when present, is characteristic of focal fat. , Hepatic steatosis and fibrosis appear similar on ultrasound and often coexist. Differentiation of hepatic steatosis from a mass or other liver pathologic process may necessitate confirmation with CT or MRI, both of which have an increased sensitivity.
CT is an imaging technique that is also fast and widely available ( Fig. 56.2 ). It is a strong diagnostic tool for hepatic steatosis because of the excellent correlation between the hepatic parenchymal CT attenuation value and the amount of hepatic triglycerides found on liver biopsy specimens. .
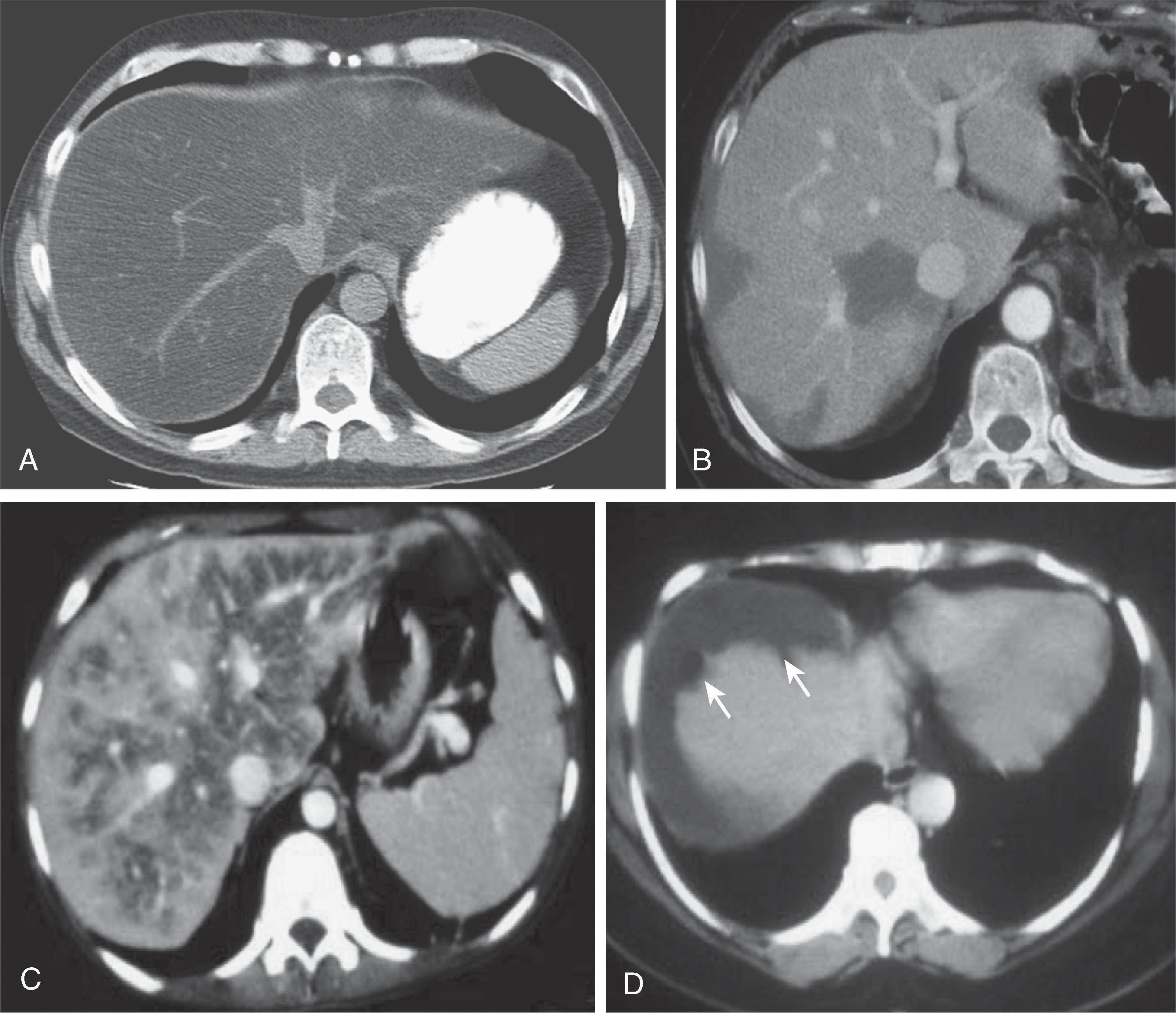
On noncontrast-enhanced scans, the normal liver appears homogeneous with an attenuation value ranging from 50 to 70 Hounsfield units (HU), which is 8 to 10 HU greater than the attenuation of the spleen. , The diffusely steatotic liver has a reduced attenuation value of less than 40 HU and appears and measures less dense than the spleen. The accumulation of other substances, such as iron, copper, glycogen, drugs (including amiodarone and gold therapy), and even cirrhosis or edema, can increase the attenuation value of the liver and underestimate the presence of fat. CT is also insensitive for mild steatosis, which makes it a poor screening tool for early disease. ,
Hepatic steatosis has a wide spectrum of CT appearances. Although diffuse parenchymal involvement is commonly seen, fat may also be deposited in a focal, lobar, segmental, or nonpattern-like distribution. Fatty infiltration of the liver can obscure the detection of focal liver lesions, such as neoplasm or abscess, by lowering the background liver attenuation to a level similar to that of an otherwise hypoattenuating mass or fluid collection.
When fatty infiltration is lobar, segmental, or wedge shaped, differentiation from other focal hepatic disease is straightforward. In such cases, it usually has a straight-line margin, typically extending to the liver capsule without associated bulging of the contour to suggest an underlying mass. When the steatosis is focal or nodular, differentiation from metastatic disease can be challenging. Absolute attenuation values are affected by multiple parameters other than fat as previously