Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Proteins, carbohydrates, and fats in the diet provide energy for various body functions or for storage and later use. Stability of body weight and composition over long periods requires that a person’s energy intake and energy expenditure be balanced. When a person is overfed, and energy intake persistently exceeds expenditure, most of the excess energy is stored as fat, and body weight increases; conversely, loss of body mass and starvation occur when energy intake is insufficient to meet the body’s metabolic needs.
Because different foods contain different proportions of proteins, carbohydrates, fats, minerals, and vitamins, appropriate balances must also be maintained among these constituents so that all of the body’s metabolic systems can be supplied with the requisite materials. This chapter discusses the mechanisms by which food intake is regulated in accordance with the body’s metabolic needs and some of the problems of maintaining balance among the different types of foods.
The energy liberated from each gram of carbohydrate as it is oxidized to carbon dioxide and water is 4.1 Calories (1 Calorie equals 1 kilocalorie), and that liberated from fat is 9.3 Calories. The energy liberated from metabolism of the average dietary protein as each gram is oxidized to carbon dioxide, water, and urea is 4.35 Calories. Also, these substances vary in the average percentages that are absorbed from the gastrointestinal tract: about 98% of carbohydrate, 95% of fat, and 92% of protein. Therefore, the average physiologically available energy in each gram of these three foodstuffs is as follows:
| Calories | |
|---|---|
| Carbohydrate | 4 |
| Fat | 9 |
| Protein | 4 |
Although considerable variation exists among different individuals, and even in the same person from day to day, the usual diet of Americans provides about 15% of the total energy intake from protein, 40% from fat, and 45% from carbohydrate. In most non-Western countries, the quantity of energy derived from carbohydrates far exceeds that derived from proteins and fats. Indeed, in some parts of the world where meat is scarce, the energy received from fats and proteins combined may be no greater than 15% to 20%.
Table 72-1 lists the compositions of selected foods, especially demonstrating the high proportions of fat and protein in meat products and the high proportion of carbohydrate in most vegetable and grain products. Fat is deceptive in the diet because it usually exists as nearly 100% fat, whereas proteins and carbohydrates are mixed in watery media, so that each of these normally represents less than 25% of the total weight. Therefore, the fat of one pat of butter mixed with an entire helping of potato sometimes contains as much energy as the potato itself.
| Food | Protein (%) | Fat (%) | Carbohydrate (%) | Fuel Value per 100 Grams (Calories) |
|---|---|---|---|---|
| Apples | 0.3 | 0.4 | 14.9 | 64 |
| Asparagus | 2.2 | 0.2 | 3.9 | 26 |
| Bacon, fat | 6.2 | 76.0 | 0.7 | 712 |
| Bacon, broiled | 25.0 | 55.0 | 1.0 | 599 |
| Beef (average) | 17.5 | 22.0 | 1.0 | 268 |
| Beets, fresh | 1.6 | 0.1 | 9.6 | 46 |
| Bread, white | 9.0 | 3.6 | 49.8 | 268 |
| Butter | 0.6 | 81.0 | 0.4 | 733 |
| Cabbage | 1.4 | 0.2 | 5.3 | 29 |
| Carrots | 1.2 | 0.3 | 9.3 | 45 |
| Cashew nuts | 19.6 | 47.2 | 26.4 | 609 |
| Cheese, cheddar, American | 23.9 | 32.3 | 1.7 | 393 |
| Chicken, total edible | 21.6 | 2.7 | 1.0 | 111 |
| Chocolate | 5.5 | 52.9 | 18.0 | 570 |
| Corn (maize) | 10.0 | 4.3 | 73.4 | 372 |
| Haddock | 17.2 | 0.3 | 0.5 | 72 |
| Lamb, leg (average) | 18.0 | 17.5 | 1.0 | 230 |
| Milk, fresh whole | 3.5 | 3.9 | 4.9 | 69 |
| Molasses | 0.0 | 0.0 | 60.0 | 240 |
| Oatmeal, dry, uncooked | 14.2 | 7.4 | 68.2 | 396 |
| Oranges | 0.9 | 0.2 | 11.2 | 50 |
| Peanuts | 26.9 | 44.2 | 23.6 | 600 |
| Peas, fresh | 6.7 | 0.4 | 17.7 | 101 |
| Pork, ham | 15.2 | 31.0 | 1.0 | 340 |
| Potatoes | 2.0 | 0.1 | 19.1 | 85 |
| Spinach | 2.3 | 0.3 | 3.2 | 25 |
| Strawberries | 0.8 | 0.6 | 8.1 | 41 |
| Tomatoes | 1.0 | 0.3 | 4.0 | 23 |
| Tuna, canned | 24.2 | 10.8 | 0.5 | 194 |
| Walnuts, English | 15.0 | 64.4 | 15.6 | 702 |
Twenty to 30 grams of the body proteins are degraded daily and used to produce other body chemicals. Therefore, all cells must continue to form new proteins to take the place of those that are being destroyed, and a supply of protein is necessary in the diet for this purpose. An average person can maintain normal stores of protein if the daily intake is greater than 30 to 50 grams .
