Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
| American College of Obstetricians and Gynecologists | ACOG |
| American Diabetes Association | ADA |
| Australian Carbohydrate Intolerance Study in Pregnant Women | ACHOIS |
| Biophysical profile | BPP |
| Continuous subcutaneous insulin infusion | CSII |
| Depot medroxyprogesterone acetate | DMPA |
| Diabetic ketoacidosis | DKA |
| Disposition index | DI |
| Free fatty acid | FFA |
| Gestational diabetes mellitus | GDM |
| Glucose tolerance test | GTT |
| Glucose transporter | GLUT |
| Hemoglobin A1c | HbA1c |
| High-density lipoprotein | HDL |
| Hyaline membrane disease | HMD |
| Infant of a diabetic mother | IDM |
| Insulin-dependent diabetes mellitus | IDDM |
| Low-density lipoprotein | LDL |
| Maternal-Fetal Medicine Unit | MFMU |
| Maternal serum α-fetoprotein | MSAFP |
| Maturity-onset diabetes of the young | MODY |
| National Institute of Child Health and Human Development | NICHD |
| Nonstress test | NST |
| Oral contraceptive | OC |
| Phosphatidylglycerol | PG |
| Randomized controlled trial | RCT |
| Respiratory distress syndrome | RDS |
| Self-monitoring of blood glucose | SMBG |
| Total urinary protein excretion | TPE |
| Tumor necrosis factor alpha | TNF-α |
| Urinary albumin excretion | UAE |
| Very-low-density lipoprotein | VLDL |
Insulin therapy was introduced nearly 100 years ago and remains perhaps the most important landmark in the care of pregnancy for the diabetic woman. Before insulin became available, pregnancy was not advised because it was likely to be accompanied by fetal mortality and also came with a substantial risk for maternal death. Since the introduction of insulin, a variety of advances continued to contribute to improved outcomes for the pregnancy complicated by diabetes mellitus (DM). Technologic innovations and management techniques have been developed that can prevent many complications associated with the diabetic pregnancy. These advances, based on our understanding of the pathophysiology, have resulted in perinatal mortality rates in optimally managed cases that approach those of the normal population. This dramatic improvement in perinatal outcome can be largely attributed to clinical efforts to establish improved maternal glycemic control both before conception and during gestation ( Fig. 45.1 ). Excluding major congenital malformations, which continue to plague pregnancies in women with preexisting (type 1 and type 2) DM, perinatal loss for the woman with diabetes has fortunately become an uncommon event.
Although the benefit of careful regulation of maternal glucose levels is well accepted, failure to establish optimal glycemic control—as well as other factors such as maternal obesity—continues to result in significant perinatal morbidity. Clinical experience has also emphasized the impact that vascular complications can have on pregnancy and the manner in which pregnancy can affect these disease processes. With current management techniques and a skilled, organized team approach, successful pregnancies have become the norm even for women with significant vascular disease of DM.
Gestational diabetes mellitus (GDM), the most common type of diabetes found in pregnancy, is increasing in frequency worldwide. GDM continues to represent a significant challenge for both clinicians and investigators. After nearly 60 years since the concept of GDM was introduced and as a result of large-scale observational studies and treatment trials, the clinical significance of this disorder is currently accepted. However, controversy still remains concerning screening techniques, diagnostic criteria, thresholds for the initiation of insulin, and whether oral hypoglycemic agents are a suitable treatment.