described and alone are not diagnostically reliable. The previously mentioned differentiating features of focal fat, including characteristic location, lack of contour abnormality, and normal course of the hepatic vessels, are helpful and are only rarely simulated in metastatic tumor lesions. On occasion, discrete focal areas of fatty replacement have a central core of normal-appearing tissue, the reverse of the typical tumor necrosis pattern.
Because of its unique ability to characterize the presence of fat, MRI is often required. It is the most reliable diagnostic tool in differentiating any geographic pattern of hepatic steatosis or fatty sparing from metastatic disease.
MRI is the most sensitive modality for hepatic steatosis and has the highest correlation with histopathologic evaluation with a sensitivity of 76.1% to 95.3%. , In addition, MRI can be used to quantify the amount of fat present. Severity of liver fat or steatosis is categorized by a four-point grading system for histopathologic liver fat of 0 to 3, representing less than 5%, 6% to 33%, 34% to 66%, and more than 66%, respectively. The grading system incorporates the accepted normal value of liver fat, which is less than 5%. Limitations of MRI include increased cost, longer time to be performed, and less widespread availability. MRI also requires the patient’s cooperation, such as the ability to lie still and breath-hold.
Proton chemical shift imaging, also termed opposed-phase gradient-echo imaging , is a rapidly acquired, high-resolution, commonly used sequence to confirm the presence of microscopic fat within multiple organs including the liver, kidney, and adrenal gland ( Fig. 56.3A and B ). This technique capitalizes on the difference in precession frequency between fat and water protons (3.7 ppm). Imaging is performed when their signals are additive (in-phase) and opposite or subtractive (opposed-phase). The presence of water alone in a solid organ will have the same signal intensity on both the in-phase and opposed-phase images. When fat and water are present, signal will be lost in the corresponding area on the opposed-phase image only because of cancellation of the contribution of signal from both water and fat in opposite phases or direction.