Some proteins have inadequate quantities of certain essential amino acids and therefore cannot be used to replace the degraded proteins. Such proteins are called partial proteins , and when they are present in large quantities in the diet, the daily protein requirement is much greater than normal. In general, proteins derived from animal foodstuffs are more complete than are proteins derived from vegetable and grain sources. For example, the protein of corn has inadequate amounts of tryptophan and lysine, two of the essential amino acids. Therefore, individuals who consume cornmeal as their principal source of protein sometimes develop the protein-deficiency syndrome called kwashiorkor , which consists of failure to grow, lethargy, depressed mentality, and edema caused by low plasma protein concentration. On the other hand, food legumes, such as chick peas and beans, provide a relatively rich source of tryptophan and lysine but contain inadequate amounts of methionine, another essential amino acid. Therefore the proteins of corn and legumes complement each other and together provide all of the essential amino acids in the diet.
When a person’s diet contains an abundance of carbohydrates and fats, almost all the body’s energy is derived from these two substances, and little is derived from proteins. Therefore, carbohydrates and fats are said to be protein sparers . Conversely, in the state of starvation, after the carbohydrates and fats have been depleted, the body’s protein stores are consumed rapidly for energy, sometimes at rates approaching several hundred grams per day rather than the normal daily rate of 30 to 50 grams.
When carbohydrates are metabolized with oxygen, exactly one carbon dioxide molecule is formed for each molecule of oxygen consumed. This ratio of carbon dioxide output to oxygen usage is called the respiratory quotient , so the respiratory quotient for carbohydrates is 1.0.
When fat is oxidized in the body’s cells, an average of 70 carbon dioxide molecules are formed for each 100 molecules of oxygen consumed. The respiratory quotient for the metabolism of fat therefore averages 0.70. When proteins are oxidized by the cells, the average respiratory quotient is 0.80. The reason that the respiratory quotients for fats and proteins are lower than those for carbohydrates is that a portion of the oxygen metabolized with these foods is required to combine with the excess hydrogen atoms present in their molecules, so less carbon dioxide is formed in relation to the oxygen used.
Now let us see how one can use the respiratory quotient to determine the relative utilization of different foods by the body. First, recall from Chapter 40 that the output of carbon dioxide by the lungs divided by the uptake of oxygen during the same period is called the respiratory exchange ratio . During a period of 1 hour or more, the respiratory exchange ratio exactly equals the average respiratory quotient of the metabolic reactions throughout the body. If a person has a respiratory quotient of 1.0, he or she is metabolizing carbohydrates almost exclusively, because the respiratory quotients for both fat and protein metabolism are considerably less than 1.0. Likewise, when the respiratory quotient is about 0.70, the body is metabolizing mostly fats, to the exclusion of carbohydrates and proteins. And, finally, if we ignore the normally small amount of protein metabolism, respiratory quotients between 0.70 and 1.0 describe the approximate ratios of carbohydrate to fat metabolism. To be more exact, one can first determine the protein utilization by measuring nitrogen excretion, as discussed in the next section. Then, using the appropriate mathematical formula, one can calculate the utilization of the three foodstuffs.
Some of the important findings from studies of respiratory quotients are the following:
Immediately after a mixed meal containing carbohydrates as well as protein and fat, almost all the food that is metabolized is carbohydrates, so the respiratory quotient at that time approaches 1.0.
About 8 to 10 hours after a meal, the body has already used up most of its readily available carbohydrates, and the respiratory quotient approaches that for fat metabolism, about 0.70.