Before considering these clinical issues, it is important to review the metabolic effects of pregnancy in relation to the pathophysiology of DM.
Significant alterations occur in maternal metabolism during pregnancy, which provide for adequate maternal nutritional stores in early gestation to meet the increased maternal and fetal metabolic demands of late gestation and lactation. Although we are apt to think of DM as a disorder exclusively of maternal glucose metabolism, in fact DM affects all aspects of nutrient metabolism, including amino acid and lipid metabolism. In this section, we consider maternal glucose metabolism as it relates to pancreatic β-cell production of insulin and insulin clearance, endogenous (i.e., primarily hepatic) glucose production, and suppression with insulin and peripheral glucose insulin sensitivity. The important role of adipokines and various placental hormones affecting these metabolic changes will also be discussed. Lastly, the effects of these alternations on maternal metabolism are examined as they relate to maternal energy expenditure and fetal growth.
Normal pregnancy has been described as a “diabetogenic state” because of the progressive increase in postprandial glucose levels and increased insulin response in late gestation. However, early gestation can be viewed as an anabolic state characterized by increases in maternal fat stores and decreases in free fatty acid (FFA) concentration, particularly in normal weight and obese women. Garcia-Patterson and colleagues have described a significant decline in maternal insulin requirements in early gestation in women with type 1 diabetes ( Fig. 45.2 ). The mechanism for the decrease in insulin requirements has been ascribed to various factors including increased insulin sensitivity—decreased substrate availability secondary to factors such as nausea, the fetus acting as a glucose sink, and enhanced maternal insulin secretion; however, the exact mechanism is not known. Longitudinal studies in women with normal glucose tolerance have shown significant alterations in all aspects of glucose metabolism as early as the end of the first trimester.
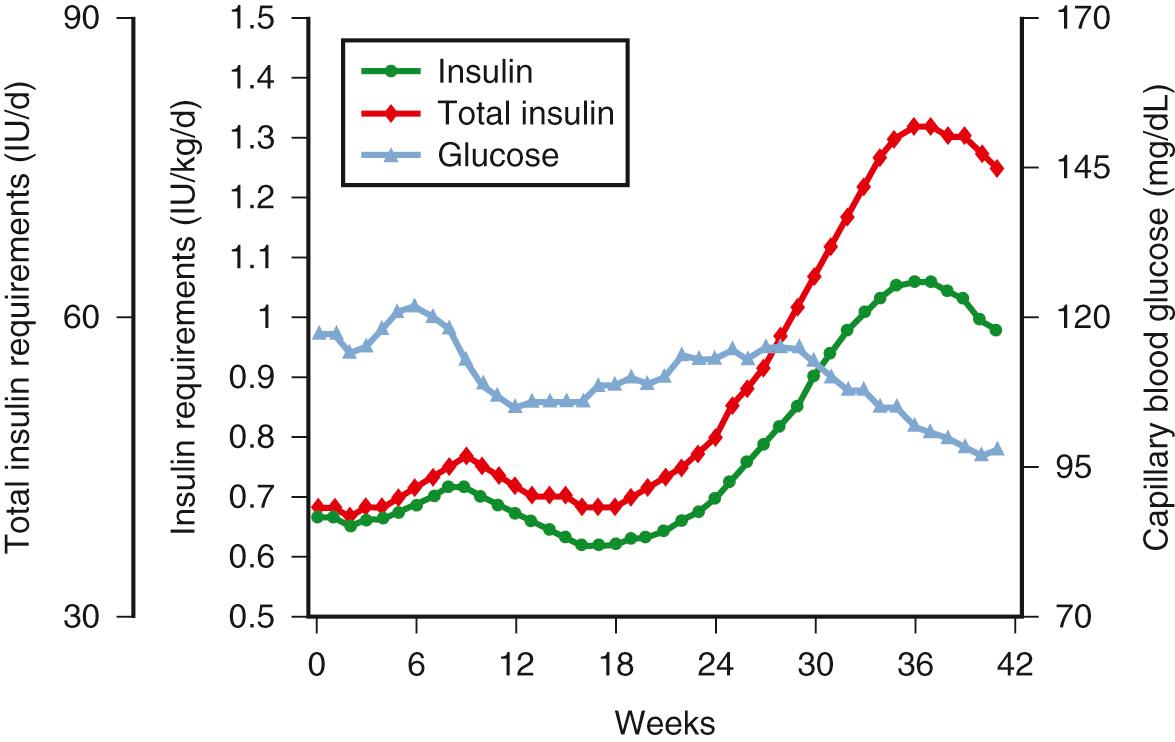
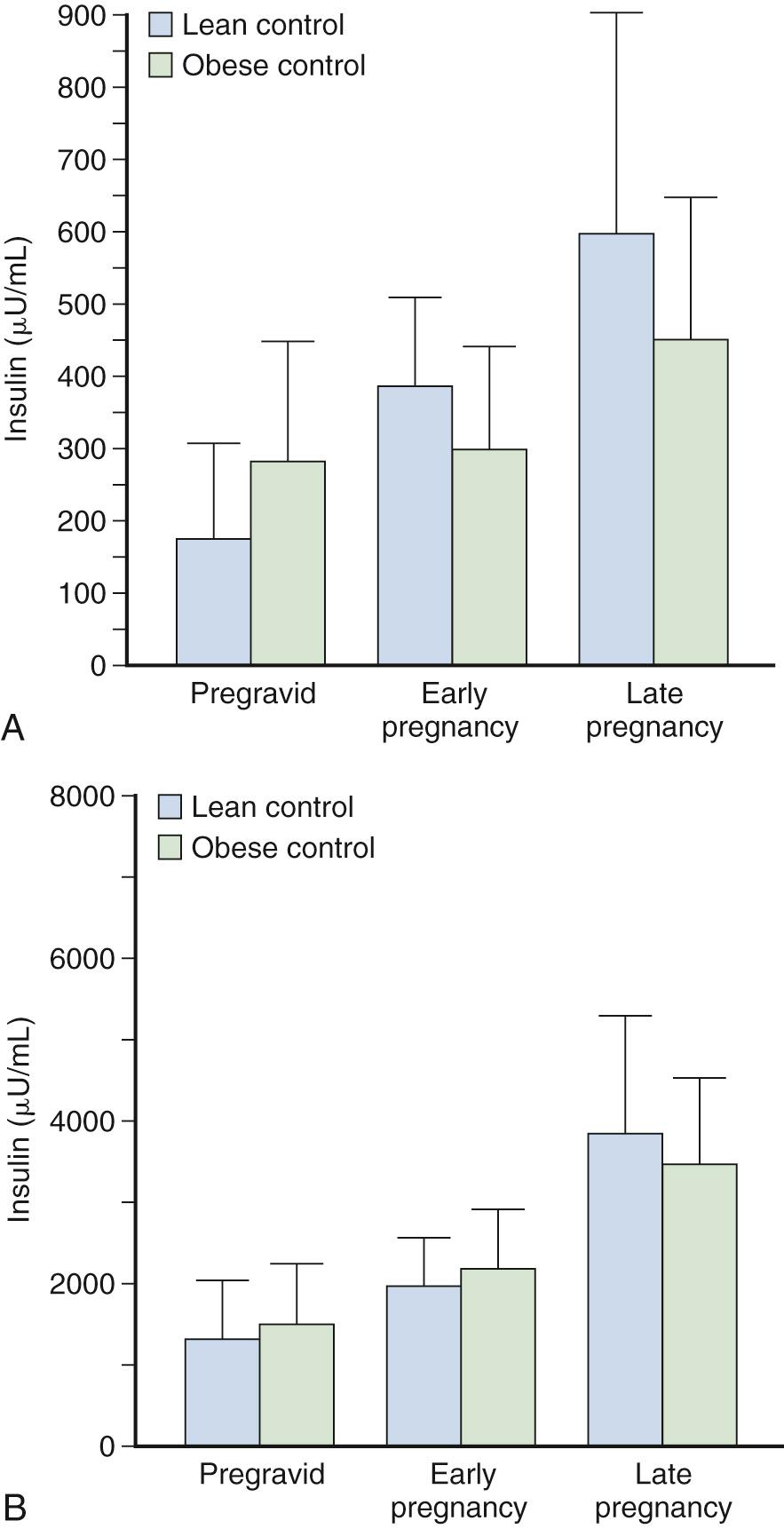
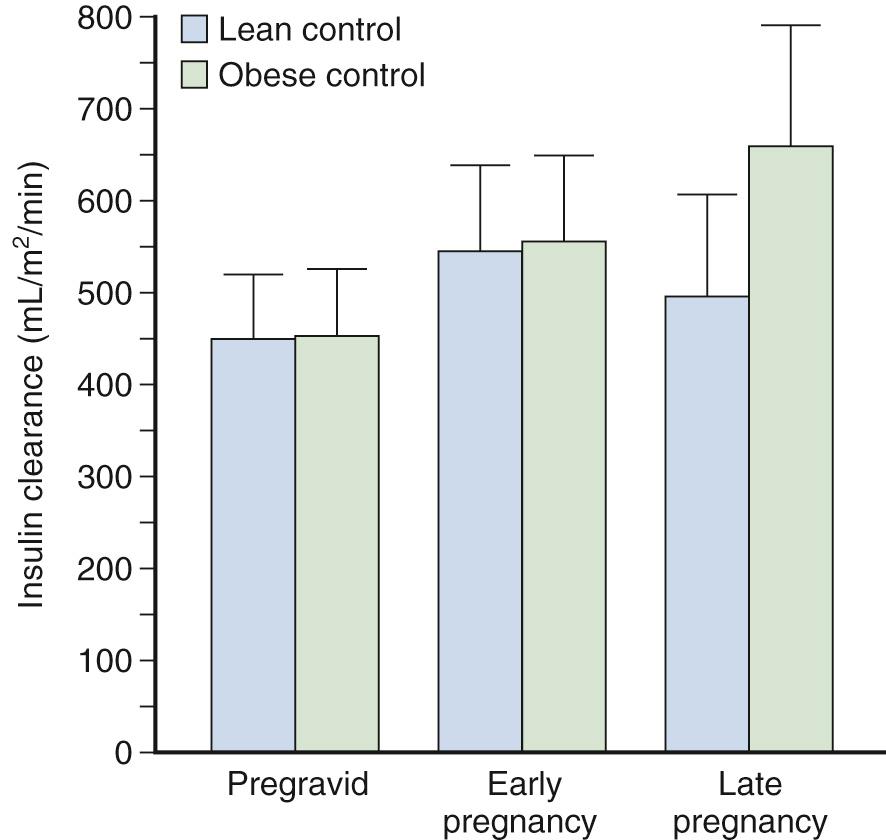
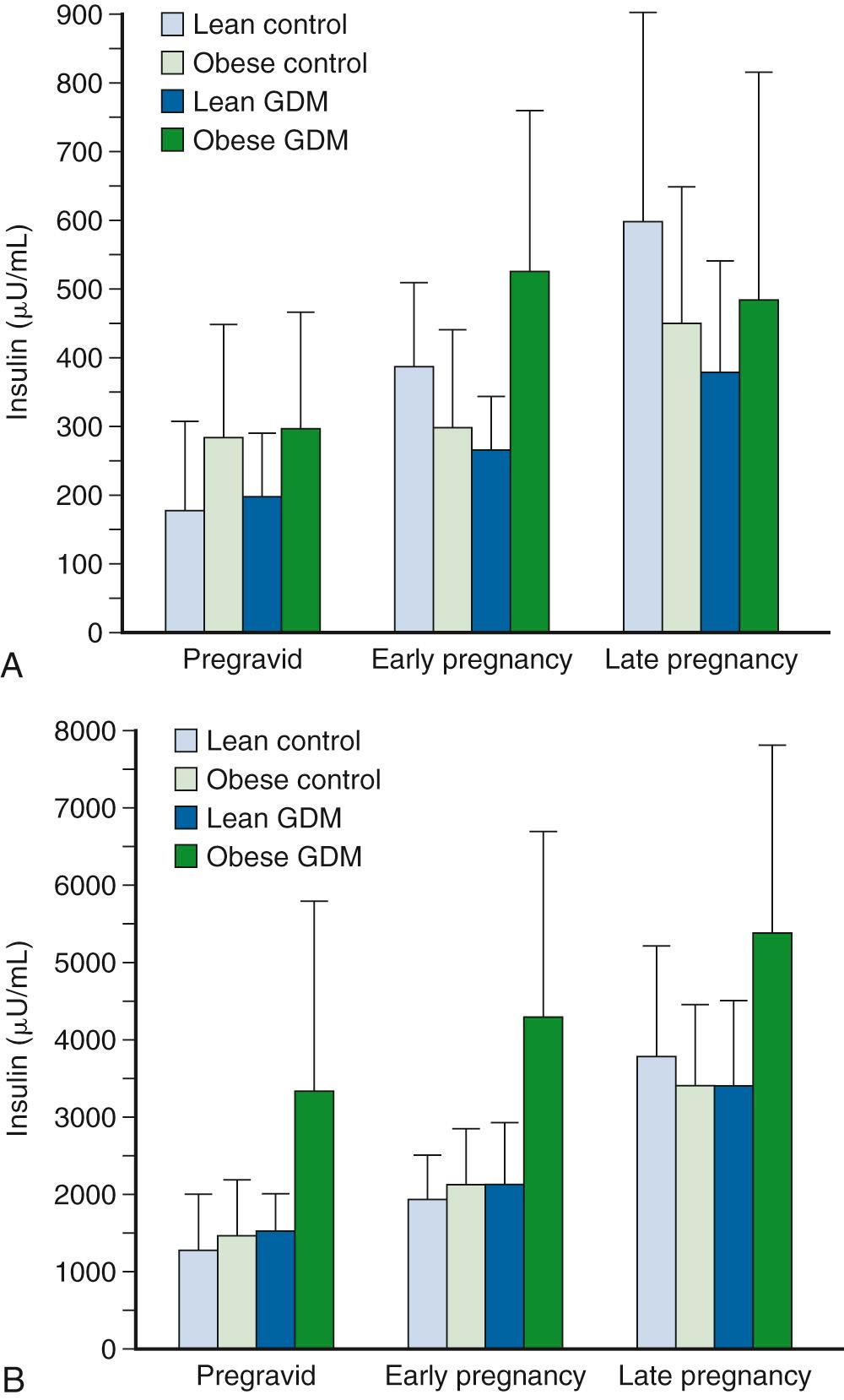
Progressive increases are seen in insulin secretion in response to an intravenous (IV) glucose challenge with advancing gestation ( Fig. 45.3 ). The increases in insulin concentration are more pronounced in lean women, compared with obese women, most probably as a response to the greater decreases in insulin sensitivity in lean women, as will be described later. The increases in insulin response in early pregnancy, regardless of the changes in insulin sensitivity, are correlated with increasing concentrations of leptin. Leptin concentrations are increased in early pregnancy, prior to significant increases in maternal fat mass, and are most likely related to placental sources. Leptin concentrations remain elevated throughout gestation. Data regarding insulin clearance in pregnancy are limited. In separate studies, Bellman, Lind, and colleagues and Burt and Davidson reported no difference in insulin disappearance rate when insulin was infused intravenously in late gestation compared with nongravid subjects. In contrast, using a radiolabeled insulin, Goodner and Freinkel described a 25% increase in insulin turnover in a pregnant compared with a nonpregnant rat model. Using the euglycemic clamp, Catalano and associates reported a 20% increase in insulin clearance in lean women and a 30% increase in insulin clearance in obese women by late pregnancy ( Fig. 45.4 ). Although the placenta is rich in insulinase, the exact mechanism for the enhanced insulin clearance in pregnancy remains speculative.