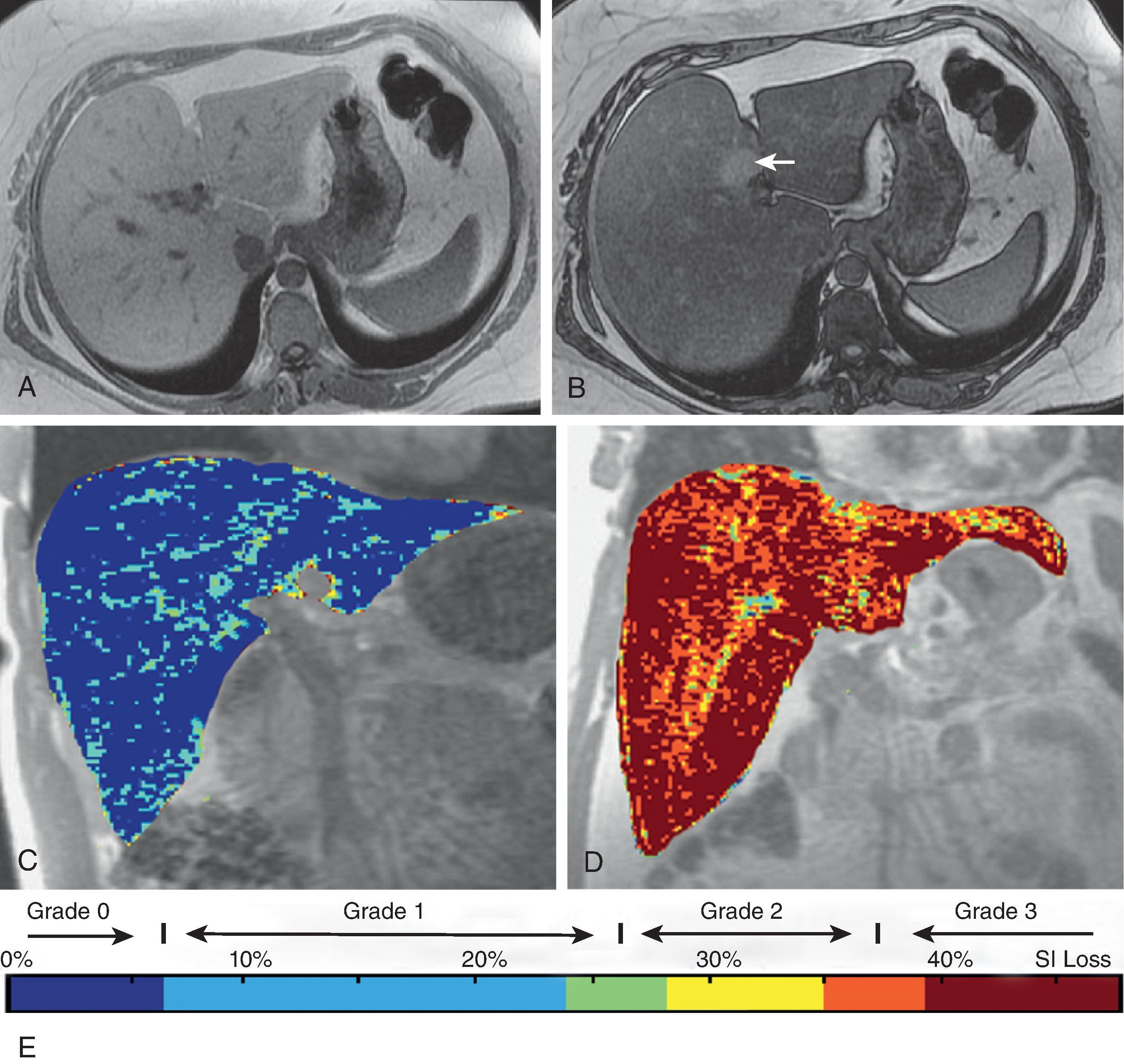
In normal liver, the lipid fraction is less than 10% and is without significant signal loss on opposed-phase imaging. Clinically significant hepatic steatosis is often greater than 20% , and can be detected with proton chemical shift imaging qualitatively with a visualized signal loss or quantitatively by creation of a fat signal fraction map ( Fig. 56.3C–E ). Chemical shift imaging is limited in the detection of a low amount of fat (<10%); at higher levels, fat amount is detected but ambiguous, with 40% fat appearing the same as 60%, reducing accurate quantification at these levels. Additional methods to detect fat include fat suppression with either T1 or T2, which performs as well as or better than chemical shift imaging, and MR spectroscopy, which is sensitive to small amounts of fat and has high accuracy but with limitations of difficulty to perform and to analyze, limited availability, high cost, small spatial sample region, and sampling error with repeated examinations.
