Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
In craniosynostosis, skull growth is arrested in the direction perpendicular to the fused suture and expanded at the sites of unaffected sutures (Virchow's law), leading to characteristic calvarial deformations. In addition, the skull base and calvarial development are interrelated, and changes at one location may affect the growth parameters at the other location.
Intracranial hypertension may accompany craniosynostosis and is a function of the number of affected sutures, ranging from approximately 8% to 14% for single-suture synostosis to roughly 47% in multisuture synostosis. Children suspected of having elevated intracranial pressure may present with irritability, feeding difficulties, failure to thrive, headache, developmental delays, visual changes, calvarial towering, supraorbital recession, or lack of circumferential skull growth. Computed tomography scan changes may include a “beaten copper” appearance or erosion of the inner table of the skull as well as compression of the ventricles and cisterns. Hydrocephalus and Chiari malformation may also be associated with children with syndromic craniosynostosis (eg, Crouzon, Apert, Pfeiffer syndromes).
An increasing number of growth factor receptors (fibroblast growth factor receptor [FGFR], transforming growth factor [TGF]-βR), growth factors (FGF2, TGF-β, bone morphogenetic protein [BMP]), as well as transcription factors (MSX-2 and TWIST) have been implicated in the pathogenesis of craniosynostosis, and this list will undoubtedly continue to grow in the future. Gastrointestinal tract malformations have also been linked in cases of FGFR-associated syndromic craniosynostosis.
The optimal timing of craniosynostosis surgery, due to the paucity of objective criteria, remains controversial. Nevertheless, the majority of craniofacial surgeons operate when patients are between 3 and 12 months of age. Because the normal brain and skull grow most rapidly in the first 2 years of life, early surgery attempts to take advantage of this rapid period of growth and facilitates cranial volume expansion. Endoscopically assisted minimally invasive approaches for suture release are generally performed at an earlier age (1–4 months) than open calvarial reconfiguration.
Posterior deformational plagiocephaly, secondary to a supine sleeping position, generally resolves with positional changes, physiotherapy, or helmet therapy and is rarely a surgical condition.
Craniosynostosis is defined as the premature closure of one or more of the fibrous cranial sutures of the skull. Simple craniosynostosis is a term used when a single suture prematurely fuses, whereas complex craniosynostosis indicates involvement of multiple sutures. Under normal conditions the infant's skull expands uniformly to accommodate the growing brain. Premature fusion of a cranial suture restricts growth in directions perpendicular to the fused suture. However, the skull continues to expand at sites of the unaffected sutures (Virchow's law). This may lead to not only cosmetic deformities but also potential underlying difficulties with brain development. Although the likelihood of developing elevated intracranial pressure currently appears low in patients with simple craniosynostosis, the risk is considerably higher in cases of complex craniosynostosis or delayed presentations of simple craniosynostosis. Over the course of time, the earlier diagnosis of craniosynostosis and its subsequent treatment have led to improved cosmetic and clinical results while reducing morbidity. The goals of surgery for craniosynostosis are still to release the affected suture(s) and to permit the normalization of skull growth to accommodate the underlying brain's development. Today's neurosurgeons and craniofacial surgeons are equipped with an expansive armamentarium of innovative technologies including endoscopic surgical approaches, distraction osteogenesis, spring-assisted cranioplasty, and computer-assisted modeling. Furthermore, the tools and techniques available for treating craniosynostosis continue to evolve and mature with time and experience.