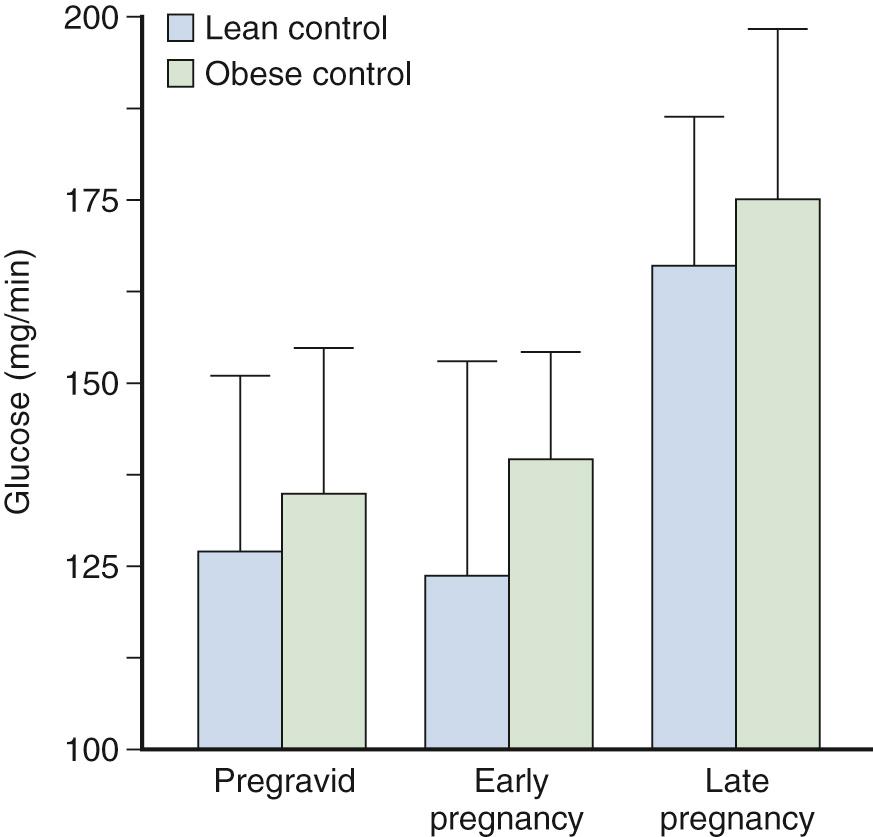
There is a progressive decrease in fasting glucose with advancing gestation, most likely resulting from the increase in plasma volume in early gestation and greater fetoplacental glucose use in late gestation. Using various stable isotope methodologies in cross-sectional study designs, Kalhan and Cowett were the first to describe increased fasting hepatic glucose production in late pregnancy. In addition, using a stable isotope of glucose in a prospective longitudinal study design, Catalano and coworkers reported a 30% increase in maternal fasting hepatic glucose production with advancing gestation ( Fig. 45.5 ), which remained significant even after adjusting for maternal weight gain. Tissue sensitivity to insulin involves both liver and peripheral tissues, primarily skeletal muscle. The increase in fasting maternal hepatic glucose production occurs despite a significant increase in fasting insulin concentration, indicating a decrease in maternal hepatic glucose sensitivity in women with normal glucose tolerance in late pregnancy. In obese women, insulin suppression of hepatic glucose production was further reduced in late gestation, consistent with a more significant decline in hepatic insulin sensitivity in these patients.
Estimates of peripheral insulin sensitivity in pregnancy have included measurements of insulin response to a fixed oral or an IV glucose challenge or the ratio of insulin to glucose under a variety of experimental conditions. Methodologies such as the minimal model and the euglycemic-hyperinsulinemic clamp have improved our ability to quantify peripheral insulin sensitivity. In lean women in early gestation, Catalano and colleagues reported a mean 10% decrease in maternal peripheral insulin sensitivity using the euglycemic-hyperinsulinemic clamp, with wide variation among individuals. However, when adjusted for changes in insulin concentrations during the clamp and residual hepatic glucose production, insulin sensitivity decreased only 10% ( Fig. 45.6 ). In contrast, a 15% increase was seen in the insulin sensitivity index in obese women in early pregnancy compared with pregravid estimates. Hence the decrease in insulin requirements in early gestation observed in some women may be in part a consequence of an increase in insulin sensitivity, particularly in women with decreased insulin sensitivity before conception.
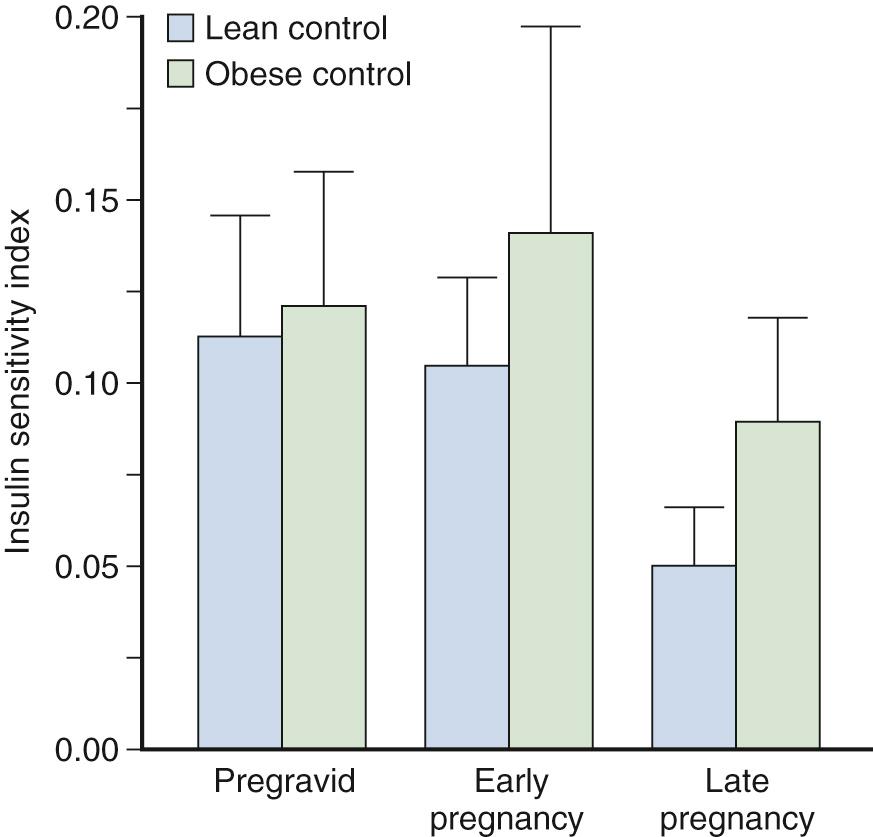
Compared with the varied metabolic alterations in early pregnancy, there is consensus regarding the decrease in peripheral insulin sensitivity in late gestation. Spellacy and Goetz were among the first investigators to report an increase in insulin response to a glucose challenge in late gestation. In addition, Burt demonstrated that pregnant women experienced less hypoglycemia in response to exogenous insulin in comparison with nonpregnant subjects. Later research by Fisher and associates (using a high-dose glucose infusion test), by Buchanan and colleagues (using the Bergman minimal model), and by Ryan and coworkers and Catalano and associates (using the euglycemic-hyperinsulinemic clamp) all demonstrated a decrease in insulin sensitivity ranging from 33% to 78% in late gestation. However, all these quantitative estimates of insulin sensitivity are overestimates because of non–insulin-mediated glucose disposal by the fetus and placenta. Hay and colleagues reported that in the pregnant ewe model, approximately one-third of maternal glucose use was accounted for by uterine, placental, and fetal tissue. In addition, Marconi and coworkers observed that, based on human fetal blood sampling, fetal glucose concentration was a function of fetal size and gestational age in addition to maternal glucose concentration.
The decrease in insulin sensitivity during pregnancy has been ascribed to an increased production of various placental and maternal hormones, such as human placental lactogen, progesterone, estrogen, cortisol, and prolactin. However, recent evidence has focused on the role of several new mediators of insulin resistance such as leptin, tumor necrosis factor α (TNF-α), placental growth hormone, and resistin.
Adiponectin is the most abundant adipocyte-derived adipokine, with circulating concentrations in the nanomolar range. Adiponectin is unique in that this adipokine is not made by the placenta and is inversely related to insulin sensitivity. Adiponectin concentrations decrease with advancing gestation and maternal adiposity and are correlated with decreased regulation of glucose utilization. Clinically, adiponectin concentration prepregnancy and in early gestation has been used as a marker of decreased insulin sensitivity and of women at increased risk of GDM.
