Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Venous thromboembolism (VTE) is a common complication in patients with cancer, affecting approximately 15% of patients during their clinical course. VTE is fivefold to sevenfold more likely to develop in patients with cancer than in patients without cancer.
VTE is the second most common cause of mortality among patients with cancer and is associated with a threefold increased risk of death compared with patients without cancer.
The incidence of VTE varies by cancer type and extent of disease. High-risk cancers include pancreatic, brain, and gastric tumors, whereas breast, head and neck, and prostate cancers are associated with a lower risk. Metastatic cancer is associated with a twofold-increased risk of VTE. Lymphoma and myeloma are also associated with a high risk of VTE.
Surgery, chemotherapy, hormonal therapy, erythropoietic stimulatory agents, and central venous catheters (CVCs) increase the risk of cancer-associated VTE.
In a prospective observational study of more than 2300 patients with cancer who underwent surgery, VTE was responsible for 46% of deaths, making it the most common cause of death within the first 30 days after surgery.
Pharmacologic VTE prophylaxis is recommended for all surgical and medical oncology patients without contraindications. Optimally managed mechanical prophylaxis should be used when pharmacologic prophylaxis is contraindicated.
Adjusted dose of warfarin (international normalized ratio, 1.3–1.9), prophylactic doses of nadroparin and semuloparin, and a therapeutic dose of dalteparin have been shown to reduce the risk of VTE in ambulatory patients with cancer who are receiving chemotherapy. Although no survival advantage has been demonstrated with the use of prophylactic anticoagulation, the studies have not been powered to do so.
The Khorana risk score that is calculated on the basis of tumor type, prechemotherapy platelet count, and white blood cell count, hemoglobin, use of erythropoietic stimulatory agents, and body mass index can be used to assess the risk of VTE among ambulatory patients with cancer who are starting chemotherapy. This score may help to identify ambulatory medical oncology patients in whom outpatient VTE prophylaxis may be beneficial.
Enoxaparin, 40 mg daily, and dalteparin, 5000 units daily for 28 days, have been shown to reduce the incidence of VTE compared with prophylaxis for 6 to 10 days in patients with cancer who have undergone surgery.
Extended outpatient pharmacologic VTE prophylaxis should be considered for high-risk surgical oncology patients. Risk factors for VTE in surgical oncology patients include age above 60 years, anesthesia time exceeding 2 hours, bed rest exceeding 3 days, advanced cancer stage, and a previous history of VTE.
Diagnosis of VTE in patients with cancer relies primarily on objective imaging with duplex ultrasonography and computed tomography (CT) angiography. In patients with negative duplex studies in whom there is a high suspicion of DVT, CT venography should be considered.
Low-molecular-weight heparin (LMWH) is recommended for the initial and long-term treatment of VTE in most patients with cancer who have VTE. Anticoagulation should be continued for a minimum of 3 months or while cancer is present or until there is no evidence of active cancer and therapy is completed.
While direct oral anticoagulants (DOACs) have demonstrated excellent efficacy and safety in the general VTE population, limited data are available in the cancer patient population. Two recently published studies suggest that the efficacy is good in active cancer patients but with an increased risk of bleeding compared to low molecular weight heparins.
Catheter-directed pharmacomechanical thrombolysis is a consideration in patients with cancer who do not have contraindications to its use, but it is reserved for those who have extensive or limb- or life-threatening DVT. Catheter-directed pharmacomechanical thrombolysis is associated with an increased risk of bleeding.
Systemic thrombolytic therapy should be considered for patients with hemodynamically significant pulmonary embolism (PE).
Vena cava filters are effective for prevention of PE but are also associated with an increased risk of DVT and inferior vena cava thrombosis. Therefore, inferior vena cava filters are primarily recommended for patients who have acute proximal lower extremity thrombus and are not candidates for anticoagulation or are at high risk and will be undergoing surgical procedures.
Patients with cancer who have stable PE and no signs of hemodynamic compromise can be safely treated as outpatients in the absence of other contraindications to outpatient management. Assessment of right ventricular overload by echocardiography or CT angiography and/or biomarkers can assist with decision making.
Patients with incidentally discovered PE should be managed in a similar fashion as patients with symptomatic PE because their outcomes appear to be similar.
Common causes of recurrent VTE in patients with cancer include local vascular compression, therapeutic resistance due to the hypercoagulable state of malignancy, especially if a vitamin K antagonist is used, and heparin-induced thrombocytopenia.