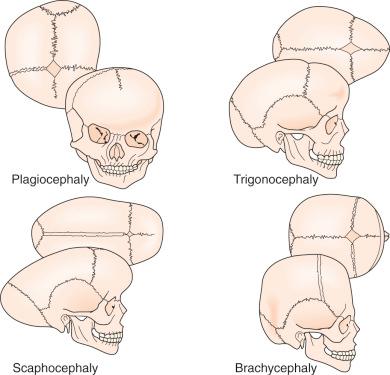
Craniosynostosis has long been recognized as an abnormal process originating at the calvarial suture. Early recognition of the importance of the skull sutures and their relationship to head shape was first made by investigators such as Hippocrates, Galen, and Celsus. In 1791, Sommerring noted that calvarial growth occurred at the suture line and that premature suture closure led to restriction of growth perpendicular to the affected suture. In addition to confirming Sommerring's findings, Virchow was the first to describe the compensatory calvarial growth that occurred in a plane parallel to the affected sutures as well as to classify the different types of skull deformities ( Fig. 9.1 ). These observations served as principal tenets directing craniosynostosis surgery over the subsequent century. Between 1910 and 1920, Crouzon and Apert subsequently observed that, in some cases, craniofacial abnormalities were only a part of more complex syndromes.
Over time the relationship between calvarial growth and the skull base became better appreciated. In 1959 Moss identified the importance of the skull base in the promotion and development of the calvarial vault. He observed that the cranial base developed prior to the calvarial vault and that characteristic abnormalities in the cranial base were associated with stereotypical sutural abnormalities. Nevertheless, subsequent experimental work in animal models demonstrated that restriction of growth at specific sutures resulted in characteristic skull deformities that mimicked shapes seen in simple (nonsyndromic) craniosynostosis. Furthermore, the advent of computed tomography (CT) in the late 1970s offered a new radiographic tool to visualize anatomic deformities more accurately than plain radiographs. As a result of these observations and radiographic advancements, the pathogenesis of craniosynostosis today is currently believed to be a combination of skull base and calvarial growth disturbances.
Craniosynostosis occurs in approximately 1 in 2000 to 1 in 2500 live births. This condition can be classified into simple versus complex or nonsyndromic versus syndromic ( Table 9.1 ). Simple craniosynostosis represents the majority of patients, and complex craniosynostosis constitutes approximately 5% to 15% of cases. As reported by large craniofacial centers, syndromic patients account for 15% to 20% of cases, whereas nonsyndromic patients constitute the majority at 80% to 85%.
| Affected Suture | Phenotypic Presentation |
|---|---|
| Sagittal | Dolichocephaly, scaphocephaly |
| Coronal (unilateral) | Anterior plagiocephaly |
| Coronal (bilateral) | Brachycephaly |
| Metopic | Trigonocephaly |
| Lambdoid | Posterior plagiocephaly |
| Multiple sutures | Cloverleaf (Kleeblatschädel), acrocephaly, oxycephaly |
Simple craniosynostosis most frequently occurs sporadically, with familial aggregation accounting for 7% to 8% of sagittal and metopic synostosis. An equal frequency is found for all ethnic populations; however, gender predilection will vary depending on the type of suture pathology. The most commonly involved location is the sagittal suture, which accounts for 45% to 68% of all individuals and is marked by a male-to-female ratio ranging from 3.5 : 1 to 7 : 1. An autosomal dominant inheritance pattern with 38% penetrance was reported for sagittal synostosis. Currently metopic synostosis is now the second most common form of craniosynostosis, occurring in 23.7% to 27.3% of cases, and shows a male predominance of 75%.
Historically, metopic synostosis accounted for 10% to 15% of all cases of craniosynostosis, but numerous investigators have noted a significant increase in its incidence, with a variety of factors being implicated (increased maternal age, changes in surgical indications, etc.) but no definitive reasons being identified to date. Unicoronal synostosis, also known as anterior plagiocephaly , accounts for approximately 18% of patients with craniosynostosis, with girls outnumbering boys by a 3 : 2 ratio. Lambdoid suture synostosis, referred to as posterior plagiocephaly , is a relatively rare event in children with an observed incidence ranging from 0.9% to 4%. Lambdoid synostosis must be distinguished from the currently ubiquitous posterior deformational plagiocephaly (also known as positional molding) in which there is occipital flattening on the affected side without associated suture fusion. This epiphenomenon is most likely related to the supine sleeping position, encouraged in young children as part of the “Back to sleep campaign” instituted in 1992 to reduce the incidence of sudden infant death syndrome (SIDS).