When signal loss is present with chemical shift imaging, the presence of fat is confirmatory. The technique is limited, however, secondary to several confounders, including magnet field inhomogeneities such as in the presence of iron, T2* decay, T1 bias (which refers to the different T1 weighting for fat versus water), and inclusion of only one spectral peak for fat when multiple smaller peaks exist and add to the fat signal. These confounders result in underestimation or lack of detection of fat. These effects are most dramatic at low fat levels, the range where screening for early disease would be important.
New MRI techniques continue to be developed to correct for these cofounders and also to better quantify the presence of fat to accurately grade steatosis. The benefit of these techniques is the potential to replace the “gold standard” for fat quantification, which currently is percutaneous biopsy, an invasive technique that samples only a small portion of the liver. These techniques may also serve as an early and accurate screening tool for asymptomatic but high-risk patients. These techniques result in accurate separation of signal created by mobile fat protons from all other mobile protons by calculating a proton density fat fraction. This fraction can be used to create a fat map of the entire liver that can be viewed with a color scale to demonstrate the variability of fat fractions throughout the parenchyma. These maps are also useful in monitoring changes over time with treatment. Early results have demonstrated high correlation of these methods with MR spectroscopy and improved ability to detect smaller amounts of fat (<5%) where the transition lies from normal to clinically significant steatosis (>5.56%). ,
Hepatitis is a general term used to describe acute or chronic inflammation of the liver. In general medical parlance, hepatitis refers to viral hepatitis, but diffuse parenchymal inflammation can result from numerous hepatic infectious or inflammatory processes. It can also be seen after inhalation, ingestion, or parenteral administration of a number of agents, including alcohol, acetaminophen, carbon tetrachloride, halothane, isoniazid, chlorpromazine, oral contraceptives, α-methyldopa, methotrexate, azathioprine, and 6-mercaptopurine.
The liver is almost invariably involved in all hematogenous viral infections. These include mononucleosis; herpes simplex; cytomegalovirus infection in patients with AIDS; yellow fever in tropical countries; and rubella, adenovirus infection, and enterovirus infection in children. The term viral hepatitis is usually reserved for hepatic infections caused by a small group of hepatotropic viruses known as hepatitis A, B, C, D, and E. Viral hepatitis is the etiology for 60% of cases of fulminant hepatic failure in the United States.
The diagnosis of viral hepatitis can usually be made on the basis of history, serologic markers, and liver function test abnormalities. Imaging is usually performed to ensure that there is no obstructive component of the patient’s hepatic dysfunction, to assess for cirrhosis and its complications, to exclude focal hepatic abnormalities such as HCC, and to assess hepatic vascular patency.
Ultrasound findings in acute hepatitis, although often present, are nonspecific. The major role of ultrasound in hepatitis is to exclude biliary obstruction as the cause of liver disease and to diagnose other major complications, such as ascites or vascular occlusion. In chronic viral hepatitis, ultrasound is also used to screen for the development of cirrhosis or HCC. Although its sensitivity for both lags in comparison to CT and MRI, ultrasound is less expensive and more readily available.
In acute viral hepatitis, the liver and spleen are frequently enlarged. When parenchymal damage is severe, the parenchymal echogenicity is decreased, and the portal venule walls are “brighter” than normal ( Fig. 56.4 ). The increased sonographic contrast between the parenchyma and the periportal collagenous tissue is probably due to increased hepatic water, hydropic swelling of hepatocytes, and inflammatory cell infiltration. The increased hepatic water (periportal edema) accentuates the acoustic mismatch between the hepatocytes and the portal tracts. Also, the decreased attenuation of the liver allows greater sound penetration to “highlight” these vessels, with increased echogenicity of the walls of peripheral portal veins. This has been termed the centrilobular pattern or “starry sky” appearance. It has been observed in up to 60% of patients with acute hepatitis and is best appreciated in thin patients. This pattern is nonspecific and has also been reported in toxic shock syndrome, cytomegalovirus infection, idiopathic neonatal jaundice, leukemia, diabetic ketoacidosis, and lymphoma. Hepatomegaly is another nonspecific finding in patients with acute hepatitis.