In untreated diabetes mellitus, little carbohydrate can be used by the body’s cells under any conditions because insulin is required for this utilization. Therefore, when diabetes is severe, most of the time the respiratory quotient remains near that for fat metabolism, which is 0.70.
The average protein contains about 16% nitrogen. During metabolism of protein, about 90% of this nitrogen is excreted in the urine in the form of urea, uric acid, creatinine, and other nitrogen products. The remaining 10% is excreted in the feces. Therefore, the rate of protein breakdown in the body can be estimated by measuring the amount of nitrogen in the urine, then adding 10% for the nitrogen excreted in the feces, and multiplying by 6.25 (i.e., 100/16) to estimate the total amount of protein metabolism in grams per day. Thus, excretion of 8 grams of nitrogen in the urine each day means that about 55 grams of protein breakdown has occurred. If the daily intake of protein is less than the daily breakdown of protein, the person is said to have a negative nitrogen balance , which means that his or her body stores of protein are decreasing daily.
Stability of the body’s total mass and composition over long periods requires that energy intake match energy expenditure. As discussed in Chapter 73 , only about 27% of the energy ingested normally reaches the functional systems of the cells, and much of this energy is eventually converted to heat, which is generated as a result of protein metabolism, muscle activity, and activities of the various organs and tissues of the body. Excess energy intake is stored mainly as fat, whereas a deficit of energy intake causes loss of total body mass until energy expenditure eventually equals energy intake or death occurs.
Although there is considerable variability in the amount of energy storage (i.e., fat mass) in different individuals, maintenance of an adequate energy supply is necessary for survival. Therefore, the body is endowed with powerful physiological control systems that help maintain adequate energy intake. Deficits of energy stores, for example, rapidly activate multiple mechanisms that cause hunger and drive a person to seek food. In athletes and laborers, energy expenditure for the high level of muscle activity may be as high as 10,000 Calories per day, compared with only about 2000 Calories per day for sedentary individuals. Thus, a large energy expenditure associated with physical work usually stimulates equally large increases in caloric intake.
What are the physiological mechanisms that sense changes in energy balance and influence the quest for food? Maintenance of adequate energy supply in the body is so critical that multiple short-term and long-term control systems exist that regulate not only food intake but also energy expenditure and energy stores. In the next few sections we describe some of these control systems and their operation in physiological conditions, as well as in the states of obesity and starvation.
The sensation of hunger is associated with a craving for food and several other physiological effects, such as rhythmic contractions of the stomach and restlessness, which cause the person to seek food. A person’s appetite is a desire for food , often of a particular type, and is useful in helping to choose the quality of the food to be eaten. If the quest for food is successful, the feeling of satiety occurs. Each of these feelings is influenced by environmental and cultural factors, as well as by physiological controls that influence specific centers of the brain, especially the hypothalamus.
Several neuronal centers of the hypothalamus participate in the control of food intake. The lateral nuclei of the hypothalamus serve as a feeding center, and stimulation of this area causes an animal to eat voraciously (hyperphagia) . Conversely, destruction of the lateral hypothalamus causes lack of desire for food and progressive inanition, a condition characterized by marked weight loss, muscle weakness, and decreased metabolism. The lateral hypothalamic feeding center operates by exciting the motor drives to search for food.
The ventromedial nuclei of the hypothalamus serve as a major satiety center . This center is believed to give a sense of nutritional satisfaction that inhibits the feeding center. Electrical stimulation of this region can cause complete satiety, and even in the presence of highly appetizing food, the animal refuses to eat (aphagia) . Conversely, destruction of the ventromedial nuclei causes voracious and continued eating until the animal becomes extremely obese, sometimes weighing as much as four times normal.
The paraventricular, dorsomedial , and arcuate nuclei of the hypothalamus also play a major role in regulating food intake. For example, lesions of the paraventricular nuclei often cause excessive eating, whereas lesions of the dorsomedial nuclei usually depress eating behavior. As discussed later, the arcuate nuclei are the sites in the hypothalamus where multiple hormones released from the gastrointestinal tract and adipose tissue converge to regulate food intake, as well as energy expenditure.
Much chemical cross talk occurs among the neurons in the hypothalamus, and together, these centers coordinate the processes that control eating behavior and the perception of satiety. These hypothalamic nuclei also influence secretion of several hormones that are important in regulating energy balance and metabolism, including those from the thyroid and adrenal glands, as well as the pancreatic islet cells.