Although the sporadic nature of simple craniosynostosis makes an accurate prediction of risk difficult to ascertain, it appears that the risk doubles for future siblings if no other family members are involved. When one parent and child are affected, the subsequent risk rises to 50%. Conversely, if both parents are unaffected and two siblings are affected, the risk for additional sibling involvement approaches 25%.
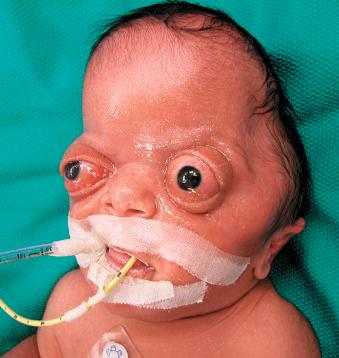
Greater than 100 syndromes have been associated with craniosynostosis, and these are often marked by an autosomal dominant mode of transmission. Among them, Crouzon, Apert, and Pfeiffer syndromes are the most frequently occurring ( Fig. 9.2 ). Syndromic synostosis is commonly associated with multiple suture closure (coronal, sagittal, etc.), and this may be combined with other systemic manifestations ( Table 9.2 ).
| Syndrome | Involved Suture | Morphologic Presentation |
|---|---|---|
| Crouzon | Coronal, sagittal | Midface hypoplasia, shallow orbits, proptosis, hypertelorism |
| Apert | Coronal, sagittal, lambdoid, others | Midface hypoplasia, shallow orbits, proptosis, hypertelorism, symmetrical syndactyly of hands and feet, choanal atresia, ventriculomegaly, genitourinary/cardiovascular anomalies |
| Pfeiffer | Coronal, sagittal | Midface hypoplasia, proptosis, hypertelorism, broad great toe/thumb |
The etiology of craniosynostosis remains elusive because of its heterogeneous nature. Nevertheless, numerous factors are now known to promote or have been implicated in the development of premature closure of the calvarial sutures. Multiple teratogens, genetic mutations, metabolic disorders, and blood dyscrasias have been associated with craniosynostosis ( Table 9.3 ). Interestingly, maternal smoking has also been associated with isolated craniosynostosis, and advanced paternal age has been found to trend with a higher frequency of metopic synostosis. Furthermore, craniosynostosis is a rare but described complication of fetal methotrexate exposure, having been described in eight cases.
| Hematologic disorders | Thalassemias Sickle cell anemia Polycythemia vera |
| Teratogens | Valproic acid Retinoic acid Aminopterin Diphenylhydantoin |
| Genetic conditions | |
| Metabolic disorders | Rickets Hyperthyroidism |
| Mucopolysaccharidoses | Hurler syndrome Morquio syndrome Mucolipidosis III |
| β-Glucuronidase deficiency | |
| Malformations | Holoprosencephaly Encephalocele Microcephaly Hydrocephalus (shunted) |
With advances in molecular genetics, candidate gene mutations as well as the molecular interactions underlying cranial deformities have been elucidated. Normal suture growth and morphogenesis are dependent on a delicate balance between the proliferation of osteoprogenitors within the suture mesenchyme and differentiation to osteoblasts at the osteogenic front ( Fig. 9.3 ). It is now known that the majority of syndromic craniosynostoses are caused by mutations in genes encoding fibroblast growth factor receptors (FGFR-1, FGFR-2, and FGFR-3) and the transcription factors TWIST and MSX-2. Moreover, these same genes are responsible for approximately 25% of all cases of craniosynostosis. In general, gain-of-function mutations are associated with the MSX2 and FGFR genes, whereas loss of function or haploinsufficiency abnormalities are found in TWIST gene mutations.
