Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Diabetic foot complications are common, costly, and impactful. The experience of many diabetic programs has demonstrated that multidisciplinary or interdisciplinary management of patients with diabetic foot ulcers (DFUs) can significantly reduce complications and produce outstanding outcomes ; participation in these groups may be one of the most beneficial roles a vascular surgeon may play. Vascular surgeons should therefore have a fundamental understanding of the spectrum of diabetic foot complications and their management.
The development of a foot ulcer—defined as a full-thickness epithelial defect on the foot (skin distal to the malleoli) lasting more than 14 days—is usually the initial complication that develops in the diabetic foot. A multitude of risk factors may predispose to the development of a foot ulcer, but sensory neuropathy is the most significant single factor. Changes in the morphology of the foot may also lead to an abnormal or uneven distribution of pressure across various areas of the foot during walking. This abnormal distribution of pressure may lead to repetitive trauma during walking and may ultimately lead to full-thickness ulceration. Although this ulcer would generally be very painful in persons with normal sensation, it often goes unnoticed in patients with sensory neuropathy; this has been termed “loss of protective sensation” (often abbreviated in medical literature as LOPS).
Once established, foot ulcers act as the portal of entry for bacterial infection of the foot. Among those with a foot ulcer, the incidence of foot infection is also twofold higher among those with peripheral arterial disease (PAD) compared with those without PAD. The incidence of foot infection is associated with foot ulcers that have been present for more than 30 days and recurrent foot ulcers, as well as penetrating trauma to the foot. In addition, the combination of foot infection and PAD triples the amputation risk in patients with diabetes.
Like ulcers, gangrenous digits can also pose a risk for foot infection. Gangrene of an entire digit occurs when oxygen and nutrient supply becomes insufficient to maintain soft tissue viability. Inflammation from local trauma or an infected ulcer may change the balance between supply and demand sufficiently to result in digit gangrene. Systemic illness (including volume overload from kidney failure or severe heart failure; hypotension requiring vasopressor support) and some tumors (via paraneoplastic syndromes) can rarely also cause digit gangrene. In most patients, foot ulcers and digit gangrene should not be ascribed to embolization of more proximal atherosclerotic lesions or to microvascular occlusive disease because these are phenomena not thought to be involved in the pathogenesis of diabetic foot complications. Similar to a foot ulcer with overlying eschar or acellular debris, the interface between viable and nonviable tissue may serve as a portal of entry for bacterial inoculation in the remainder of the foot.
Infection in the diabetic foot often has a more subtle presentation than does infection in other areas or infection in patients without diabetes. Typically, diabetic foot infections may present with erythema, swelling, and often with fluctuance. Purulent drainage is a more specific sign of infection, but serous discharge should also be viewed as suspicious for infection. Foul odor can sometimes be caused simply by necrotic tissue on the surface of the wound but should also be considered a sign of possible infection. The presence of unilateral edema in the affected foot or calf should signal the presence of an underlying foot infection, even if the appearance of the ulcer and skin immediately surrounding it is unremarkable.
Systemic symptoms and signs also help to identify infection. As with infections in other areas, such systemic signs may include fever, tachycardia, and evidence of inadequate end-organ perfusion (tachypnea, mental status changes, oliguria). However, similar to local signs of infection, only subtle symptoms or signs may be present, including malaise, anorexia, nausea, fatigue, or a degree of hyperglycemia that is unusual for a particular patient. Patients with a foot ulcer and systemic signs of infection should be assumed to have a foot infection, unless another plausible cause of infection can be clearly identified.
Infections may affect virtually any of the structures in the foot. Cellulitis and abscesses are particularly common. Diabetic foot osteomyelitis complicates approximately 20% to 30% of foot ulcers, and the risk of osteomyelitis appears to be directly correlated with the duration of a foot ulcer as well as the depth. Other things being equal, foot ulcers complicated by osteomyelitis have twofold to threefold higher rate of leg amputation than those with only soft tissue infection.