Pregnancy has been characterized as a chronic low-grade inflammatory condition because of the increase in activation of circulating blood leukocytes. The inflammation of pregnancy is further enhanced by maternal prepregnancy obesity. This increase in low-grade inflammation, particularly observed in obese women, has been related to increases in macrophage infiltration in both maternal white adipose tissue and the placenta. The increase in inflammation has been associated with higher circulating C-reactive protein and interleukin-6 levels. Higher concentrations of plasminogen activator inhibitor-1 and C-reactive protein are associated with elevated concentrations of maternal glucose, body mass index (BMI), and C-peptide in pregnant women without overt diabetes. These factors may exacerbate the increased insulin resistance in obese women with normal glucose tolerance because of their effects on the postreceptor insulin-signaling cascade. Therefore these inflammatory cytokines may contribute to increased substrate availability for the developing fetus and resultant macrosomia.
Placental glucose transport is a process that takes place through facilitated diffusion. Glucose transport is dependent on a family of glucose transporters referred to as the GLUT glucose transporter family . The principal glucose transporter in the placenta is GLUT1, which is located in the syncytiotrophoblast. GLUT1 is found on both the microvillus and basal membranes . Basal membrane GLUT1 may be the rate-limiting step in placental glucose transport. The oral hypoglycemic drug, glyburide, has been associated with an increase in the placental GLUT1 transporter. A twofold to threefold increase is seen in the expression of syncytiotrophoblast glucose transporters with advancing gestation. Although GLUT3 and GLUT4 expression have been identified in placental endothelial cells and intervillous nontrophoblastic cells, respectively, the role they may play in placental glucose transport remains speculative.
Debate has been ongoing regarding the location and/or function of insulin receptors in the placenta. In early pregnancy, insulin receptors are abundant primarily on the syncytiotrophoblast, the cellular layer in contact with maternal blood. In late gestation, insulin receptors are increased on the placental vascular endothelium (i.e., in contact with fetal blood) and less so on the syncytiotrophoblas. Enhanced maternal insulin response in early pregnancy, as is often seen in women who are obese, or increased levels of exogenous insulin, as observed in women with diabetes, are closely related to placental weight at birth. Placental weight at birth has the strongest correlation with birthweight (i.e., neonatal fat and lean body mass). The implication of these data is that early maternal pregnancy metabolism, insulin resistance, and response may affect placental growth and gene expression, which becomes clinically manifest as fetal overgrowth only late in gestation.
During pregnancy, classification of women with diabetes has often relied on the White classification, first proposed in the 1940s. This classification is based on factors such as the age of onset of diabetes and duration, as well as end-organ involvement, primarily retinal and renal ( Table 45.1 ).
| Class | Diabetes Onset Age (y) | Duration (y) | Vascular Disease | Need for Insulin or Oral Agent |
|---|---|---|---|---|
| Gestational Diabetes | ||||
| A 1 | Any | Any | − | − |
| A 2 | Any | Any | − | + |
| Pregestational Diabetes | ||||
| B | >20 | <10 | − | + |
| C | 10–19 | or 10–19 | − | + |
| D | <10 | or >20 | + | + |
| F | Any | Any | + | + |
| R | Any | Any | + | + |
| T | Any | Any | + | + |
| H | Any | Any | + | + |
All forms of diabetes can occur during pregnancy. In addition to types 1 and 2 diabetes, genetic causes of diabetes exist, the most common of which is maturity-onset diabetes of the young (MODY), characterized by β-cell dysfunction; it has an autosomal dominant mode of inheritance and usually becomes manifest in young adulthood. Mutations in the glucokinase gene are a frequent cause of MODY. Various mutations have been described, and each mutation is associated with varying degrees of disease severity. The most common of these mutations, MODY2, occurs in the European population and involves the glucokinase gene. Because the age of onset of diabetes in women with MODY coincides with the reproductive years, it may be difficult to distinguish between type 1 or type 2 DM and MODY. The glucokinase gene acts as a sensor in the β-cell, which leads to a secretory defect in insulin response. Ellard and colleagues reported that 2.5% of women with GDM in the United Kingdom have the glucokinase mutation, whereas Stoffel, in a small population in the United States, noted that 5% of patients had a glucokinase gene mutation. In another US population, Sewell and colleagues reported no cases of MODY in 72 pregnant women with GDM or recently diagnosed pregestational diabetes. If the mother has the mutation, there is an increased risk for fetal macrosomia, whereas if the mutation is inherited from the father, the fetus may exhibit significant decrease in growth secondary to a relative insulin deficiency.
Type 1 diabetes is usually characterized by an abrupt onset at a young age and absolute insulinopenia with lifelong requirements for insulin replacement. Depending on the population, the onset of type 1 diabetes may also occur in individuals in their third or fourth decades of life. Although the phenotype of the individual with type 1 diabetes has often been conceptualized as being thin, in an 18-year follow-up study, over time, the prevalence of being overweight increased by 47% and the prevalence of obesity rose sevenfold. Patients with type 1 diabetes may have a genetic predisposition for autoantibodies directed against their pancreatic islet cells. The degree of concordance for the development of type 1 diabetes in monozygotic twins is approximately 50%, suggesting that the events subsequent to the development of autoantibodies and appearance of glucose intolerance are also related to environmental factors. Because of the complete dependence on exogenous insulin, pregnant women with type 1 diabetes are at increased risk for the development of diabetic ketoacidosis (DKA). In addition, because intensive insulin therapy is emphasized in women with type 1 diabetes to reduce the risk for spontaneous abortion and congenital anomalies in early gestation, and excessive fetal growth and other morbidities in late gestation, these women are more likely to experience hypoglycemic reactions. Studies by Diamond and Rosenn have shown that the likelihood for hypoglycemia is more problematic in patients with type 1 diabetes during pregnancy because of the diminished counterregulatory response including both epinephrine and glucagon. The deficiency in this counterregulatory mechanism may in part result from an independent effect of pregnancy.
The alterations in glucose metabolism in women with type 1 diabetes are not well characterized. Because of maternal insulinopenia, insulin response during gestation can only be estimated relative to pregravid requirements. Estimates of the change in insulin requirements are complicated by the degree of preconceptional glucose control and potential presence of insulin antibodies. Garcia-Patterson reported on the change in insulin requirements in women with type 1 diabetes and strict glucose control prior to conception (see Fig. 45.2 ). In early pregnancy, both insulin doses and total insulin peak at 9 weeks’ gestation and reach a nadir at 16 weeks to baseline prepregnancy levels. After 16 weeks, insulin requirements gradually rise through 37 weeks. This represents a total increase in insulin requirements of 5.19% per week and approximately a twofold increase relative to prepregnancy requirements. A 5% decrease in insulin requirements after 36 weeks’ gestation was also noted by McManus and Ryan. The decrease in insulin requirements was associated with a longer duration of DM but not with adverse perinatal outcome. More recently, Padmanabhan et al. described the relationship between a fall in insulin requirement of greater than or equal to 15% from the peak total daily dose and placental dysfunction including altered expression of placental antiangiogenic factors and preeclampsia. The fall in insulin requirements in early pregnancy in women with type 1 diabetes may also be a reflection of increased insulin sensitivity as was previously described.
Schmitz and associates have evaluated the longitudinal changes in insulin sensitivity in women with type 1 diabetes in early and late pregnancy, as well as postpartum, in comparison with nonpregnant women with type 1 diabetes. In the pregnant women with type 1 diabetes, a 50% decrease in insulin sensitivity was observed only in late gestation. No significant difference was found in insulin sensitivity in pregnant women with type 1 diabetes in early pregnancy or within 1 week of delivery compared with nonpregnant women with type 1 diabetes. Therefore, based on the available data, women with type 1 diabetes appear to have a similar decrease in insulin sensitivity when compared with women with normal glucose tolerance. Relative to the issue of placental transporters (GLUT1), a report by Jansson and Powell describes an increase in both basal GLUT1 expression and glucose transport activity in placental tissue from women with pregnancies complicated by White class D diabetes.
The pathophysiology of type 2 diabetes involves abnormalities of both insulin-sensitive tissue (i.e., both a decrease in skeletal muscle and hepatic sensitivity to insulin) and β-cell response as manifested by inadequate insulin release for a given degree of glycemia. Initially, in the course of the development of type 2 diabetes, the insulin response to a glucose challenge may be increased relative to that of individuals with normal glucose tolerance, but it is inadequate to maintain normoglycemia. Whether decreased insulin sensitivity precedes β-cell dysfunction in the development of type 2 diabetes continues to be debated. Arguments and experimental data support both hypotheses.
Despite the limitations of any classification system, certain generalizations can be made regarding women with type 2 diabetes or GDM. These individuals are typically older and more often have higher BMI compared with individuals with normal glucose tolerance. The onset of the disorder is usually insidious, with few patients complaining of the classic triad of symptoms seen at the onset of type 1 diabetes—polydipsia, polyphagia, and polyuria. Individuals with type 2 diabetes are often initially told to lose weight, increase their activity (i.e., exercise), and follow a diet low in saturated fats and high in complex carbohydrates. Oral agents are frequently used to increase insulin response, enhance insulin sensitivity, or increase renal excretion of glucose. Individuals with type 2 diabetes may eventually require insulin therapy to maintain euglycemia but are at significantly less risk for DKA. Data from monozygotic twin studies have reported a lifetime risk for both twins developing type 2 diabetes that ranges between 58% and almost 100%, suggesting that the disorder has a strong genetic component.