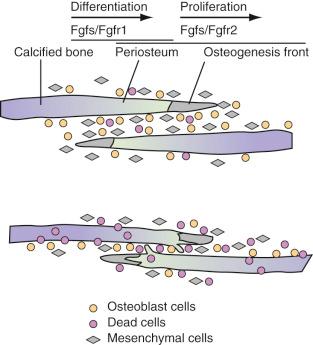
Genetic alterations in FGFR-1 and FGFR-2 have been implicated in Crouzon, Apert, Pfeiffer, and Jackson-Weiss syndromes. FGFR-1 has been found to regulate osteoblast differentiation; therefore a gain-of-function mutation may precipitate premature suture fusion through the promotion of osteoblast differentiation and bone formation. Moreover, FGFR-2 has been linked to activation of osteogenic cell apoptosis. As Chen and coworkers have shown, a gain-in-function mutation leads to increased apoptosis and results in decreased cell numbers and distance between two overlapping bones. Ultimately, this develops into physical contact of two opposing bones, eventually leading to premature closure.
Several clinical reports of FGFR-associated craniosynostosis patients and mouse mutants have been linked to gastrointestinal tract disorders. Results of investigations to determine gastrointestinal symptoms in a sample of FGFR-associated craniosynostosis syndromic patients and a mouse model of Crouzon syndrome containing the W290R mutation in FGFR-2 suggest a direct relationship between FGFR-associated craniosynostosis syndromes and gastrointestinal tract malformations. Based on these results, gastrointestinal tract malformations should be considered in children with FGFR2-associated craniosynostosis syndrome.
Alterations in growth factor receptors have also been observed in nonsyndromic craniosynostoses. FGFR-3-associated coronal synostosis, also known as Muenke-type craniosynostosis, has been identified in up to 52% of patients with nonsyndromic bicoronal synostosis, either as a result of de novo mutations or associated with an autosomal dominant inheritance pattern. Gripp and colleagues observed that 10.8% of patients with unilateral coronal synostosis were positive for an FGFR-3 mutation and subsequently recommended testing of all patients with unilateral coronal synostosis to assess the risk of recurrence. These guidelines stem from the observation that Muenke-type craniosynostosis has been associated with a reoperation rate of at least 43%.
Studies have implicated transforming growth factor-beta receptors (TGF-βR) in syndromic and intrauterine head constraint–related craniosynostosis. Loeys and coworkers have discovered a link between mutations of TGF-βR1 and TGF-βR2 and a syndrome of altered cardiovascular, craniofacial, neurocognitive, and skeletal development. Hunenko and colleagues demonstrated up-regulation of TGF-βR1 and TGF-βR2 in mice undergoing intrauterine constraint leading to coronal suture synostosis. Such data point to the ability of mechanical forces to alter growth factor–mediated signaling during craniofacial growth and development.
In addition to the aforementioned growth factor receptors, their corresponding ligands have been found to be an integral component of calvarial osteoblast proliferation and subsequent sutural fusion. FGF2 has been shown to enhance proliferation rates in rat fetal osteoblasts, promote premature fusion of frontal sutures in calvarial organ cultures, and correlate with intrauterine constraint-related coronal suture synostosis. Inverse patterns of TGF-β isoform expression between fusing and patent sutures have been demonstrated in animal and human models. Opperman and colleagues demonstrated declining levels of TGF-β3 but continued expression of TGF-β1 and TGF-β2 posterior frontal suture fusion. Bone morphogenetic proteins (BMPs), members of the TGF-β superfamily, are involved in a broad range of developmental roles, including bone formation, skeletal patterning, and limb development. Several investigators have demonstrated the critical role of BMPs and their antagonists in dictating cranial suture biology. In particular, in situ hybridization of mouse cranial sutures localized expression of BMP-2 and BMP-4 to the osteogenic fronts and BMP-4 to suture mesenchyme and dura mater in the sagittal and posterior frontal sutures. Nacamuli and coworkers found BMP-3, a bone morphogenic protein antagonist, to be decreased in normally fusing posterior frontal sutures and increased in normally patent sagittal sutures.