Management of recurrent VTE is variable and depends on the type and intensity of anticoagulation that the patient was using when the recurrent VTE occurred.
Prospective studies have noted that symptomatic central venous catheter thrombosis occurs in 4% of patients with cancer.
Risk factors for central access catheter (CAC)–associated VTE include left-sided insertion, CAC outer diameter and number of lumens, and catheter tip position above or below the superior vena cava–right atrial junction.
Prophylactic doses of LMWH and low-dose warfarin are ineffective for CAC-associated deep venous thrombosis (DVT) and should not be prescribed. Adjusted-dose warfarin (international normalized ratio, 1.5–2) was associated with a reduced incidence of CAC thrombosis at a cost of increased bleeding.
In general, CAC-associated thrombosis can be managed by anticoagulation alone without CAC removal. Anticoagulation should be continued for at least 3 months or as long as the CAC is in place.
LMWH is usually used for treatment as with other types of VTE. One study in which a DOAC was used to treat CAC-associated VTE in a small number of cancer patients was concerning because of the development of fatal PE.
Patients with primary and metastatic brain tumors without evidence of hemorrhage typically can be cautiously treated with anticoagulation for VTE. Metastatic central nervous system tumors at high risk for bleeding include metastatic melanoma, renal cell carcinoma, thyroid carcinoma, and choriocarcinoma. Current data are from retrospective studies.
Patients with metastases that have been treated appear to have no difference in risk of bleeding when treated with LMWH for VTE compared with patients without central nervous system (CNS) disease.
Patients with primary CNS tumors have an increased risk of VTE.
The seminal description of the association between cancer and venous thromboembolism (VTE) by Trousseau was made more than 150 years ago. Consequently, the combination of the cancer and VTE is still commonly referred to as Trousseau syndrome. In addition to deep venous thrombosis (DVT) and pulmonary embolism (PE), a wide range of clinically significant thromboembolic events have been observed in patients with cancer, including visceral thrombosis, catheter-related thrombosis, arterial thromboembolism, nonbacterial (marantic) thrombotic endocarditis, migratory thrombophlebitis, hepatic venoocclusive disease (VOD), and disseminated intravascular coagulation (DIC). Since the pivotal observation by Trousseau in 1865, our understanding of the prevalence and pathophysiology of VTE in patients with cancer has significantly improved, novel and advanced diagnostic techniques have become readily available for daily clinical use, and effective preventive and therapeutic interventions have been incorporated into standard clinical practice. Nonetheless, the intimate complex bidirectional relationship between VTE and cancer has not been completely deciphered, and VTE continues to be an important and a commonly underrecognized contributor to morbidity and mortality in patients with cancer. This chapter discusses the epidemiology, pathogenesis, diagnosis, prevention, and treatment of cancer-associated VTE.
Since the early observations by Trousseau and others, the strong association between malignancy and development of VTE has been confirmed in multiple retrospective and observational prospective studies. The estimated annual incidence of VTE in patients with cancer is 0.5%, compared with 0.1% in the general population. During the clinical course of cancer, the cumulative incidence of symptomatic VTE has been reported to be approximately 15%, with a range of 3.8% to 30.7%. It has been estimated that up to one-fifth of all patients with VTE have an underlying cancer. As a group, malignancies are associated with a fourfold to sevenfold increase in VTE, with a 28-fold increase in risk of VTE in certain types of cancer. Researchers from the Netherlands observed a sevenfold increase in risk of VTE among patients with malignancy compared with persons without cancer. In a large population-based study, the presence of a malignant neoplasm was associated with an odds ratio (OR) of 6.5 for VTE compared with control subjects without cancer. In addition, incidental asymptomatic VTE is noted on routine imaging in 1.5% to 6.3% of patients.