Type 2 pregestational diabetes is usually classified as class B diabetes according to the White classification system. Women who develop GDM—glucose intolerance first diagnosed in the second or third trimester of pregnancy that is not clearly either preexisting type 1 or type 2 diabetes—share many of the metabolic characteristics of women with type 2 diabetes. Although earlier studies reported a 10% to 35% incidence of islet cell antibodies in women with GDM as measured by immunofluorescence techniques, research using specific monoclonal antibodies has described a much lower incidence, on the order of 1% to 2%, suggesting a low risk for type 1 diabetes in women with GDM. Furthermore, postpartum studies of women with GDM have demonstrated defects in insulin secretory response and decreased insulin sensitivity, indicating that the typical abnormalities in glucose metabolism seen in type 2 diabetes are present in women with GDM and may well have existed prior to pregnancy complicated by GDM. The alterations in insulin secretory response and insulin resistance in women with a previous history of GDM compared with a weight-matched control group may differ depending on whether the women with previous GDM are lean or obese. Thus, in women with GDM, the hormonal events of pregnancy may unmask a genetic susceptibility to type 2 diabetes.
Significant alterations in glucose metabolism are present in women who develop GDM relative to women with normal glucose tolerance. Decreased insulin response to a glucose challenge has been demonstrated by Yen, Fisher, and Buchanan and their colleagues in women with GDM in late gestation. In prospective longitudinal studies of both lean and obese women with GDM, Catalano and associates also showed a progressive decrease in first-phase insulin response in late gestation in lean women who develop GDM compared with a weight-matched control group ( Fig. 45.7 ). In contrast, in obese women who develop GDM, no difference in first-phase insulin response was reported, but rather a significant increase was seen in second-phase insulin response to an IV glucose challenge compared with that of a weight-matched control group (see Fig. 45.7 ). These differences in insulin response may be related to the ethnicity of the various study groups. Although the metabolic clearance rate of insulin is increased with advancing gestation, no evidence suggests that a significant difference exists between women with normal glucose tolerance and those with GDM.
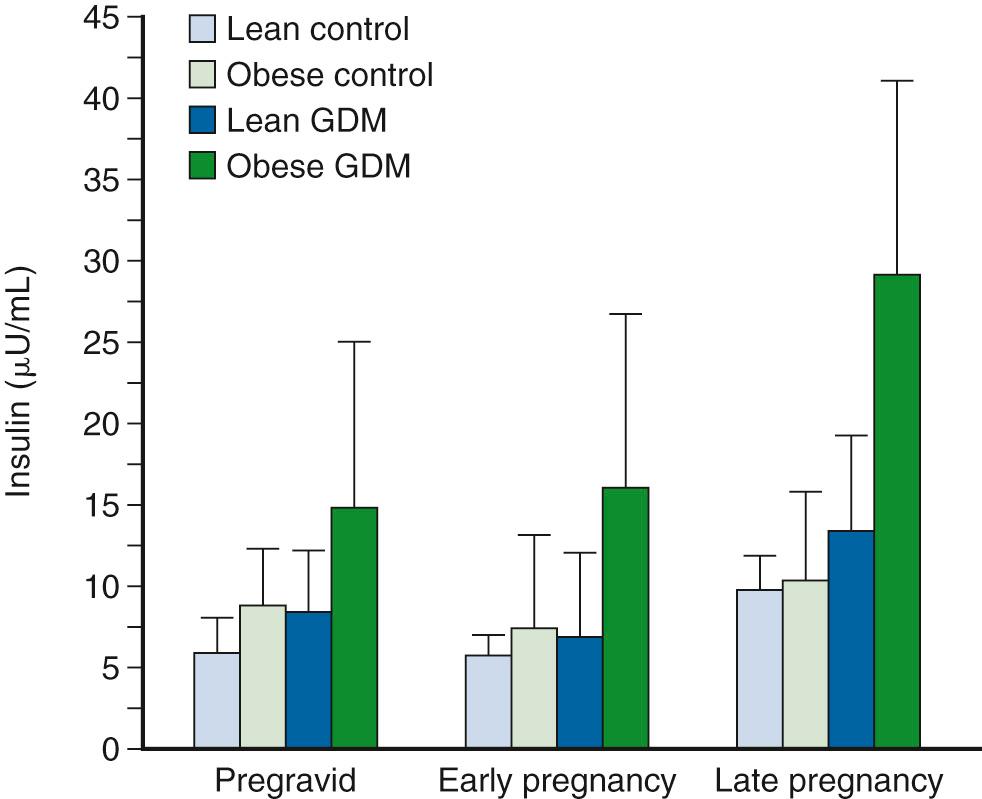
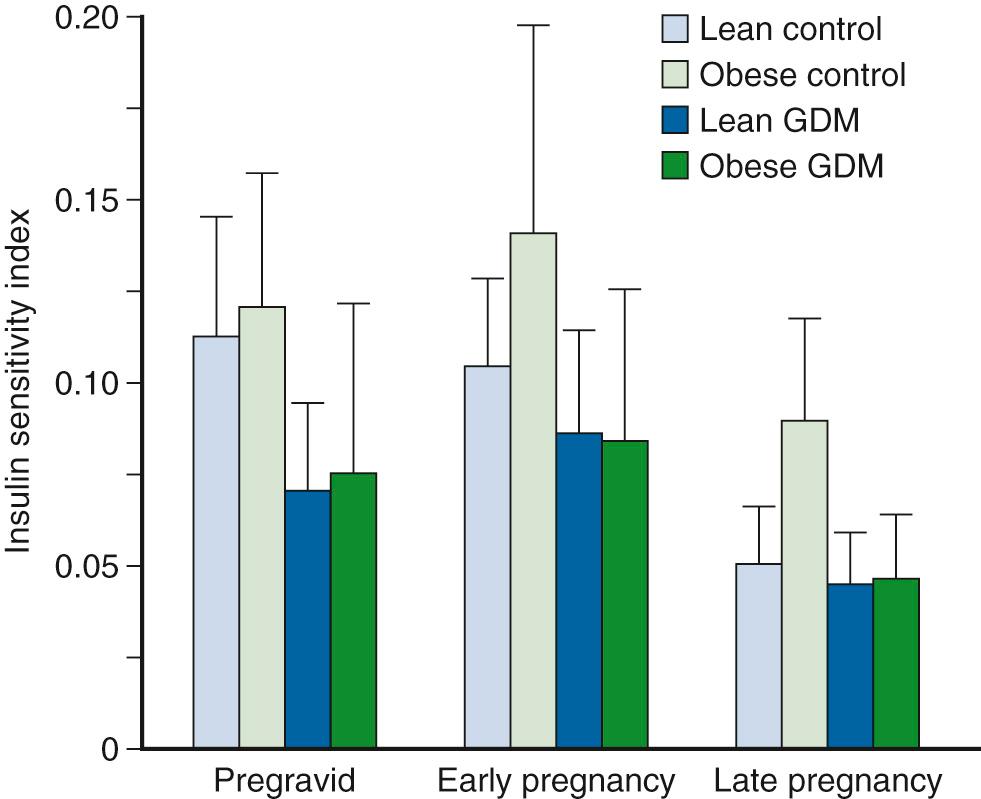
Fasting glucose concentrations decrease with advancing gestation in women who develop mild GDM characterized by normal fasting glucose levels in late pregnancy; however, hepatic glucose production increases in women with GDM in comparison with a matched control group. At this time in gestation, women with GDM have increased fasting insulin concentrations ( Fig. 45.8 ) and less suppression of hepatic glucose production during insulin infusion, indicating decreased hepatic insulin sensitivity compared with a weight-matched control group. In the studies of Xiang and associates, a significant correlation was found between fasting FFA concentrations and hepatic glucose production, which suggests that increased FFA concentrations may contribute to hepatic insulin resistance. As described previously, decreased insulin sensitivity is an important characteristic of GDM. Ryan and colleagues were the first to report a 40% decrease in insulin sensitivity in women with GDM compared with a pregnant control group in late pregnancy using a hyperinsulinemic-euglycemic clamp. Xiang and associates found that women with GDM who had normal glucose tolerance within 6 months of delivery had significantly decreased insulin sensitivity as estimated by the glucose clearance rate during a hyperinsulinemic-euglycemic clamp compared with that of a matched control group. Using similar techniques, Catalano and coworkers described the longitudinal changes in insulin sensitivity in both lean and obese women who developed GDM in comparison with a matched control group. Women who developed GDM had decreased insulin sensitivity compared with that of the matched control group ( Fig. 45.9 ). The differences in insulin sensitivity were greatest before and during early gestation; by late gestation, the differences in insulin sensitivity between the groups were less pronounced but still significant. Of interest, an increase in insulin sensitivity was noted from the time before conception through early pregnancy (12 to 14 weeks), particularly in those women with the greatest decreases in insulin sensitivity before conception. The changes in insulin sensitivity from the time before conception through early pregnancy were significantly correlated with changes in maternal weight gain and energy expenditure. The relationship between these alterations in maternal glucose insulin sensitivity and weight gain and energy expenditure may help explain the decrease in maternal weight gain and insulin requirements in women with diabetes in early gestation. In summary, the various degrees of decreased insulin sensitivity observed in late pregnancy in women with normal glucose tolerance or GDM are a reflection of their individual prepregnancy insulin sensitivity. Unless unforeseen severe metabolic events occur during pregnancy, relatively uniform decreases are apparent in insulin sensitivity with advancing gestation in all women.