Mutations of transcription factors have also been implicated in causing syndromic forms of craniosynostosis. Liu and coworkers linked a gain-of-function mutation in the MSX-2 transcription factor with Boston-type craniosynostosis. Loss-of-function mutations in TWIST proteins, transcription factors activating osteoblast differentiation, have been found to cause Saethre-Chotzen syndrome. Woods and colleagues have recommended TWIST1 mutation screening of all patients with either bicoronal or unicoronal synostosis, given that this genetic alteration confers a greater risk of recurrent intracranial hypertension and subsequent reoperation than nonsyndromic synostosis of the same sutures.
Skull development can be divided into neurocranium and viscerocranium formation, a process starting between 23 and 26 days of gestation. Neurocranium growth leads to cranial vault development via membranous ossification, whereas viscerocranium expansion leads to facial bone formation by ossification. Cranial sutures form by 16 weeks' gestation at the junction of numerous osteogenic fronts and are particularly active areas of bone formation and deposition, directly affected by underlying tension forces of brain growth and dural reflections as well as local growth factors.
The calvarium grows most rapidly during the first 12 months, with the brain doubling in volume in the first 6 months and again by the second birthday. Although calvarial expansion is most pronounced during the first 2 years, growth continues in a linear fashion until the age of 6 to 7 years, at which time the cranium is 90% of the adult size. Most of this cranial growth takes place in the sutures between the bone plates. Within the center of the sutural area, a population of proliferating osteoprogenitor cells is maintained. A portion of these cells enters the pathway of osteogenic differentiation, forming bone-matrix-secreting osteoblasts at the bone edges and contributing to skull expansion. Normal cranial suture closure occurs from front to back and from lateral to medial, with the metopic suture usually closing between 9 and 11 months of age and the remaining sutures fusing in adulthood.
A disturbance in the balance between proliferation, differentiation, and apoptosis causes premature ossification within the suture and its synostosis. Factors disturbing this balance include genetic or acquired changes in growth factor receptor/ligand profiles, loss of direct contact between dural and sutural cells, and increased external mechanical forces. As mentioned previously, many of the syndromic forms of craniosynostosis are attributed to alterations in the FGF/FGFR, TGF-β/TGF-βR, and BMP cascades. Both cerebral hypoplasia and overshunted hydrocephalus have been associated with secondary craniosynostosis, a phenomenon likely attributed to loss of dural contact. Both breech positioning and twin pregnancies have been associated with intrauterine constraint-related craniosynostosis, stemming from mechanical force signal transduction.
Preoperative assessment for craniosynostosis includes a detailed medical history, physical examination, and radiographic imaging. Medical history should elicit for asymmetrical calvarial deformities noted by friends or family members, family history of calvarial deformities, and symptoms of intracranial hypertension including, but not limited to, headache/vomiting, delayed developmental milestones, irritability, and oculomotor paresis. Physical examination should evaluate for characteristic calvarial shapes and asymmetries, premature closure of the anterior fontanelle (normally open until 12–15 months of age), perisutural ridging (calcification), and signs of intracranial hypertension such as papilledema, supraorbital retrusion, severe towering, and severe frontal/occipital bossing. Routine funduscopic examination for papilledema is an accurate predictor of raised pressure in the older child but may not be 100% sensitive for children younger than 8 years old (25% false negatives). Craniofacial asymmetries should be documented in the form of head circumferences, cranial indices, and anthropometric measurements. The history combined with the examination is often confirmatory in an experienced primary care physician/nurse or craniofacial surgeon's initial evaluation.