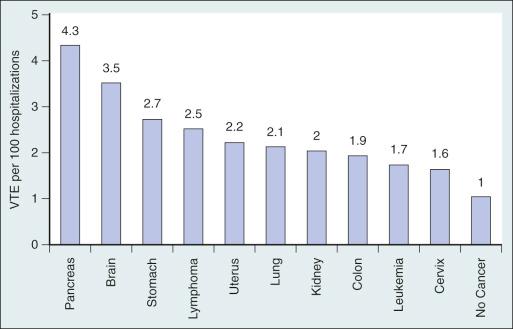
The incidence of VTE is not uniformly distributed among different cancer types. In general, pancreatic, gastric, brain, ovarian, renal, and hematologic malignancies have been associated with a higher risk of VTE, whereas cancers of the head and neck, breast, prostate, and esophagus are associated with lower VTE rates ( Fig. 33.1 ). However, because of the higher incidence and prevalence of lung, colon, prostate, and breast cancers, these malignancies are associated with the highest absolute number of VTEs. The histologic type and extent of the malignancy also influence VTE risk. For example, lung adenocarcinoma is associated with a greater risk of VTE than is squamous cell carcinoma of the lung. Metastatic cancers are more likely to be associated with VTE than are localized malignancies. For the most common cancer types, the relative risk (RR) of symptomatic VTE has been estimated to be fourfold to more than twentyfold higher for metastatic malignancies. Even within the same patient with cancer, VTE risk varies throughout the course of the person's disease because of effects of other intrinsic and extrinsic factors such as cancer stage, surgery and length of anesthesia, chemotherapeutic and hormonal therapies, age, the presence of indwelling central venous catheters (CVCs), immobilization, inherited thrombophilia, a previous personal history of VTE, and infections. The first year after cancer diagnosis, and especially the first few months, is particularly associated with the development of VTE, although the risk of VTE remains elevated for many years after the initial cancer diagnosis. Blom and colleagues noted an adjusted OR for VTE of 53.5 (95% confidence interval [CI], 8.6–334.3) during the first 3 months after cancer diagnosis, with a subsequent reduction to 14.3 (95% CI, 5.8–35.2) 3 to 12 months after diagnosis, and a reduction to 3.6 (95% CI, 2.0–6.5) 1 to 3 years after diagnosis, but the increased VTE risk persisted for 15 years after cancer diagnosis.
Similar to solid tumors, hematologic malignancies are also associated with an increased incidence of cancer-associated VTE. In fact, in a population-based study, patients with hematologic malignancies had the highest risk of VTE among different tumor types (OR, 28 [95% CI, 4–200]). Among patients with hematologic malignancies, patients with multiple myeloma (MM) are at a higher risk for VTE due to cancer and treatment-related factors. It has been reported that cancer-associated VTE complicates the course of MM in at least 10% of patients. In an analysis from the California Cancer Registry, the 1-year cumulative incidence of VTE in persons with acute myeloid leukemia, lymphoma, chronic lymphocytic leukemia, acute lymphoblastic leukemia, and chronic myeloid leukemia were 3.7%, 2.8%, 2.7%, 2.6%, and 1.5%, respectively.
The relationship between cancer and VTE is bidirectional; VTE, especially idiopathic VTE, can be a harbinger of an occult malignancy. Population-based observational studies have documented an increased risk of malignancy after a first episode of idiopathic VTE. In an analysis of the Swedish Cancer Registry, Baron and colleagues found that at the time of VTE diagnosis or during the first year of follow-up, there was a large increase in the risk for virtually all cancers (standardized incidence ratio of 4.4 [95% CI, 4.2–4.6]) The increased risk of future cancer diagnosis in patients with VTE compared with those without VTE persisted for 2 through 25 years after admission to the hospital with the index VTE. In a large analysis of the Danish Cancer Registry, an increased standardized incidence ratio of 1.3 (95% CI, 1.21–1.33) for cancer diagnosis was found for patients with a VTE episode. The risk was substantially elevated only during the first 6 months of follow-up and declined rapidly thereafter to a constant level slightly above 1.0 a year after the VTE episode. The risk of cancer was twofold higher for patients diagnosed with idiopathic versus triggered VTE. The association was most pronounced for cancers of the pancreas, ovary, liver, and brain. Unfortunately, extensive cancer screening at the time of VTE diagnosis is not associated with improved outcome because most cancers associated with VTE are metastatic at the time of VTE diagnosis. Consequently, the possibility of an underlying cancer among patients with an idiopathic VTE always should be taken into consideration, but cancer screening should be limited to age-appropriate screening procedures in the absence of obvious signs of an underlying malignancy.