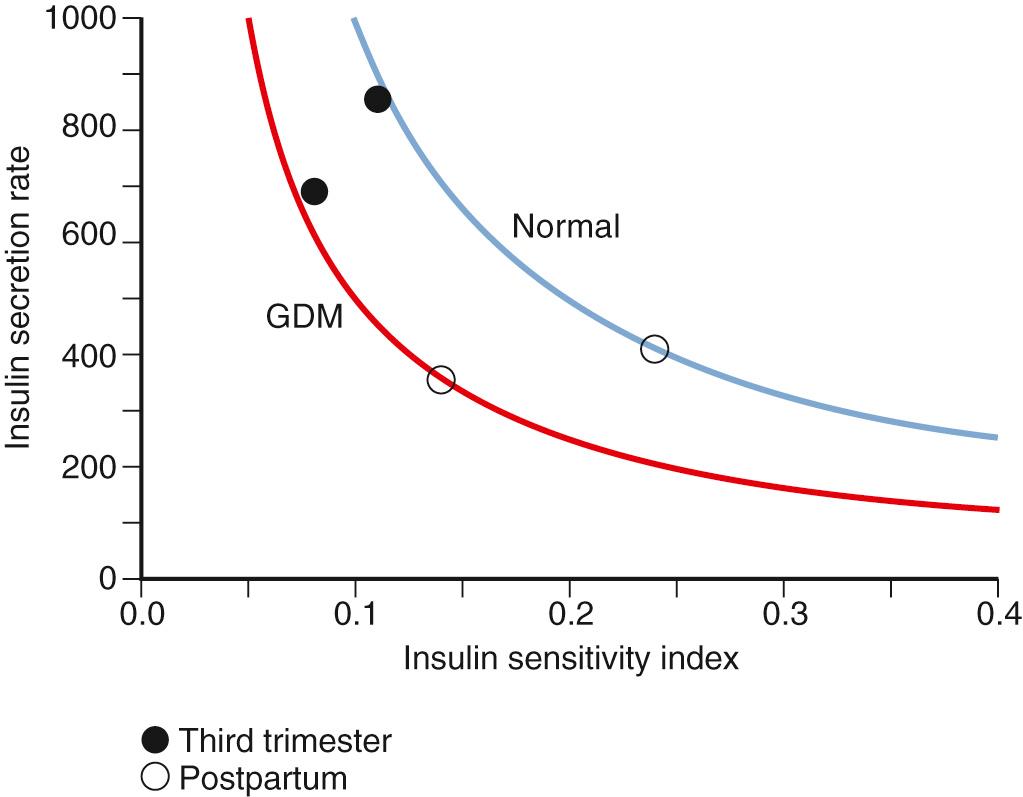
The interactions of β-cell response and insulin sensitivity are hallmarks of the metabolic adaptations of pregnancy. As described by Bergman, the relationship between insulin response and insulin resistance is fixed in nonpregnant individuals and follows a hyperbolic curve (i.e., the disposition index [DI]; Fig. 45.10 ). Buchanan described a similar relationship between insulin response and insulin action during pregnancy. Indeed, when the DI has been compared between women with normal glucose tolerance and GDM both during and after pregnancy, the failure of the β-cell to compensate for insulin resistance in GDM has been similar to the hyperbolic changes in the control group (see Fig. 45.10 ). However, this relationship between insulin sensitivity and insulin resistance may not hold in early pregnancy, when both an increase in insulin sensitivity and insulin response may be evident.
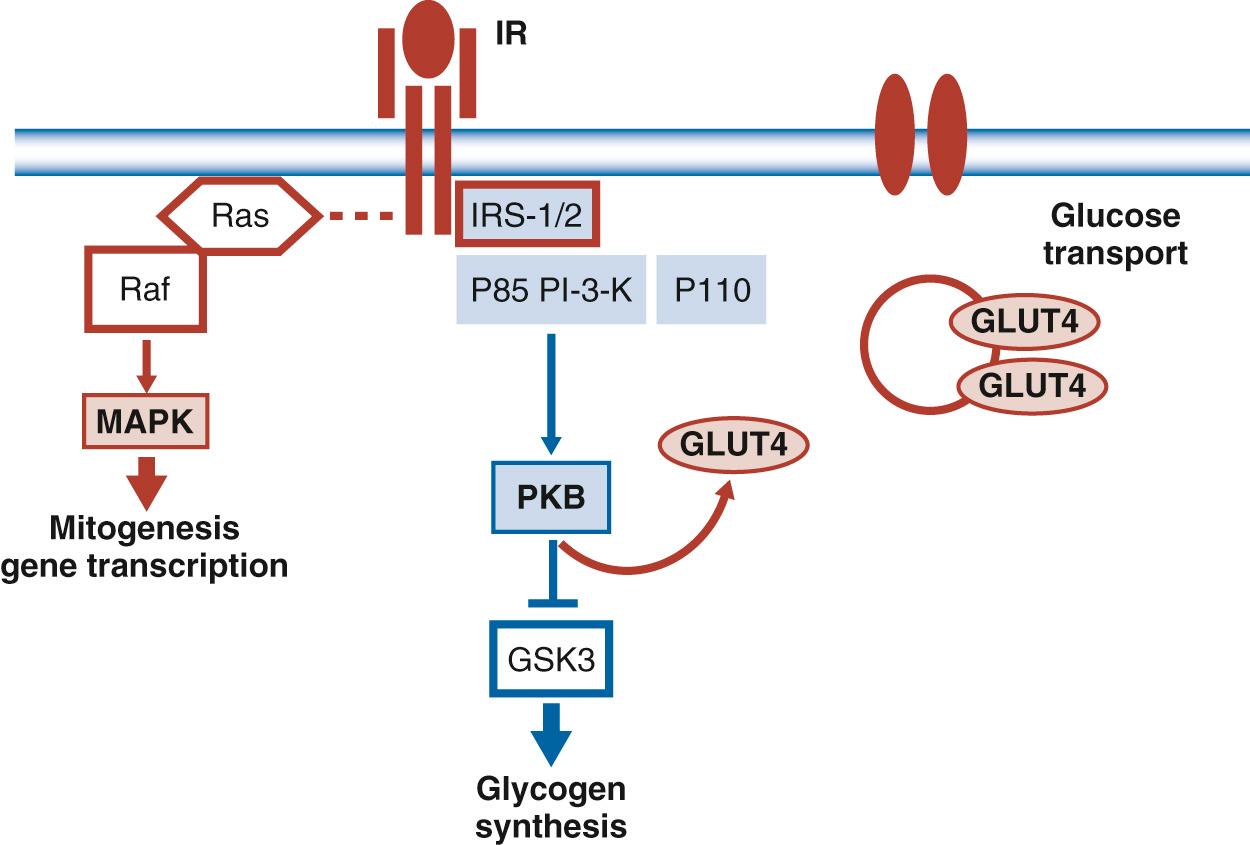
Studies in human skeletal muscle and adipose tissue have demonstrated that postreceptor defects in the insulin-signaling cascade are related to decreased insulin sensitivity in pregnancy. Garvey and colleagues were the first to demonstrate that there were no significant differences in the glucose transporter (GLUT4) responsible for insulin action in skeletal muscle in pregnant compared with nonpregnant women. Based on the studies of Friedman and colleagues in pregnant women with normal glucose tolerance, GDM, as well as in weight-matched nonpregnant control subjects, defects were apparent in the postreceptor insulin-signaling cascade. All pregnant women appeared to have a decrease in expression of insulin receptor substrate 1 (IRS1). The downregulation of the IRS1 protein closely parallels the decreased ability of insulin to induce additional steps in the insulin-signaling cascade, which results in movement of the GLUT4 to the cell surface membrane to facilitate glucose transport into the cell. The downregulation of IRS1 protein closely parallels the ability of insulin to stimulate 2-deoxyglucose uptake in vitro. In addition to the previous mechanisms, women with GDM demonstrate a distinct decrease in the ability of insulin receptor - β , that component of the insulin receptor not on the cell surface, to undergo tyrosine phosphorylation. The additional defect in the insulin-signaling cascade results in a 25% lower glucose transport activity ( Fig. 45.11 ). Kirwan and coworkers reported that TNF-α was inversely correlated with the changes in insulin sensitivity from the time before conception through late gestation. In combination with other placental hormones, multivariate stepwise regression analysis revealed that TNF-α was the strongest independent predictor of insulin sensitivity in pregnancy, accounting for about half of the variance in the decrease in insulin sensitivity during gestation.
Although glucose is the primary source of energy for the fetus and placenta, no appreciable amounts of glucose are stored as glycogen in the fetus or placenta. However, accretion of protein is essential for growth of fetal and placental tissue. Nitrogen retention is increased in pregnancy in both maternal and fetal compartments, and this increase results in approximately 0.9 kg of maternal fat-free mass by 27 weeks. A significant decrease is apparent in most basal maternal amino acid concentrations in early pregnancy before the accretion of significant maternal or fetal tissue. These anticipatory changes in amino acid metabolism occur after a shorter period of fasting in comparison with nonpregnant women and may be another example of the accelerated starvation of pregnancy described by Freinkel and coworkers. Furthermore, amino acid concentrations, such as those of serine, correlate significantly with fetal growth in both early and late gestation. Maternal amino acid concentrations were significantly decreased in mothers of small-for-gestational-age (SGA) neonates in comparison with maternal amino acid concentrations in appropriately grown neonates.