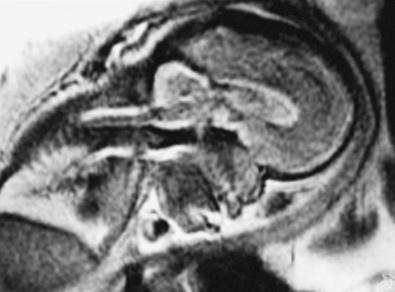
The role of the radiologic workup for craniosynostosis varies among clinicians. Currently, it is not uncommon for prenatal ultrasound to document craniosynostosis in utero. In addition to the ultrasound evaluation, fetal magnetic resonance imaging (MRI) ( Fig. 9.4 ) at some centers has offered significant prenatal definition. Radiologic investigation may be necessary to corroborate the diagnosis and rule out any associated intracranial abnormalities in the postnatal consultation period. CT studies remain the most sensitive barometer of bony fusion, as skull plain films suffer from poor sensitivity and a high false positive rate. Low-radiation protocols are the standard of care at most hospitals, and the amount of radiation delivered for a routine CT scan is 0.8 to 5 mSv with common doses from general cosmic radiation exposure at sea level approximating 0.24 vSv/year. The advent of three-dimensional CT (3D CT) has provided an excellent view of affected suture(s) as well as overall head shape, thereby simplifying the diagnosis and helping with surgical planning. This modality is not mandatory but rather is reserved for multiple/complicated suture pathology, confirmation of diagnosis, or demonstration of skull base pathology.
CT scans may also provide radiologic evidence for raised intracranial pressure. The presence of intracranial hypertension is dependent on the number of affected sutures as well as patient age, ranging from approximately 8% to 14% for single-suture synostosis to approximately 47% for multiple-suture synostosis,. Although few children will manifest clinical symptoms of increased intracranial pressure, it is not uncommon to visualize erosion of the inner calvarial table (beaten copper appearance) on CT scan. A diffuse beaten copper appearance has been associated with greater intracranial pressure, as reported by Tuite and coworkers. Though it is common to see expanded subarachnoid spaces in all types of craniosynostoses, these spaces usually spontaneously resolve and are not felt to unequivocally represent an increase in intracranial pressure. If there is any evidence for elevated pressure, surgical consideration should be expedited. This is especially vital in patients with syndromic synostosis such as Crouzon, Apert, and Pfeiffer syndromes. These patients are also at a greater risk for hydrocephalus and Chiari malformations with associated intracranial hypertension.
CT and MRI studies are also helpful in evaluating the underlying brain for any structural or functional abnormalities. Unrecognized intracranial abnormalities may exist in a small number of patients and may include hydrocephalus, partial agenesis of the corpus callosum, holoprosencephaly, or focal cortical dysplasias. Investigators have reported that up to 5% of their patients with sagittal craniosynostosis had unrecognized underlying intracranial pathology.
The fundamental objectives of surgical intervention of craniosynostosis are correction of calvarial contour deformities and the potential prevention of psychosocial dysfunction, intracranial hypertension, and mental retardation. Historically, surgical intervention for simple craniosynostosis was undertaken primarily because of cosmetic and psychosocial considerations. However, due to growing evidence gathered since the 1990s, sutural release in simple craniosynostosis has also been considered in the setting of increased concerns regarding elevated intracranial pressure as well as mild but significant developmental delay in the aging child with uncorrected single-suture synostosis. In contrast to simple craniosynostosis, patients with complex or syndromic craniosynostosis present with more severe neurologic and cosmetic symptoms. Therefore surgical intervention in these infants is considered more imperative.
The optimal timing for reconstructive surgery in craniosynostosis remains controversial, as the age at surgery has different effects on intraoperative hemodynamics, anesthetic risk, postoperative cranial growth, and subsequent mental development. With regard to intraoperative hemodynamics, Meyer and coworkers demonstrated that older patient age (>6 months) was associated with decreased blood loss. In addition to benefiting from decreased blood loss, older infants can tolerate extensive blood loss better than younger infants. From the perspective of long-term skull growth, the data are less clear. In 1987 Whitaker demonstrated that as surgical age increased, the likelihood of secondary surgery was also elevated. On the other hand, Fearon and colleagues found that older patient age (≥12 months) was associated with less diminished cranial growth following correction of all types of single sutural craniosynostosis. These findings must be weighed against the need to fully reconstruct any advanced postoperative skull defects in children over 12 months of age, because dura will not regenerate bone as readily. From the standpoint of mental development, Arnaud and coworkers reported that postoperative mental outcome was significantly better when surgery was performed before the patient reached 12 months of age.