The organisms involved in both soft tissue infections and osteomyelitis are similar; approximately one-third of foot infections are monomicrobial, whereas the remainder are polymicrobial, with two to five organisms commonly found. Approximately 60% of isolates are gram-positive aerobic bacteria, 20% are gram-negative aerobic bacteria, and 15% are anaerobic bacteria. Staphylococcus aureus is the most common species of organism isolated in most series, with methicillin-resistant and methicillin-sensitive strains (MRSA and MSSA, respectively) being found in a near equal distribution. Compared with MSSA osteomyelitis, MRSA osteomyelitis has been associated with a higher fever, more pronounced leukocytosis, fetid odor, a higher prevalence of cutaneous necrosis, as well as a higher incidence of radiographic evidence of bony changes suggestive of osteomyelitis. Patients with MRSA osteomyelitis also have longer hospital stays and slower healing times. However, MRSA osteomyelitis is not associated with higher rates of treatment failure or leg amputation, when compared with MSSA infections.
In contrast to MRSA, two gram-negative aerobic bacteria— Escherichia coli and Pseudomonas aeruginosa —have been associated with higher rates of treatment failure and leg amputation compared with other organisms. These organisms are not as common in North America (generally <5% to 10% of isolates) compared with other areas in the world but still should be treated aggressively with surgery and appropriate isolate-specific antimicrobial therapy when found.
Fungal isolates are rare, not often found as the sole isolate, and are generally considered a contaminant or colonizing organism rather than a pathogen. Anaerobic bacteria generally comprise only 10% to 20% of isolates in series that use conventional techniques for cultures of operative specimens. However, one study that used 16s rRNA to identify organisms at a higher sensitivity found anaerobes in 87% of bone specimens. The role of anaerobes in foot osteomyelitis will need to be clarified with further study.
The surgeon must play a critical role in the early treatment of foot infections: identification and operative drainage of foot infection. Specifically, the surgeon should be available to rapidly evaluate for the signs of infection. Aside from cellulitis or osteomyelitis without associated soft tissue infection, the surgeon should have a low threshold to operate on a patient with a suspected diabetic foot infection. Surgeons from all of the appropriate specialties, including vascular, general, orthopedic, podiatric, and plastic surgeons, should be sufficiently trained to be capable of evaluating and treating deep soft tissue infections.
It has been recognized since at least 1931 that foot infections usually track along longitudinally oriented soft tissue structures (including tendon sheaths, the plantar aponeurosis, and fascial planes separating the compartments of the foot); therefore transectioning the forefoot transversely to treat infection using partial foot amputations is not be the most effective means of draining these longitudinally oriented infections. Rather, amputation should initially be limited to only obvious areas of irreversible necrosis. Probing with a metal instrument and incising along the identified sinus tract will help to establish drainage of abscess tracts. Drains can be placed through these tracts and eventually be used to facilitate opening them along their entire length ( Fig. 62.1 ).
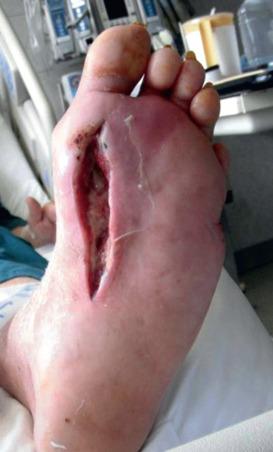
Deep soft tissue infection must be drained aggressively. Even with an initial aggressive approach, the need for reoperation at 24 to 72 hours is frequent and should not be viewed as signaling a poor prognosis. When performed comprehensively, operative drainage is very effective at controlling a septic diabetic foot. Severity of infection should only be the indication for a major amputation in rare situations. In addition to operative drainage of any suppurative infection, broad-spectrum antibiotics should be initiated and continued until culture results are obtained from surgery. They can help to tailor an isolate-specific antibiotic regimen. All organisms identified from a deep operative culture (including Staphylococcus epidermidis and other organisms not typically considered pathogenic) should be covered by this therapeutic antibiotic regimen. Societal guidelines have recommended up to 1 to 2 weeks of antibiotics for soft tissue infections or osteomyelitis that has been resected to a negative margin, 6 weeks for osteomyelitis with grossly normal but histologically positive margins, and more than 3 months for osteomyelitis treated nonsurgically or with residual dead or grossly infected bone.
The surgical drainage of deep soft tissue infection should not be postponed by studies to assess arterial blood flow. Such evaluations should generally be done after drainage, although it may be reasonable to combine surgical drainage for focal and more limited infections and angiography with possible endovascular intervention in centers with the available resources and expertise to offer this approach.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here