Studies have shown that diagnosis of VTE in patients with malignancy is associated with worse outcomes and shortened survival. VTE is the second most common cause of mortality after cancer itself among patients with malignancy. In fact, 15% of deaths occurring in hospitalized patients with cancer are attributable to PE. Chew and colleagues analyzed approximately 235,000 patients with cancer in the California Cancer Registry who were linked to the California Patient Discharge Data Set and found in a multivariate analysis that diagnosis of VTE during the first year of follow-up was a significant predictor of death and decreased survival for most cancer types and stages. The 1-year survival of patients with cancer who were diagnosed with VTE is one-third (12% versus 36%) that of patients with cancer who do not have VTE. Levitan and colleagues noted a threefold increase in 6-month mortality among patients with cancer who had VTE compared with those without VTE.
In addition to symptomatic VTE, unsuspected asymptomatic PE found on routine cancer staging computed tomography (CT) was found to adversely affect survival in patients with cancer. In a retrospective matched cohort study, the hazard ratio (HR) for death among patients who had cancer with unsuspected PE detected on staging CT was 1.51 (95% CI, 1.01–2.27), with the risk attributable to proximal rather than subsegmental unsuspected PE. Another retrospective study showed that patients with cancer who were diagnosed with and treated for incidental PE had similar rates of recurrent VTE, bleeding, and mortality compared with patients with cancer who had symptomatic PE. Moreover, several studies in patients with cancer have correlated increases in biomarkers of thrombin generation, even in absence of documented VTE, with more aggressive cancer biology and worse outcomes. Despite ongoing anticoagulation therapy, patients with cancer have a 3.2-fold increased risk of recurrent VTE compared with patients without cancer (12-month cumulative VTE incidence 20.7% versus 6.8%). In addition, the incidence of major bleeding is 2.2-fold higher in patients with cancer compared with patients without cancer (12.4% versus 4.9%). The risks of recurrent VTE and bleeding appear to correlate with the extent of the malignancy. As expected, the development of VTE in patients with cancer is associated with a significant increase in the consumption of health care resources.
Multiple factors contribute to the hypercoagulable state associated with malignancy. In general terms, these factors can be divided into three broad categories: factors intrinsic to the cancer (tumor-specific factors), patient-related factors (host-specific factors), and environmental factors.
Malignancy is characterized by a bidirectional interrelationship connecting cancer growth, progression, and metastasis with activation of the coagulation cascade and subsequent thrombin generation and inflammation. As discussed earlier, tumor-associated factors such as site, histology, and stage all influence the risk of thrombosis. Cancer cells can disrupt the hemostatic balance via several different pathways, including production of procoagulant; profibrinolytic, proproteolytic, and proaggregating activities; expression of adhesion molecules that mediate direct interactions with host vascular and blood cells; and secretion of proinflammatory and proangiogenic cytokines. Fig. 33.2 depicts some of the tumor-specific factors that contribute to the hypercoagulable state of cancer. Cancer procoagulant is a cysteine protease expressed only by malignant cells that can directly activate factor X independent of factor VII. In addition, cancer procoagulant has been demonstrated to activate platelets, further adding to its prothrombotic potential. Cancer procoagulant has been identified in a wide variety of cancer types and has been noted to have increased activity at disease onset with a subsequent slow decline.
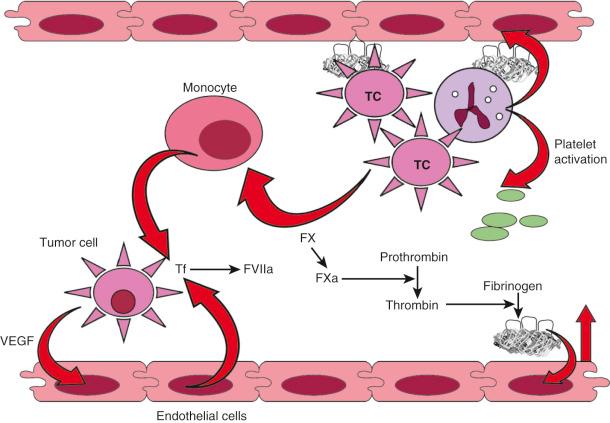
Tissue factor (TF) is the principal initiator of the coagulation protease cascade and is normally expressed as a transmembrane glycoprotein on vascular subendothelial cells. Therefore TF is not normally in contact with blood unless vascular endothelial integrity is compromised or if its expression is induced on endothelial cells by inflammatory stimuli. Many types of cancer cells such as pancreatic adenocarcinoma and malignant glioma express high levels of TF, which can subsequently lead to activation of both factor X and factor IX, thrombin formation, and ultimately, fibrin clot formation. TF, which can also be produced by cells in the cancer microenvironment depending on cancer type and context, has been associated with cancer initiation, metastasis, progression, and angiogenesis, in addition to activation of coagulation. Laboratory studies have demonstrated a correlation between increasing TF expression in glioma cells and histologic grade, progression, and the risk of intravascular thrombosis. In pancreatic adenocarcinoma, it has been shown that fibrinogen and plasminogen activator inhibitors (PAIs) 1, 2, and 3 exist throughout the tumor stroma and that tumor