The hypothalamus receives (1) neural signals from the gastrointestinal tract that provide sensory information about stomach filling; (2) chemical signals from nutrients in the blood (glucose, amino acids, and fatty acids) that signify satiety; (3) signals from gastrointestinal hormones; (4) signals from hormones released by adipose tissue; and (5) signals from the cerebral cortex (sight, smell, and taste) that influence feeding behavior. Some of these inputs to the hypothalamus are shown in Figure 72-1 .
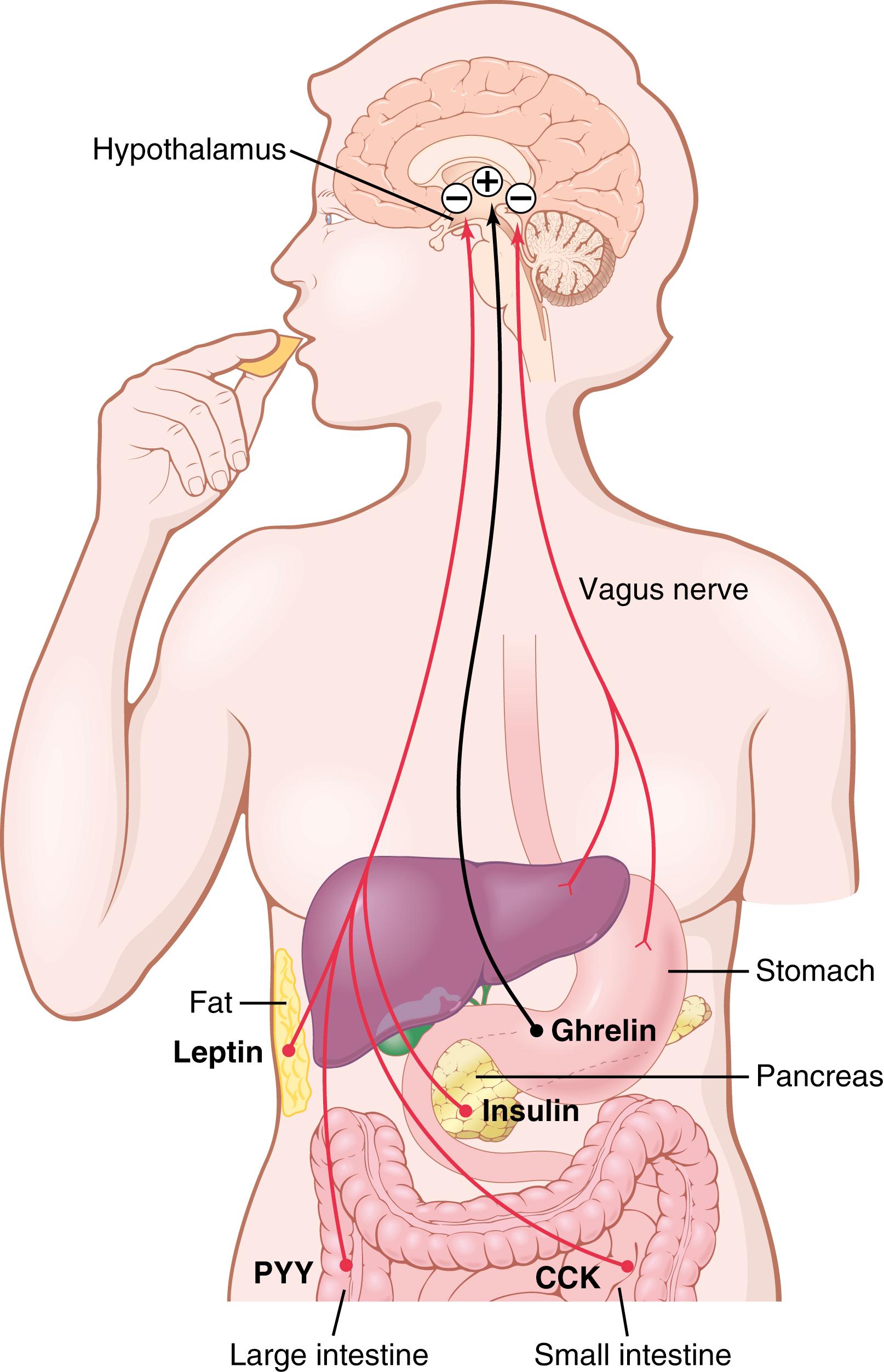
The hypothalamic feeding and satiety centers have a high density of receptors for neurotransmitters and hormones that influence feeding behavior. A few of the many substances that have been shown to alter appetite and feeding behavior in experimental studies are listed in Table 72-2 and are generally categorized as (1) orexigenic substances that stimulate feeding or (2) anorexigenic substances that inhibit feeding.