Although the literature is inconclusive regarding the appropriate timing for correction of craniosynostosis, the majority of craniofacial surgeons operate when the patient is between 3 and 12 months of age. The specific time period is dependent on the type of surgical approach used. Endoscopic corrections are generally done by 3 to 4 months of age. In contrast, open surgical corrections are often done later. Fearon and colleagues perform treatment at 4 months of age for sagittal synostosis and 9 months of age for all other single-suture synostoses (metopic, coronal, and lambdoid). Marchac and associates reviewed their craniofacial experience with 983 patients operated on over 20 years, discussing their timing of surgical operations. Children with brachycephaly underwent a floating forehead procedure between 2 and 4 months of age, infants with sagittal synostosis underwent parasagittal craniectomies between 2 and 4 months of age or a frontocranial remodeling procedure if presenting between 6 and 9 months of age, and infants with either metopic or unicoronal synostoses had a frontocranial remodeling procedure between 6 and 9 months of age. The results of a 12-year retrospective review of long-term outcomes in 212 patients who underwent open craniofacial surgery at our center support the merits of surgical delay, targeting an age of 6 months or older. In our series surgical reconstruction was performed at a mean age of 11.3 months, including nonsyndromic patients at an average age of 10.6 months and syndromic patients at an average age of 19.3 months. The likelihood of additional surgery appeared to be influenced by age and was less in the older patients.
Experimental data from animal models have raised concerns about long-term effect of anesthesia on the developing brain and neurocognitive development. As a result, a number of large-scale prospective pediatric anesthetic studies have been initiated to assess any potential deleterious effects of general anesthesia on the developing brain in the infant. Although the GAS study comparing general versus regional anesthesia for herniorrhaphy is still under way assessing 5-year Wechsler Preschool and Primary Scale of Intelligence, third edition (WPPSI-III), Full Scale IQ, the early results have failed to demonstrate a significant problem. Furthermore, the Pediatric Anesthesia and Neurodevelopment Assessment (PANDA) study compared siblings in which one underwent inguinal hernia surgery before 3 years of age; the results failed to demonstrate any IQ differences between siblings. No difference in secondary outcomes concerning memory, attention, executive function, language, motor/processing speed, or behavior could be demonstrated. A subgroup analysis also failed to show any difference due to longer anesthetic exposure or anesthesia received during the first, second, or third year of life.
There is a continuing debate in the literature regarding the optimal type of operation for correction of craniosynostosis. An open craniofacial approach was proposed as early as 1890, with Lannelogue advocating early open surgical release of a fused suture to prevent intracranial hypertension. Building on the principles of Lannelogue, a number of centers have reported their large-volume experience with open cranial vault remodeling procedures for all types of craniosynostoses. In 421 intracranial operations with movement of one or both orbits, Whitaker and coworkers in 1979 reported a 2.2% rate of mortality, 6.2% rate of infection, and 2.2% frequency of CSF leak. Eight years later with increased experience and refinement in technique, Whitaker and colleagues reported a 0% mortality rate, 3.7% infection rate, and 1.2% CSF leak rate in a 1987 report of 164 open craniofacial procedures for craniosynostosis. Regarding longevity of the open craniofacial procedure, McCarthy and associates identified a 13.5% rate of reoperation for simple craniosynostoses and 36.8% revision rate for complex craniosynostoses during a 20-year experience. Reoperation rates have also decreased with experience and refinement of the open surgical technique, with Sloan and coworkers reporting a 7.2% overall rate of reoperation in 250 patients, and Fearon and associates noting a 2% rate of revision in 248 cases of simple craniosynostosis. The reoperation rate at our own institution is 3.9% for single suture synostoses and increases to 5.4% when including bicoronal synostoses in “apparent” nonsyndromic patients.