cells stain for TF, PAI, prothrombin, and several other coagulation factors. In another study, expression of TF was found to correlate strongly with the degree of histologic differentiation in pancreatic adenocarcinoma. Tumors with stronger immunoreactivity for TF were more poorly differentiated. Based on these observations, it has been theorized that local coagulation activation may regulate growth and progression of pancreatic adenocarcinoma. TF can drive cancer progression by coagulation-dependent and coagulation-independent mechanisms. The circulating form of TF has been proposed to contribute more significantly to the pathogenesis of cancer-associated VTE than TF expressed on primary cancer cells. In a retrospective study, patients with cancer who had circulating TF-expressing microparticles had a 1-year cumulative incidence of VTE of 34.8% versus 0% in those without detectable TF-bearing microparticles. This and other similar observations suggest that cancer-derived TF-bearing microparticles are thrombogenic in vivo and are likely to play a central role in the pathogenesis of cancer-associated VTE. The clinical utility of this assay has not been demonstrated yet, and it remains an investigational tool at the current time.
Most patients with cancer have been found to exhibit increased levels of coagulation factors V, VIII, IX, and XI, and biomarkers of thrombin generation such as prothrombin fragment 1+2 and D dimer. As mentioned previously, elevated biomarkers of thrombin generation have been associated with aggressive cancer biology and worse clinical outcomes. The fibrinolytic system can also be significantly dysregulated in patients with cancer. Malignant cells can express different proteins in the fibrinolytic system, including urokinase-type and tissue-type plasminogen activators (UPA and TPA, respectively) and PAIs. For example, acute promyelocytic leukemia is often associated at onset with a life-threatening coagulopathy associated with hypofibrinogenemia, increased thrombin generation and fibrin degradation, and prolonged prothrombin and thrombin times. The leukemic cells in acute promyelocytic leukemia can express the UPA receptor on their surface, which activates UPA and TPA, contributing to the fibrinolytic state characteristic of the disease. Other leukemic cells are also capable of expressing various fibrinolytic and proteolytic enzymes that can mediate the bleeding complications seen in some patients with acute leukemia. Similarly, plasminogen activators, PAIs, and other proteins that regulate the fibrinolytic system are also expressed by solid tumor cells, and the resulting imbalance in fibrinolysis may contribute to the hypofibrinolytic and procoagulant state seen in some affected patients. In addition, cancers can be associated with deficiencies in the natural anticoagulants, antithrombin, protein C, and protein S, further promoting the cancer-associated thrombogenic state. The degree of activation of the coagulation cascade and fibrinolysis differs among various tumor types. As noted earlier, some patients with cancer will exhibit clinically evident manifestations of activated coagulation such as DIC and/or VTE or arterial thromboembolism, whereas many other patients with cancer will only have laboratory markers of a procoagulant state such as elevated D dimer.
Cancer cells can also modulate the hemostatic balance in an indirect fashion through their interaction with host immune cells such as monocytes and macrophages, leading to activation of platelets and factors X and XII. Tumor cells can directly produce inflammatory cytokines or indirectly stimulate their production by host cells (leukocytes and endothelial cells), promoting a hypercoagulable state. These inflammatory cytokines, such as tumor necrosis factor–α (TNF-α), interleukin-1 (IL-1), and vascular endothelial growth factor (VEGF) can induce TF production by endothelial cells and monocytes, stimulate PAI-1 production, and downregulate expression of the natural anticoagulant protein, thrombomodulin, on endothelial cells. In addition, these cytokines can lead to vascular endothelial cell damage and conversion of vascular lining into a thrombogenic surface. In addition to activation of TF production by endothelial cells and monocytes, the effects of VEGF include induction of angiogenesis and increased local vascular permeability, therefore increasing the exposure of TF and promoting cancer-associated thrombogenesis. The acute-phase reactants induced by inflammatory cytokines in patients with cancer include procoagulants such as von Willebrand factor (vWF), factor VIII, and fibrinogen, therefore favoring a thrombogenic hemostatic milieu. Tumor cells can also promote nonenzymatic activation of factor X through the sialic acid moieties of mucin produced by adenocarcinomas. Although increased levels of factor VIII, vWF, fibrinogen, PAI, and markers of thrombin generation and fibrin degradation have been associated with more advanced cancers and worse outcomes, these biomarkers have not shown any benefit to date in selecting patients with cancer who might benefit from primary anticoagulant VTE prophylaxis.