Based on a review of various studies, Duggleby and Jackson have estimated that during pregnancy, protein synthesis is similar to that in nonpregnant women in the first trimester. However, a 15% increase in protein synthesis occurs during the second trimester, and a further increase is seen in the third trimester, by approximately 25%. In addition, interindividual differences at each time point are marked, and these differences have a strong relationship with fetal growth: mothers who had increased protein turnover in midpregnancy had babies who had increased lean body mass after adjustment for important covariables.
Amino acids can be used for protein accrual, or they can be oxidized as an energy source. Estimation of urea synthesis using stable isotopes has been performed in a number of studies. In general, a modest shift in oxidation occurs in early pregnancy with an accrual of amino acids for protein synthesis in late gestation. Kalhan and colleagues reported significant pregnancy-related adaptations in maternal protein metabolism early in gestation before any significant increase in fetal protein accretion. Catalano and associates have also described decreased amino acid insulin sensitivity based on reduced suppression of leucine turnover during insulin infusion in late gestation. Some evidence suggests an increase in basal leucine turnover in women with GDM compared with that of a matched control group. Whether these decreases in amino acid insulin sensitivity are related to decreased whole-body and liver protein synthesis or increased breakdown are not known at this time.
Cetin and associates reported that placental amino acid exchange is altered in pregnancies complicated by GDM. Ornithine concentrations were significantly increased in women with GDM compared with controls, and in the cord blood of infants of women with GDM, significant increases were observed in multiple amino acids, including phenylalanine and leucine, while decreases in glutamate were also found. The investigators speculate that in infants of women with GDM, the altered in utero fetal milieu affects fetal growth through multiple mechanisms that impact various nutrient compartments.
Amino acids are actively transported across the placenta from mother to fetus through energy-requiring amino acid transporters. These transporters are highly stereospecific, but they have low substrate specificity. In addition, they may vary in location between the microvillus and basal membranes. Decreased amino acid concentrations have been reported in growth-restricted neonates in comparison with appropriately grown neonates, and decreased amino acid transporter activity has been implicated as a possible mechanism. However, the potential role, if any, of placental amino acid transporters in the development of fetal macrosomia in women with diabetes is currently unknown.
Although ample literature supports the changes observed in glucose metabolism during gestation, the data regarding the alterations in lipid metabolism are meager by comparison. Darmady and Postle measured serum cholesterol and triglyceride before, during, and after pregnancy in 34 normal women and observed a decrease in both cholesterol and triglyceride at approximately 7 weeks’ gestation. Both cholesterol and triglyceride concentrations increased progressively until term, and then a decrease was seen in serum triglyceride postpartum. The decrease was more rapid in women who breastfed compared with those women who bottle-fed their infants. In addition, Knopp and coworkers have reported that a twofold to fourfold increase occurs in total triglyceride concentration and a 25% to 50% increase is seen in total cholesterol concentration during gestation. A 50% increase in LDL cholesterol and a 30% increase in high-density lipoprotein (HDL) cholesterol has been documented by midgestation, but these levels then decrease slightly in the third trimester. Maternal triglyceride and very-low-density lipoprotein (VLDL) triglyceride levels in late gestation are positively correlated with maternal estriol and insulin concentrations.
A study by Vahratian and associates examined the changes in lipid levels during pregnancy in normal-weight compared with overweight and obese women from 6 to 10 through 32 to 36 weeks’ gestation. The levels of total cholesterol, triglycerides, and LDL and HDL cholesterol increased throughout gestation. Although the concentrations in the overweight and obese women were generally higher in early pregnancy, the rate of change of LDL cholesterol and total cholesterol was lower in later gestation.
FFAs have been associated with fetal overgrowth, particularly of fetal adipose tissue. A significant difference in the arteriovenous FFA concentration is seen at birth, much the same as with arteriovenous glucose concentration. Multiple clinical studies suggest the contribution of maternal lipids to fetal growth and in particular to adiposity. Knopp and coworkers reported that neonatal birthweight was positively correlated with triglyceride and FFA concentrations in late pregnancy. Similar conclusions were reached by Ogburn and colleagues, who showed that higher insulin levels decrease FFA concentrations, inhibit lipolysis, and result in increased fat deposition. Kleigman reported that infants of obese women had an increased birthweight and skinfold thickness and higher FFA levels compared with infants of lean women. DiCianni and associates observed that in women with a positive glucose screen but normal glucose tolerance, their serum triglycerides and prepregnancy BMI had a significant correlation with birthweight at term. In Australia, Nolan and coworkers showed that nonfasting maternal triglycerides measured at 9 to 12 weeks’ gestation were significantly correlated with neonatal birthweight ratio at term. Finally, in a well-controlled German GDM population, Schaeffer-Graf and colleagues reported that maternal FFA concentrations correlated with ultrasound estimates of neonatal abdominal circumference and anthropometric estimates of neonatal fat mass at delivery. Maternal FFA concentrations were also positively correlated with cord FFA. Although FFA concentrations were higher in cord blood of large-for-gestational-age (LGA) neonates compared with either appropriately grown or SGA neonates, a paradoxical negative correlation of cord triglycerides and birthweight was reported. The authors speculated that SGA newborns have a lower lipoprotein lipase activity and hence were unable to hydrolyze triglycerides. In contrast, the LGA neonates have lower cord triglyceride concentrations because of enhanced lipoprotein lipase activity resulting from their increased number of fat cells. Similar findings were noted by Merzouk and coworkers in growth-restricted infants. Lastly, the placentas of women with GDM have increased expression of genes related to inflammation and lipid metabolism in comparison with those of a BMI-matched control group.
In summary, in women with evidence of decreased insulin sensitivity (obesity and/or GDM) in addition to glucose, maternal lipid metabolism accounts for a significant proportion of fetal growth, particularly adiposity. These data support the original work by Freinkel, which proposed that fetal growth or overgrowth is a function of multiple nutritional factors in addition to glucose.
Lipid metabolism in women with DM is influenced by whether the woman has type 1 or type 2 diabetes. This also applies when these women become pregnant. In women with type 2 diabetes and GDM, Knopp and coworkers reported an increase in triglyceride and a decrease in HDL concentrations. However, Montelongo and colleagues observed little change in FFA concentrations through all three trimesters after a 12-hour fast. Koukkou and coworkers noted an increase in total triglyceride but lower LDL cholesterol in women with GDM. Increased triglyceride concentrations during pregnancy have also been reported to be related to the development of GDM and preeclampsia in women with normal pregravid glucose tolerance, both of which are related to increased insulin resistance. In women with type 1 diabetes, no change was observed in total triglycerides, but a lower cholesterol concentration was reported secondary to a decrease in HDL. This is of interest because HDL acts as a plasma antioxidant, and thus a lower HDL level may be related to the increase in congenital malformations in women with type 1 diabetes. Oxidative stress has been implicated as a potential factor contributing to the increased incidence of anomalies in women with type 1 diabetes.
Hyperinsulinemic-euglycemic clamp studies in pregnant women with normal glucose tolerance and GDM have revealed a decreased ability of insulin to suppress plasma FFAs with advancing gestation. Insulin's ability to suppress plasma FFAs was lower in women with GDM when compared with women with normal glucose tolerance.
Taken together, these studies demonstrate reduced nutrient insulin sensitivity in all women with advancing gestation. These decreases in insulin sensitivity are further exacerbated by the presence of decreased pregravid maternal insulin sensitivity, which manifests in later pregnancy as GDM and results in greater nutrient availability and higher ambient insulin concentrations for the developing fetoplacental unit, which may eventually result in fetal overgrowth.
Estimates of the energy cost of pregnancy range from a requirement of 80,000 kcal to a net savings of up to 10,000 kcal. As a result, the recommendations for nutritional intake in pregnancy differ and depend on the population being evaluated. Furthermore, recommendations for individuals within a population may be more diverse than previously believed, making general guidelines for nutritional intake difficult.
The theoretic energy cost of pregnancy was originally estimated by Hytten and Leitch using a factorial method. The additional cost of pregnancy consisted of (1) the additional maternal and fetoplacental tissue accrued during pregnancy and (2) the additional “running cost” of pregnancy (e.g., the work of increased cardiac output). In Hytten's model, the greatest increases in maternal energy expenditure occur between 10 and 30 weeks’ gestation, primarily because of maternal accretion of adipose tissue. Each kilogram increase of adipose tissue represents approximately 10,000 kcals. However, the mean increases in maternal adipose tissue vary considerably among various ethnic groups. Forsum and associates reported a mean increase of more than 5 kg of adipose tissue in Swedish women, whereas Lawrence and colleagues found no increase in adipose tissue stores in women from The Gambia.
Basal or resting metabolic rate accounts for 60% to 70% of total energy expenditure in individuals not engaged in competitive physical activity, and this correlates well with total energy expenditure. As with the changes in maternal accretion of adipose tissue, wide variations are seen in the change in maternal basal metabolic rate during gestation, not only in different populations but again within relatively homogeneous groups. The cumulative energy changes in basal metabolic rate range from a high of 52,000 kcal in Swedish women to a net savings of 10,700 kcal in women from The Gambia without nutritional supplementation. The mean increase in basal metabolic rate in Western women relative to a nonpregnant, nonlactating control group averages approximately 20% to 25%. However, the coefficient of variation of basal metabolic rate ranges from 93% in women in the United Kingdom to more than 200% in Swedish women. However, when assessing energy intake in relation to energy expenditure, estimated energy intake remains lower than the estimates of total energy expenditure. These discrepancies have usually been explained by factors such as increased metabolic efficiency during gestation, decreased maternal activity, and unreliable assessment of food intake.