| Decrease Feeding (Anorexigenic) | Increase Feeding (Orexigenic) |
|---|---|
| α–Melanocyte-stimulating hormone | Neuropeptide Y |
| Leptin | Agouti-related protein |
| Serotonin | Melanin-concentrating hormone |
| Norepinephrine | Orexins A and B |
| Corticotropin-releasing hormone | Endorphins |
| Insulin | Galanin |
| Cholecystokinin | Amino acids (glutamate and γ-aminobutyric acid) |
| Glucagon-like peptide | Cortisol |
| Cocaine- and amphetamine-regulated transcript | Ghrelin |
| Peptide YY | Endocannabinoids |
Two distinct types of neurons in the arcuate nuclei of the hypothalamus are especially important as controllers of both appetite and energy expenditure ( Figure 72-2 ): (1) pro-opiomelanocortin (POMC) neurons that produce α–melanocyte-stimulating hormone (α-MSH) together with cocaine- and amphetamine-related transcript (CART); and (2) neurons that produce the orexigenic substances neuropeptide Y (NPY) and agouti-related protein (AGRP). Activation of the POMC neurons decreases food intake and increases energy expenditure, whereas activation of the NPY-AGRP neurons has the opposite effects, increasing food intake and reducing energy expenditure. Considerable cross talk occurs among these neurons and, as discussed later, POMC/CART and AGRP/NPY neurons appear to be the major targets for several hormones that regulate appetite, including leptin , insulin , cholecystokinin (CCK), and ghrelin . In fact, the neurons of the arcuate nuclei appear to be a site of convergence of many of the nervous and peripheral signals that regulate energy stores.
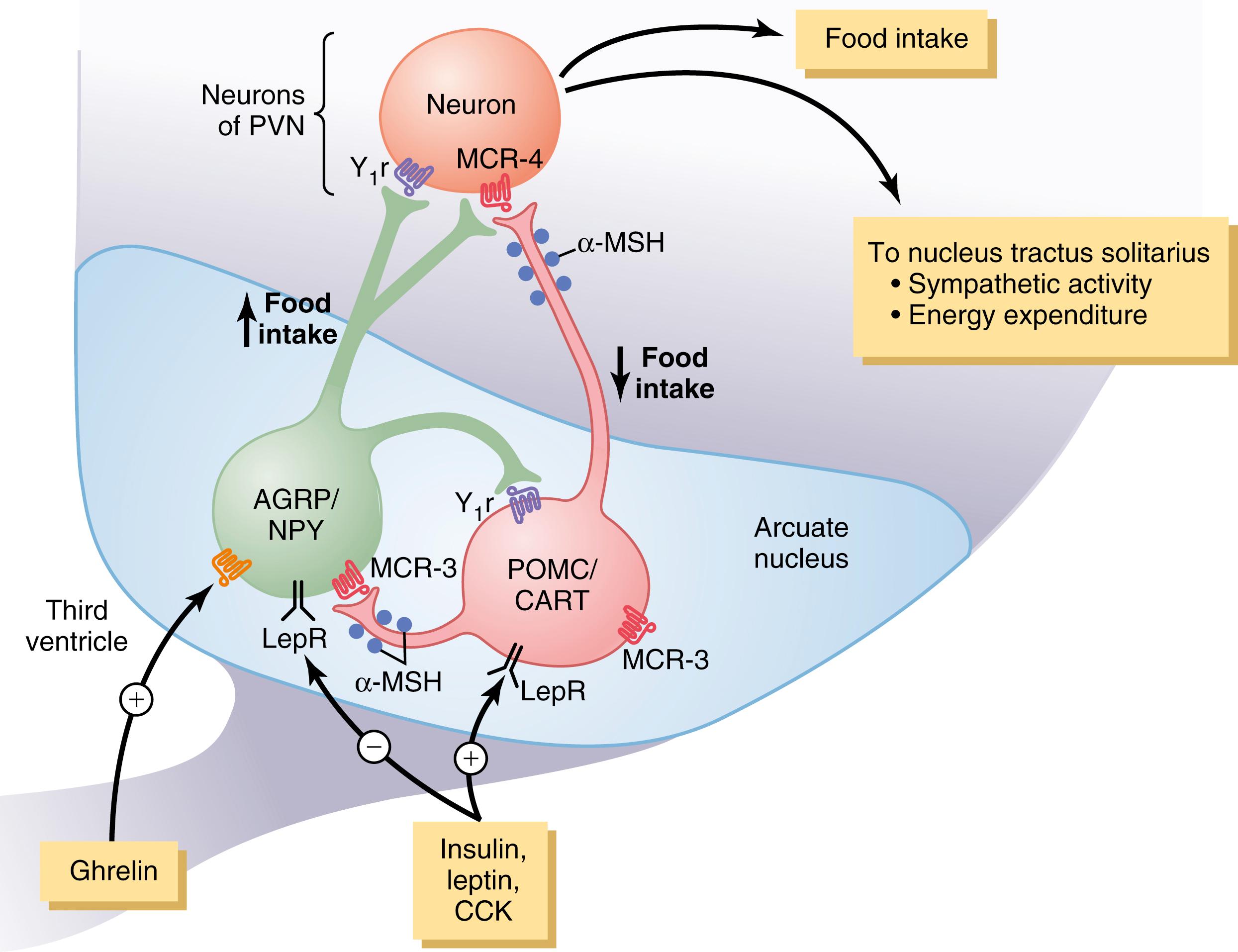
The POMC neurons release α-MSH, which then acts on melanocortin receptors found especially in neurons of the paraventricular nuclei . Although at least five subtypes of melanocortin receptors (MCR) exist, MCR-3 and MCR-4 are especially important in regulating food intake and energy balance. Activation of these receptors reduces food intake while increasing energy expenditure. Conversely, inhibition of MCR-3 and MCR-4 greatly increases food intake and decreases energy expenditure. The effect of MCR-4 activation to increase energy expenditure appears to be mediated, at least in part, by activation of neuronal pathways that project from the paraventricular nuclei to the nucleus tractus solitarius (NTS) and stimulate sympathetic nervous system activity. However, POMC neurons and MCR-4 are also found in brainstem neurons, including the NTS, where they also regulate food intake and energy expenditure.