Tumor cells can also directly aggregate platelets and secrete important platelet aggregation agonists such as thrombin and adenosine diphosphate. In addition to these biochemical procoagulant mechanisms, direct cell-cell interactions and the local mass effect of tumors contribute to VTE pathogenesis in patients with cancer. Vascular invasion and physical compression by the tumor can mechanically obstruct venous blood flow, leading to venous stagnation, endothelial lining damage, and local activation of the coagulation cascade, all of which predispose to VTE. In patients with myeloma, increased plasma viscosity, elevated levels of circulating immunoglobulins, autoantibodies targeting natural anticoagulants, and secretion of inflammatory mediators with procoagulant activity have all been proposed to contribute to the pathogenesis of VTE.
Studies have shown that some host-specific factors can also modify the risk of VTE. Older age (≥65 years) has been modestly associated with cancer-associated VTE in hospitalized patients. Poor performance status in patients with lung cancer who are receiving chemotherapy, which might be a surrogate for limited mobility, has been prospectively associated with increased VTE risk. Patient race (African Americans have a higher risk, whereas Asians have a lower risk) and comorbidities such obesity and renal disease have also been associated with increased risk of cancer-associated VTE, whereas gender does not significantly influence risk. ABO blood group status, which was found to affect the risk of VTE in the general population, has also been found to modify the risk of VTE in patients with malignant glioma. Although the pathophysiology of the ABO blood group's association with VTE is not fully clarified, ABO blood group status does influence factor VIII and vWF levels, which might mediate this effect.
The presence of prothrombotic genetic alterations has been also shown to influence the risk of VTE in patients with cancer. In a large population-based, case-control study of 3220 consecutive patients aged 18 to 70 years in the Netherlands, patients with cancer who were carriers of factor V Leiden or the prothrombin gene 20210A mutation had a fourfold increased risk of VTE compared with patients with cancer who did not have these mutations. In contrast, a smaller study from Brazil found no significant difference in the prevalence of four thrombophilic genetic mutations/polymorphisms (factor V Leiden, prothrombin gene 20210A mutation, FXIII Val34Leu polymorphism, and methylenetetrahydrofolate reductase [MTHFR] C677T polymorphism) in patients with cancer who did and did not have VTE. A third study in patients with gastrointestinal adenocarcinoma found a significant association between VTE and factor V Leiden mutation but not with prothrombin gene 20210A mutation or the MTHFR C677T polymorphism. A matched nested, case-control study of breast cancer prevention using tamoxifen found no significant association between the factor V Leiden mutation or the prothrombin gene 20210A mutation and VTE risk.
Environmental factors significantly modulate VTE risk in patients with cancer. As expected, many modifiable VTE risk factors increase VTE risk in patients with and without cancer.
Major surgery, a well-recognized VTE risk factor, has been associated with a twofold increased risk of VTE in patients with cancer compared with patients without cancer. Furthermore, patients with cancer have a fourfold increased risk of fatal PE after undergoing surgery compared with patients without cancer. Factors such as the duration of anesthesia and the procedure, the complexity of the surgery, increasing age, and late mobilization can all modify the risk of postoperative VTE in patients with cancer. In contrast to surgery, conflicting data exist regarding the contribution of radiation therapy to the risk of cancer-associated VTE. For example, adjuvant radiation therapy in combination with surgery or chemotherapy was associated with an increased incidence of VTE in patients with glioma or rectal carcinoma, whereas no increased VTE risk was found in otherwise healthy patients with early-stage uterine cervical cancer who received radiation therapy. In a large observational database study by Blom and colleagues, no additional risk of VTE was conferred by radiation therapy in patients with cancer. These data suggest that radiotherapy is inconsistently associated with VTE and that the impact of radiotherapy on VTE risk may be influenced by tumor type and treatment context.