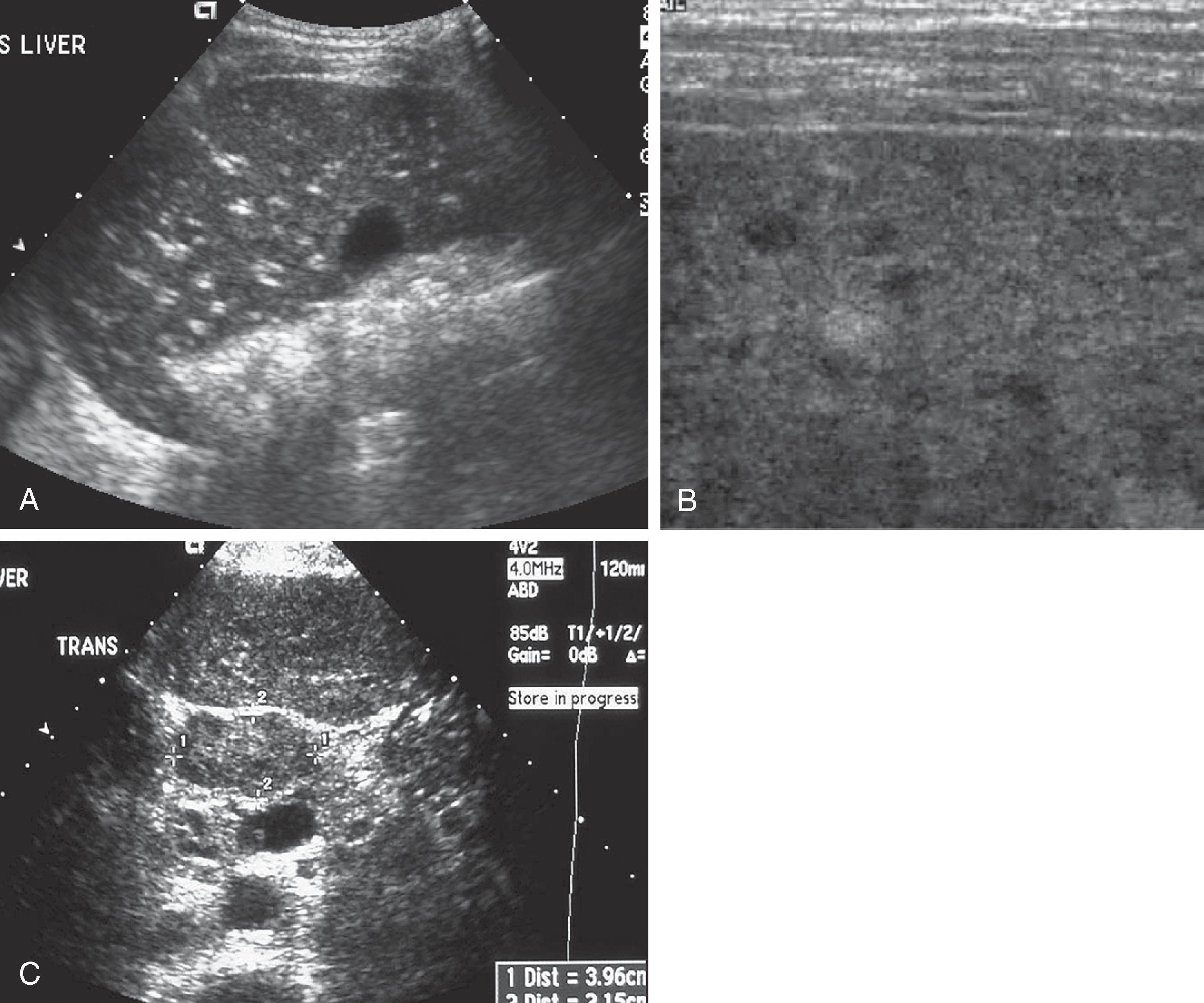
When it is severe, chronic hepatitis produces increased parenchymal echogenicity and loss of mural definition of the portal veins. This is attributed to “silhouetting out” of the portal vein walls by the echogenic fibrotic and inflammatory process surrounding the lobules that abut the portal triads. These findings are nonspecific and can also be seen in patients with fatty infiltration and cirrhosis. One useful differentiating feature is the presence of adenopathy in the hepatoduodenal ligament in chronic active hepatitis, which has been attributed to direct spread of the hepatic inflammatory process.