The hypothalamic–brainstem melanocortin system plays a powerful role in regulating energy stores of the body, and defective signaling of this pathway is associated with extreme obesity. In fact, mutations of POMC and MCR-4 represent the most common known monogenic (single-gene) causes of human obesity, and some studies suggest that POMC and MCR-4 mutations may account for as much as 5% to 6% of early-onset severe obesity in children. In contrast, excessive activation of the melanocortin system reduces appetite. Some studies suggest that this activation may play a role in causing the loss of appetite for food (anorexia) associated with severe infections, cancer tumors, or uremia.
AGRP released from the orexigenic neurons of the hypothalamus is a natural antagonist of MCR-3 and MCR-4 and probably increases feeding by inhibiting the effects of α-MSH to stimulate melanocortin receptors (see Figure 72-2 ). Although the role of AGRP in normal physiological control of food intake is unclear, excessive formation of AGRP in mice and humans due to gene mutations is associated with increased food intake and obesity.
NPY is also released from orexigenic neurons of the arcuate nuclei. When energy stores of the body are low, orexigenic neurons are activated to release NPY, which stimulates appetite. At the same time, firing of the POMC neurons is reduced, thereby decreasing the activity of the melanocortin pathway and further stimulating appetite.
Another aspect of feeding is the mechanical act of the feeding process. If the brain is sectioned below the hypothalamus but above the mesencephalon, the animal can still perform the basic mechanical features of the feeding process. It can salivate, lick its lips, chew food, and swallow. Therefore, the actual mechanics of feeding are controlled by centers in the brainstem . The function of the other centers in feeding, then, is to control the quantity of food intake and to excite these centers of feeding mechanics to activity.