Chemotherapy, hormonal therapy, and hematopoietic growth factors have all been associated with an increased risk of VTE. Patients with cancer who undergo chemotherapy have been found to have a higher risk of VTE and recurrent VTE when compared with patients with cancer who do not undergo chemotherapy. As described earlier, the risk of VTE after cancer therapy depends on the interaction between therapeutic agents, the type and stage of malignancy, and the presence of other VTE risk factors such as advanced age, surgery, immobilization, and the use of CVCs. Although the causal role of cancer therapies in VTE is well recognized, the pathogenic mechanisms underlying the augmented prothrombotic state and increased risk of VTE are poorly understood despite many years of active research. Multiple mechanisms are likely to be involved depending on the specific chemotherapeutic agent and its interaction with other patient variables. These mechanisms can involve direct vascular endothelial cell damage, increased endogenous procoagulant and decreased anticoagulant levels, altered fibrinolytic activity, platelet activation and aggregation, and increased TF expression by direct and indirect effects. Prechemotherapy platelet count and chemotherapy-associated neutropenia in hospitalized patients with cancer also have been associated with increased VTE risk.
The causal relationship between chemotherapy and VTE in patients with cancer has been most studied in the context of breast cancer clinical trials. When treated with adjuvant chemotherapy, the risk of DVT in patients with early-stage breast cancer increases to 10% from a baseline risk of less than 1% to 2%. In a study of adjuvant epirubicin and cyclophosphamide, a 10% incidence of VTE was found. Saphner and colleagues reviewed the records of 2673 patients for the occurrence of vascular complications; these patients received treatment as part of seven consecutive Eastern Cooperative Oncology Group studies of adjuvant therapy for breast cancer. The authors found a significantly higher risk of thrombosis (venous and arterial combined) of 5.4% among patients who received adjuvant therapy in comparison with 1.6% among patients who did not receive adjuvant therapy. Premenopausal patients who received chemotherapy with tamoxifen had a significantly higher rate of VTE than did those who received chemotherapy without tamoxifen (2.8% versus 0.8%). Postmenopausal patients who received tamoxifen and chemotherapy had a significantly higher rate of VTE than did those who received tamoxifen alone (8% versus 2.3%) or those who were observed (8% versus 0.4%). The authors concluded that combining chemotherapy with tamoxifen was associated with more venous and arterial thromboembolic complications than chemotherapy alone in premenopausal patients and with more venous thrombi than tamoxifen alone among postmenopausal patients. Other studies confirmed a higher risk of VTE with chemotherapy–hormonal therapy combinations compared with tamoxifen alone in adjuvant therapy for postmenopausal patients with breast cancer. In a randomized trial comparing a chemotherapy–hormonal therapy regimen for 12 weeks versus 36 weeks of chemotherapy in patients with stage II breast cancer, VTE developed in 6.8% of treated patients, all during therapy. Although aromatase inhibitors such as anastrozole have been associated with a lower risk of VTE than tamoxifen, anastrozole has been associated with a 1% to 2% incidence of VTE when used as first-line therapy for advanced hormone receptor–positive breast cancer in postmenopausal women. In the Arimidex, Tamoxifen, Alone or in Combination (ATAC) study evaluating adjuvant endocrine treatment for postmenopausal women with hormone receptor–positive early breast cancer, the rate of VTE after almost 5.5 years of follow-up was 4.5% for patients who received tamoxifen versus 2.8% for those who received anastrozole.
The agent 5-fluorouracil, which has been associated with VTE in one out of every seven patients with colorectal cancer who have been treated with the drug, has been proposed to induce a prothrombotic state by decreasing protein C levels, increasing fibrinogen proteolysis, and possibly causing vascular endothelial toxicity. l -Asparaginase has been associated with a 4% to 14% incidence of VTE in adults with acute lymphoblastic leukemia. l -Asparaginase is associated with reductions in fibrinogen, protein C, protein S, antithrombin, plasminogen, and factors IX and XI, but it increases levels of factors V and VIII. Platinum-based regimens have been associated with increased VTE risk in patients with germ cell tumors, non–small cell lung cancer (NSCLC), cervical cancer, and ovarian cancer. Other agents such as bleomycin and mitomycin C and the use of high-dose chemotherapy conditioning for hematopoietic stem cell transplantation (HSCT) have all been also associated with VTE. Corticosteroids, a class of drugs commonly used in cancer therapy, especially for lymphoid malignancies and MM, can increase the risk of VTE, especially when high doses are used in patients with germ cell tumors and MM. Glucocorticoids have been found to increase factor VII, VIII, XI, and fibrinogen levels, which may contribute to the reported increased risk of VTE in patients using glucocorticoids on a chronic basis.