To address concerns regarding the extent of incision length, operative blood loss, and length of stay for open craniofacial procedures, minimally invasive techniques that rely on dynamic cranial vault alteration have been proposed. Techniques include endoscopic sutural release, spring-assisted cranioplasty, and distraction osteogenesis. A brief overview of each of these evolving techniques follows.
Jimenez and Barone pioneered endoscopic surgery for craniosynostosis in the mid-1990s, and it has been used by many surgeons who treat craniosynostosis. Although the technique varies at different institutions, in general, the idea is to perform a minimally invasive endoscopically assisted strip craniectomy of the fused suture at an early age (preferentially before 3–4 months of age). The surgery is done through one or two small incisions. The child then wears a cranial molding helmet for several months, which in the setting of the child's rapid head growth helps to correct the head shape gradually over the course of this time. Endoscopic strip craniectomy can be used for single suture or multisuture craniosynostosis. The specifics of this surgical technique are described later in this chapter in sections specific to each type of craniosynostosis.
Summarizing the data from studies by Jimenez and Barone, average age at surgery is about 3 to 4 months, mean operating time is around 60 minutes, average estimated blood loss (EBL) is approximately 30 mL or approximately 5% estimated blood volume (EBV), transfusion rate is around 10%, average length of stay is generally 1 day, and complication rates range from 0% to 6%. These numbers are far lower than the blood loss of 25% to 500% of EBV, blood transfusion volume of 25% to 500% of EBV, and 2- to 5-day length of stay associated with open craniofacial techniques. MacKinnon and coworkers also found that children treated with endoscopic repair of unicoronal craniosynostosis may have less severe eye findings, such as V-pattern strabismus, than children treated with fronto-orbital advancement at a later age. Redo rates for endoscopic surgery (5%–8%) are similar to that seen for open vault remodeling.
Results from a retrospective analysis of 111 cases of nonsyndromic endoscopically assisted strip craniectomy in The Netherlands further supports the “good” outcomes of this technique. In this series, 64 procedures were performed for sagittal craniosynostosis, 34 procedures for metopic craniosynostosis, and 13 procedures for unicoronal craniosynostosis. The mean age of surgery was 3.9 months, mean operative time was 58 minutes, and mean estimated blood loss was 34 mL. The mean length of hospital stay was 2.6 days, and the mean duration of postoperative helmet therapy was 10 months.
Studies have also shown that endoscopic surgery and postoperative helmet therapy can be more cost effective than open surgical techniques. Endoscopic craniosynostosis surgery has become an important treatment for young infants (<3–4 months) diagnosed with craniosynostosis. Studies have demonstrated that this surgical approach followed by helmet therapy provides excellent results in a safe and efficacious manner when performed by a trained surgeon.
Spring-assisted cranioplasty was first undertaken on human subjects by Lauritzen in 1997, based on success in the rabbit model by Persing and coworkers. This technique relies on a standard open access incision, osteotomies at the sites of stenosed sutures, placement of omega-shaped tension springs across the osteotomy sites, and possible placement of compressive springs along areas of compensatory growth ( Fig. 9.5 ). Implantable springs are typically removed 4 to 7 months postoperatively. In 2008 Lauritzen and coworkers reported their large-volume experience with spring-assisted cranioplasty for all forms of craniofacial surgery. Results included an average operative time of 97 to 215 minutes, mean blood loss ranging from 143 to 503 mL, average length of stay between 5 and 6 days, mean cephalic index of 74 in scaphocephalic infants 6 months following cranioplasty, 6% reoperation rate, 3% rate of intracranial hypertension secondary to compressive springs, and a 0% mortality rate. On the heels of this study, David and coworkers reported their experience with the first 75 spring-assisted surgeries for scaphocephaly. With a mean follow-up period of 46 months, the mean cephalic index was 75.4, which is comparable to patients with open cranial vault reconstruction and was maintained at 3- and 5-year follow-ups. Optimism for this emerging modality must be balanced against the need for a second operation for spring removal as well as the lack of three-dimensional control of spring action.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here