Neural centers higher than the hypothalamus also play important roles in the control of feeding, particularly in the control of appetite. These centers include the amygdala and the prefrontal cortex , which are closely coupled with the hypothalamus. Recall from the discussion of the sense of smell in Chapter 54 that portions of the amygdala are a major part of the olfactory nervous system. Destructive lesions in the amygdala have demonstrated that some of its areas increase feeding, whereas others inhibit feeding. In addition, stimulation of some areas of the amygdala elicits the mechanical act of feeding. An important effect of destruction of the amygdala on both sides of the brain is a “psychic blindness” in the choice of foods. In other words, the animal (and presumably the human being as well) at least partially loses the appetite control that determines the type and quality of food it eats.
Regulation of the quantity of food intake can be divided into short-term regulation , which is concerned primarily with preventing overeating at each meal, and long-term regulation , which is concerned primarily with maintenance of normal quantities of energy stores in the body.
When a person is driven by hunger to eat voraciously and rapidly, what turns off the desire to eat when he or she has eaten enough? There has not been enough time for changes in the body’s energy stores to occur, and it takes hours for enough nutritional factors to be absorbed into the blood to cause the necessary inhibition of eating. Yet, it is important that the person not overeat and that he or she eat an amount of food that approximates nutritional needs. Several types of rapid feedback signals are important for these purposes, as described in the following sections.
When the gastrointestinal tract becomes distended, especially the stomach and the duodenum, stretch inhibitory signals are transmitted mainly by way of the vagi to suppress the feeding centers, thereby reducing the desire for food and providing a negative feedback mechanism to help limit meal size (see Figure 72-1 ).
CCK, which is released mainly in response to fat and proteins entering the duodenum, enters the blood and acts as a hormone to influence several gastrointestinal functions such as gallbladder contraction, gastric emptying, gut motility, and gastric acid secretion as discussed in Chapter 63, Chapter 64, Chapter 65 . However, CCK also activates receptors on local sensory nerves in the duodenum, sending messages to the brain via the vagus nerve that contribute to satiation and meal cessation. The effect of CCK is short-lived, and chronic administration of CCK by itself has no major effect on body weight. Therefore, CCK functions mainly to prevent overeating during meals but may not play a major role in the frequency of meals or the total energy consumed.
Peptide YY (PYY) is secreted from the entire gastrointestinal tract, but especially from the ileum and colon. Food intake stimulates release of PYY, with blood concentrations rising to peak levels 1 to 2 hours after ingesting a meal. These peak levels of PYY are influenced by the amount and composition of the food, with higher levels of PYY observed after meals with a high fat content. Although injections of PYY into mice have been shown to decrease food intake for 12 hours or more, the importance of this gastrointestinal hormone in regulating appetite in humans is still unclear.
For reasons that are not entirely understood, the presence of food in the intestines stimulates them to secrete glucagon-like peptide (GLP), which in turn enhances glucose-dependent insulin production and secretion from the pancreas. GLP and insulin both tend to suppress appetite. Thus, eating a meal stimulates the release of several gastrointestinal hormones that may induce satiety and limit further intake of food (see Figure 72-1 ).
Ghrelin is a hormone released mainly by the oxyntic cells of the stomach but also, to much less of an extent, by the intestine. Blood levels of ghrelin rise during fasting, peak just before eating, and then fall rapidly after a meal, suggesting a possible role in stimulating feeding. Also, administration of ghrelin increases food intake in experimental animals, further supporting the possibility that it may be an orexigenic hormone.
When an animal with an esophageal fistula is fed large quantities of food, even though this food is immediately lost to the exterior, the degree of hunger is decreased after a reasonable quantity of food has passed through the mouth. This effect occurs despite the fact that the gastrointestinal tract does not become the least bit filled. Therefore, various “oral factors” related to feeding, such as chewing, salivation, swallowing, and tasting, have been postulated to “meter” the food as it passes through the mouth, and after a certain amount has passed, the hypothalamic feeding center becomes inhibited. The inhibition caused by this metering mechanism, however, is considerably less intense and of shorter duration—usually lasting for only 20 to 40 minutes—than is the inhibition caused by gastrointestinal filling.
An animal that has been starved for a long time and is then presented with unlimited food eats a far greater quantity than does an animal that has been on a regular diet. Conversely, an animal that has been force-fed for several weeks eats very little when allowed to eat according to its own desires. Thus, the biological feeding control mechanisms of the body are geared to the nutritional status of the body although multiple behavioral, social and environmental factors also influence food intake in humans.