Acute viral hepatitis can also be associated with thickening of the gallbladder wall. In early hepatitis, the gallbladder is hypertonic, whereas in patients more than 9 days after disease onset, the gallbladder is hypokinetic with a large volume and demonstrates a diminished response to a fatty meal.
The primary role of CT in patients with hepatitis is to assess for the development of cirrhosis and associated sequelae, including HCC. CT findings in hepatitis are usually nonspecific. Hepatomegaly, gallbladder wall thickening, and hepatic periportal lucency ( Fig. 56.5 ) are the major CT findings in patients with acute viral hepatitis. This lucency is due to periportal fluid and lymphedema surrounding the portal veins and has also been reported in patients with AIDS, trauma, neoplasm, liver transplants, liver transplant rejection, and congestive heart failure. Early in the disease, the liver is enlarged and heterogeneous. Multiple regenerative nodules may be seen, and the liver becomes smaller and nodular with time as fibrosis increases and progresses to cirrhosis. CT has shown that hepatic necrosis is repaired by hypertrophy of regenerative nodules rather than by an increase in the number of nodules. These nodules give rise to the macronodular pattern of postnecrotic cirrhosis. Rarely, the nodules may be hypodense and simulate metastases on CT. Cirrhosis is discussed in detail later in this chapter.
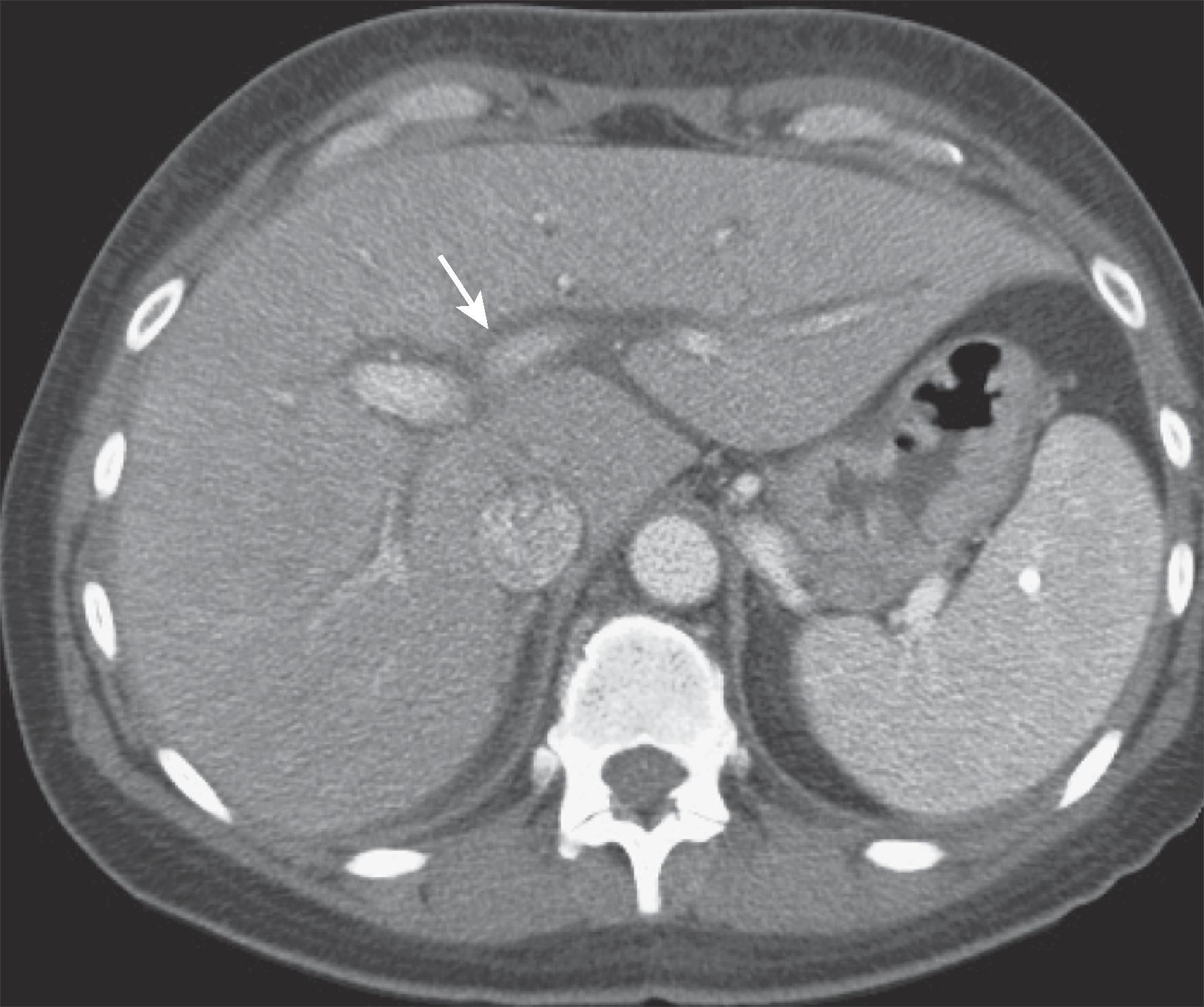
In patients with chronic active hepatitis, lymphadenopathy is commonly found in the porta hepatis, gastrohepatic ligament, and retroperitoneum; it may be the only CT abnormality in a patient with severe hepatic dysfunction. CT can also follow the course of antiviral therapy by observing a reduction in lymph node size.
On MRI, acute hepatitis may show nonspecific findings such as heterogeneous signal intensity, most apparent on T2-weighted sequences, and a heterogeneous pattern of enhancement on arterial-dominant phase spoiled gradient-echo images. This abnormal enhancement becomes more marked and can persist into the venous and delayed phases as the severity of disease increases. High-signal-intensity periportal edema can be seen on T2-weighted images. In both acute and chronic hepatitis, adenopathy in the lesser omentum may be the only abnormality identified. ,