In addition to traditional chemotherapy and hormonal therapy, some newer antineoplastic agents have also been associated with increased VTE risk. The immunomodulatory and antiangiogenic agents thalidomide and lenalidomide, which are used for treatment of various cancers, have both been associated with VTE. Thalidomide use in MM as a single agent, whether in newly diagnosed or relapsed or refractory disease, has not been associated with a significantly increased risk of VTE (an incidence of 2%–4%). In contrast, combinations of thalidomide with dexamethasone or chemotherapy have been associated with an increased risk of VTE in newly diagnosed patients with MM (an incidence of 14%–26%). Interesting to note, VTE incidence is not increased to the same degree with thalidomide and dexamethasone in patients with relapsed or refractory MM (an incidence of 2%–8%).
Similarly, lenalidomide has also been associated with increased VTE risk in patients with MM when used in combination with dexamethasone or chemotherapy, especially in newly diagnosed patients. In newly diagnosed patients with MM, lenalidomide and high-dose dexamethasone (480 mg/mo) have been associated with a 12% to 26% VTE rate compared with 6% to 12% when lenalidomide is used with low-dose dexamethasone (160 mg/kg). Most cases of VTE associated with lenalidomide occur in the first 3 months of therapy. VTE rates as high as 17% have been reported in patients with relapsed or refractory MM who have received treatment with lenalidomide. The etiology of thalidomide- and lenalidomide-associated VTE is not fully understood, although direct vascular endothelial toxicity, acquired protein C resistance, and upregulation of the potent platelet activator cathepsin G have been implicated.
Some molecularly targeted antineoplastic agents have also been associated with increased VTE risk. The novel antiangiogenic agent bevacizumab, a humanized monoclonal antibody to VEGF that is used in the treatment of several types of cancer, has been variably associated with an increased risk of VTE, arterial thromboembolism, and bleeding. In a meta-analysis of 15 randomized trials that included 7956 patients with a variety of advanced solid cancers, patients who received bevacizumab had an incidence of all-grade and high-grade VTE of 11.9% and 6.3%, respectively. Therapy with bevacizumab was associated with an RR of VTE of 1.33 (95% CI, 1.13–1.56) compared with control subjects, and that risk was increased for both all-grade and high-grade VTE. In contrast, Hurwitz and colleagues found no added risk of VTE among patients treated with regimens containing bevacizumab compared with patients receiving regimens that did not contain bevacizumab (10.9% versus 9.8%; OR, 1.14 [95% CI, 0.96–1.35]; P = .13). Axitinib, an oral receptor tyrosine kinase inhibitor of VEGF and platelet-derived growth factor receptors commonly used in patients with advanced renal cell carcinoma, has been also associated with mesenteric vein thrombosis. In a systematic review, two other oral agents targeting angiogenesis, sunitinib and sorafenib, were found to be associated with an increased risk of arterial but not venous thrombosis. The safety of continuing VEGF inhibitors in patients who have sustained a VTE while receiving therapy and subsequently undergo anticoagulation is currently unknown.
Hematopoietic growth factor therapy plays an important role in the supportive care of patients with cancer. Erythropoiesis-stimulating agents (ESAs) can reduce the severity of anemia and transfusion requirements in patients with cancer, whereas granulocyte colony-stimulating factor (G-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) can reduce neutropenic complications. The use of ESAs has been associated with an increased risk of thrombotic complications in patients with cancer. Several recent studies have demonstrated an association with tumor progression, mortality, and thrombotic complications, especially VTE, in some patients with solid tumor malignancies who received ESAs. In a systematic review of 27 randomized trials involving 3287 adult patients with cancer who received darbepoetin or epoetin, Bohlius and colleagues noted that the RR of thromboembolic complications was 1.7 (95% CI, 1.4–2.1) compared with untreated control subjects. In a randomized study of healthy volunteers, G-CSF administration enhanced platelet aggregation by 75% as measured by circulating soluble P-selectin, suggesting that G-CSF use may increase thrombotic risk in patients with cancer. Nonetheless, there is currently no conclusive evidence of an association of myeloid growth factor administration with VTE. In a retrospective analysis, transfusion of blood products was independently associated with an elevated risk of VTE, arterial thrombosis, and in-hospital mortality in hospitalized patients with cancer.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here