A decrease in blood glucose concentration has been shown in experimental studies to cause hunger, which has led to the so-called glucostatic theory of hunger and feeding regulation . Similar studies have demonstrated the same effect for blood amino acid concentration and blood concentration of breakdown products of lipids such as the keto acids and some fatty acids, leading to the aminostatic and lipostatic theories of regulation. That is, when the availability of any of the three major types of food decreases, the desire for feeding is increased, eventually returning the blood metabolite concentrations back toward normal if the appropriate foods are available.
The following observations from neurophysiological studies of function in specific areas of the brain also support the glucostatic, aminostatic, and lipostatic theories: (1) a rise in blood glucose level increases the rate of firing of glucoreceptor neurons in the satiety center in the ventromedial and paraventricular nuclei of the hypothalamus , and (2) the same increase in blood glucose level simultaneously decreases the firing of glucosensitive neurons in the hunger center of the lateral hypothalamus . In addition, some amino acids and lipid substances affect the rates of firing of these same neurons or other closely associated neurons.
When an animal is exposed to cold, it tends to increase feeding; when it is exposed to heat, it tends to decrease its caloric intake. This phenomenon is caused by interaction within the hypothalamus between the temperature-regulating system (see Chapter 74 ) and the food intake–regulating system. This is important because increased food intake in a cold animal (1) increases its metabolic rate and (2) provides increased fat for insulation, both of which tend to protect against the cold.
Most of the stored energy in the body consists of fat, the amount of which can vary considerably in different persons. What regulates this energy reserve, and why is there so much variability among individuals?
Studies in humans and in experimental animals indicate that the hypothalamus senses energy storage through the actions of leptin , a peptide hormone released from adipocytes. When the amount of adipose tissue increases (signaling excess energy storage), the adipocytes produce increased amounts of leptin, which is released into the blood. Leptin then circulates to the brain, where it moves across the blood-brain barrier by facilitated diffusion and occupies leptin receptors at multiple sites in the hypothalamus, especially the POMC and AGRP/NPY neurons of the arcuate nuclei and neurons of the paraventricular nuclei, as well as neurons in other areas of the brain including the brainstem.
Stimulation of leptin receptors in these central nervous system nuclei initiates multiple actions that decrease fat storage, including (1) decreased production in the hypothalamus of appetite stimulators, such as NPY and AGRP ; (2) activation of POMC neurons , causing release of α-MSH and activation of melanocortin receptors; (3) increased production in the hypothalamus of substances, such as corticotropin-releasing hormone , that decrease food intake; (4) increased sympathetic nerve activity (through neural projections from the hypothalamus to the vasomotor centers), which increases metabolic rate and energy expenditure; and (5) decreased insulin secretion by the pancreatic beta cells, which decreases energy storage. Thus, leptin is an important means by which the adipose tissue signals the brain that enough energy has been stored and that intake of food is no longer necessary.
In mice or humans with mutations that render their fat cells unable to produce leptin or mutations that cause defective leptin receptors in the hypothalamus, marked hyperphagia and morbid obesity occur. In most obese humans, however, there does not appear to be a deficiency of leptin production because plasma leptin levels increase in proportion with increasing adiposity. Therefore, some physiologists believe that obesity may be associated with leptin resistance ; that is, leptin receptors or postreceptor signaling pathways normally activated by leptin may be resistant to activation by leptin in obese people, who continue to overeat despite having very high levels of leptin.
Another explanation for the failure of leptin to prevent increasing adiposity in obese individuals is that there are many redundant systems that control feeding behavior, as well as social and cultural factors that can cause continued excess food intake even in the presence of high levels of leptin.
Even though our information on the different feedback factors in long-term feeding regulation is imprecise, we can make the following general statement: When the energy stores of the body fall below normal, the feeding centers of the hypothalamus and other areas of the brain become highly active, and the person exhibits increased hunger, as well as the behavior of searching for food. Conversely, when the energy stores (mainly the fat stores) are already abundant, the person usually loses the sensation of hunger and develops a state of satiety. Although the precise feedback systems that regulate food intake and energy expenditure are not fully understood, rapid advances have been made in this field of research in recent years, with the discovery of many new orexigenic and anorexigenic factors.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here