In chronic hepatitis, MRI can provide information on the degree of histologic activity of disease and help monitor the response to therapy. Homogeneous or heterogeneous increase in signal intensity has been described on T2-weighted images and reflects the presence of inflammation or necrosis of the liver parenchyma. Early patchy enhancement patterns in patients with chronic hepatitis have been shown to be associated with significant parenchymal inflammatory reaction. Absence of this early patchy enhancement correlates with low inflammatory reaction. In patients with chronic fibrosis associated with cirrhosis, there is progressive enhancement on delayed images due to leakage of gadolinium contrast from the intravascular into the interstitial space.
In patients with alcoholic hepatitis, imaging is done to exclude obstructive biliary disease and neoplasm and to evaluate parenchymal damage noninvasively.
In acute alcoholic hepatitis, the liver is hyperechoic secondary to fatty infiltration. The liver may be enlarged early in the disease process, but it becomes atrophic as cirrhosis ensues. Because cirrhosis and fatty infiltration appear similar sonographically, both with heterogeneous echotexture, increased echogenicity, and sound attenuation, CT and MRI are more sensitive in their differentiation.
CT may show fatty infiltration in a normal-sized, enlarged, or atrophic liver, depending on the extent of prior liver injury. As hepatitis progresses to cirrhosis, alcoholic cirrhosis characteristically demonstrates a micronodular pattern as well as enlargement of the caudate lobe and of the right posterior hepatic lobe notch of greater proportion than in other causes of hepatitis. HCC develops at a 5-year cumulative rate of 8%. , Biliary stasis and development of gallstones as well as periportal lymphadenopathy are common in alcoholic hepatitis.
Like CT, MRI depicts hepatic size and morphology and can be useful in depicting hepatic fibrosis, steatosis, and cirrhosis. MRI is also the most sensitive modality for detection of HCC. Phosphorus 31 MR spectroscopy has shown that livers of patients with alcoholic hepatitis and cirrhosis can be differentiated from normal liver and from each other on the basis of intracellular pH and absolute molar concentration of adenosine triphosphate. Hepatocytes are more alkaline than normal in alcoholic hepatitis and more acidic than normal in cirrhosis.
Although drug-induced liver disease represents a small proportion of all adverse drug effects, it accounts for 2% to 5% of hospital admissions for jaundice in the United States and up to 43% of admissions for “acute hepatitis.”
Anesthetics (halothane, enflurane), anticonvulsants (phenytoin), anti-inflammatory agents (gold, clometacin, sulindac), analgesics (acetaminophen), hormonal derivatives (danazol), antidepressant or antianxiety drugs (amitriptyline, amineptine), antimicrobials (isoniazid, amoxicillin, sulfamethoxazole-trimethoprim), antiulcer drugs (cimetidine, ranitidine), hypolipidemic agents (nicotinic acid, gemfibrozil), drugs for inflammatory bowel disease (sulfasalazine), and cardiovascular drugs (quinidine, amiodarone) have all been associated with parenchymal liver disease. Liver damage may be severe, exemplified by 20% to 50% of cases of fulminant hepatic failure being attributed to drugs such as halothane, acetaminophen, α-methyldopa, and phenytoin.
The liver plays a key role in the metabolism of most medications that are either parenterally or orally administered and absorbed into the portal venous system and therefore is vulnerable to possible injury as a result of exposure. Hepatic deterioration resulting from chemical toxicity can be a diagnostic dilemma in oncology patients with known liver metastases. It is often clinically difficult to differentiate chemotherapy-induced parenchymal injury from other processes, including tumor progression, tumor necrosis, portal and hepatic venous thrombosis, hepatic ischemia and infarction, transfusion reactions, viral hepatitis, and segmental biliary obstruction by tumor. Radiology plays a key role in elucidating the cause of hepatic dysfunction in these patients.
Clinical or subclinical hepatotoxicity is common during systemic chemotherapy. A large series of patients with breast cancer demonstrated that 77% of those receiving systemic adjuvant chemotherapy and 82% of those being treated for metastatic breast cancer, all with normal pretreatment liver function test results, developed abnormal hepatic laboratory values during and after therapy. The mechanism of chemotherapy agents often involves prevention or retardation of cell division, which can also damage liver cells and diminish immune response. Methotrexate is often associated with steatosis, fibrosis of the portal triads, and eventually cirrhosis. Corticosteroids and l -asparaginase may produce steatosis. Dacarbazine and 6-thioguanine can cause thrombosis of intrahepatic portal veins, producing a Budd-Chiari–like appearance. Azathioprine and 6-mercaptopurine therapy may result in intrahepatic cholestasis and variable degrees of parenchymal cell necrosis. Intra-arterial infusion of floxuridine may cause chemical hepatitis and biliary strictures simulating sclerosing cholangitis. Mithramycin is perhaps the most hepatotoxic drug used in chemotherapy and is associated with acute parenchymal necrosis. Bevacizumab (Avastin) has been associated with hepatic sinusoidal obstruction syndrome.
On cross-sectional images, the findings are nonspecific and identical to those seen in other liver injuries: hepatomegaly, cirrhosis, and fatty infiltration. Cross-sectional imaging, however, is vital in excluding biliary obstruction or worsening liver metastases as the cause of the patient’s hepatic dysfunction. MRI and MR spectroscopy may be helpful in evaluating parenchymal injury.
Hepatotoxicity caused by amiodarone is well documented and is usually manifested by mild asymptomatic elevation of serum transaminase levels. When more severe injury occurs, it can simulate alcoholic liver disease with steatosis, Mallory bodies, and cirrhosis. The length of treatment with amiodarone before recognition of hepatic damage can range from several weeks to several years.
The sonographic findings in this disorder are nonspecific and related to steatosis or cirrhosis. The CT appearance ( Fig. 56.6 ), however, is distinctive. When this drug or its metabolites, which contain iodine, accumulate in sufficient quantities in the liver, increased parenchymal attenuation simulating hemochromatosis can be identified.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here