Data in nonpregnant individuals may help to explain some of the wide variations in metabolic parameters during human gestation, even with homogeneous populations. Swinburn and colleagues reported that in the Pima Indian population, subjects with decreased insulin sensitivity gained less weight compared with more insulin-sensitive subjects (3.1 vs. 7.6 kg) over a period of 4 years. Furthermore, the percentage weight change per year was highly correlated with insulin sensitivity as estimated from clamp studies. Catalano and coworkers evaluated the changes in maternal accretion of body fat and basal metabolic rate in lean and obese women with and without GDM. Women who developed GDM had decreased insulin sensitivity in early gestation compared with a matched control group and had significantly smaller increases in body fat than women with normal glucose tolerance. A significant inverse correlation was found between the changes in fat accretion and insulin sensitivity (i.e., women with decreased pregravid insulin sensitivity had less accretion of body fat compared with women with increased pregravid insulin sensitivity). The variations in resting metabolic rate during pregnancy correlate positively with maternal fat free mass but negatively with fat mass. Hence variations in gestational weight gain may also be related to changes in resting metabolic rate. Women with smaller increases in resting metabolic rate across pregnancy have greater gestational weight gain, specifically adipose tissue, whereas women with proportionally greater increases in resting metabolic rate have less accrual of weight and adipose tissue.
In the fasting state, lean women increase the use of carbohydrate as a metabolic fuel, whereas in obese women, there is an increased use of lipids for oxidative needs. However, with the decrease in insulin sensitivity in late gestation, all women have an increase in fat oxidation and decrease in nonoxidative glucose metabolism (e.g., glycogen [storage]). These increases in lipid oxidation are positively correlated with the increases in maternal leptin concentrations, possibly accounting for a role of leptin in human pregnancy. The ability of women with decreased pregravid glucose insulin sensitivity (obese women and women with GDM) to conserve energy, not significantly increase body fat, and make sufficient nutrients available to produce a healthy fetus supports the hypothesis that lower maternal insulin sensitivity may have a reproductive metabolic advantage in women when food availability is marginal (i.e., the thrifty gene hypothesis). In contrast, decreased maternal insulin sensitivity before conception in areas where food is plentiful and a sedentary lifestyle is more common may manifest as GDM, increasing the long-term risk for both diabetes and obesity in the woman and her offspring.
In the past, sudden and unexplained stillbirth occurred in 10% to 30% of pregnancies complicated by type 1 diabetes; although currently relatively uncommon, such losses still plague the pregnancies of patients who do not receive optimal care. Mathiesen and colleagues reported 25 stillbirths among 1361 singleton births of women with type 1 diabetes. In this series the offspring of women with type 1 or type 2 diabetes were five times more likely to be stillborn compared with those of mothers without diabetes. Starikov reviewed seven series of women with type 1 diabetes and reported a fivefold increased risk for stillbirth and a relative risk (RR) of 2.5 to 4.5 for women with type 2 diabetes compared with the nondiabetic population. Cundy has reported that stillbirths are currently more common in type 2 diabetic pregnancies than in women with type 1 disease. Stillbirths have been observed most often after the 36th week of pregnancy in patients with poor glycemic control, hydramnios, fetal macrosomia, vascular disease, or preeclampsia. Women with vascular complications may develop fetal growth restriction (FGR) and intrauterine fetal demise as early as the second trimester.
Excessive stillbirth rates in pregnancies complicated by diabetes have been linked to chronic intrauterine hypoxia. Extramedullary hematopoiesis, frequently observed in stillborn infants of diabetic mothers (IDMs), supports chronic intrauterine hypoxia as a likely cause of these intrauterine fetal deaths. Studies of fetal umbilical cord blood samples in pregnant women with type 1 diabetes have demonstrated relative fetal erythremia and lactic acidemia. Maternal diabetes may also produce alterations in red blood cell (RBC) oxygen release and placental blood flow. Reduced uterine blood flow is thought to contribute to the increased incidence of FGR observed in pregnancies complicated by diabetic vasculopathy. Ketoacidosis and preeclampsia, two factors known to be associated with an increased incidence of intrauterine deaths, may further decrease uterine blood flow.
Alterations in fetal carbohydrate metabolism also may contribute to intrauterine asphyxia. Considerable evidence links hyperinsulinemia and fetal hypoxia. Hyperinsulinemia induced in fetal lambs by an infusion of exogenous insulin produces an increase in oxygen consumption and a decrease in arterial oxygen content. Persistent maternal-fetal hyperglycemia occurs independent of maternal uterine blood flow, which may not be have the capacity to further increase to allow for enhanced oxygen delivery in the face of increased metabolic demands, especially in the macrosomic fetus. Thus hyperinsulinemia appears to increase the metabolic rate and oxygen requirement in the fetus of women with diabetes. Other factors such as hyperglycemia, ketoacidosis, preeclampsia, and maternal vasculopathy can also reduce placental blood flow and fetal oxygenation.
Congenital malformations are the most important cause of perinatal loss in pregnancies complicated by type 1 and type 2 DM. In the past, these anomalies were responsible for only 10% of all perinatal deaths. Malformations currently account for 30% to 50% of perinatal mortality. Neonatal deaths exceed stillbirths in pregnancies complicated by pregestational diabetes, and fatal congenital malformations are responsible for this pattern.
Most studies have documented a twofold to sixfold increase in major malformations in infants of type 1 and type 2 mothers with diabetes. A large population-based cohort study of Canadian women revealed a 23% decline in congenital malformations in diabetic pregnancies from 1996 through 2010; however, the RR for malformations remained elevated in women with preexisting diabetes (RR, 2.33; 95% confidence interval [CI], 1.59 to 3.43). In the Diabetes in Early Pregnancy Study in the United States, the incidence of major anomalies was 2.1% in 389 control patients and 9% in 279 women with diabetes. A recent case-control study of 13,030 infants with congenital anomalies and 4895 controls revealed a prevalence in type 1 diabetes of 2.2% versus 0.5% in women with normal glucose tolerance and, in type 2 diabetes, of 5.1% versus 3.7% in controls. In general, the incidence of major malformations in worldwide studies of offspring of women with diabetes has ranged from 7.5% to 10% ( Table 45.2 ).
| Study (year) | N | % |
|---|---|---|
| Mills et al. (1988) | 25/279 | 9.0 |
| Greene (1993) | 35/451 | 7.7 |
| Steel (1982) | 12/239 | 7.8 |
| Fuhrmann et al. (1983) | 22/292 | 7.5 |
| Simpson et al. (1983) | 9/106 | 8.5 |
| Albert et al. (1996) | 29/289 | 10.0 |
The insult that causes malformations in IDMs affects most organ systems and must act before the seventh week of gestation. Central nervous system malformations—particularly anencephaly, open spina bifida, and holoprosencephaly—are increased 10-fold. Cardiac anomalies are the most common malformations seen in IDMs (35% to 40%), with ventricular septal defects and complex lesions such as transposition of the great vessels increased fivefold. Other lesions observed include double-outlet right ventricle, truncus arteriosus, and tricuspid atresia. A recent population-based cohort study from Sweden found an incidence of cardiac malformations of 4.9% in offspring of 2458 women with type 1 diabetes compared with 1.5% in infants of women with normal glucose tolerance . The congenital defect thought to be most characteristic of diabetic embryopathy is sacral agenesis or caudal dysplasia, an anomaly found 200 to 400 times more often in offspring of women with diabetes ( Fig. 45.12 ). However, this defect is not pathognomonic for diabetes because it also occurs in nondiabetic pregnancies.

Impaired glycemic control and associated derangements in maternal metabolism appear to contribute to abnormal embryogenesis. Maternal hyperglycemia has been proposed by most investigators as the primary teratogenic factor, but hyperketonemia, hypoglycemia, somatomedin inhibitor excess, and excess free oxygen radicals have also been suggested ( Box 45.1 ). The profile of a woman most likely to produce an anomalous infant would include a patient with poor periconceptional glucose control, long-standing diabetes, and vascular disease. Genetic susceptibility to the teratogenic influence of diabetes may also be a factor.
Hyperglycemia
Ketone body excess
Somatomedin inhibition
Arachidonic acid deficiency
Free oxygen radical excess
Historically, several mechanisms have been proposed by which the previously mentioned teratogenic factors produce malformations. Freinkel and colleagues first suggested that anomalies might arise from inhibition of glycolysis, the key energy-producing process during embryogenesis. They found that the addition of D-mannose to the culture medium of rat embryos inhibited glycolysis and produced growth restriction and derangement of neural tube closure. Freinkel and his group stressed the sensitivity of normal embryogenesis to alterations in these key energy-producing pathways, a process he labeled “fuel-mediated” teratogenesis. Goldman and Baker suggested that the mechanism responsible for the increased incidence of neural tube defects (NTDs) in embryos cultured in a hyperglycemic medium may involve a functional deficiency of arachidonic acid, because supplementation with arachidonic acid or myoinositol will reduce the frequency of NTDs in this experimental model. Pinter and Reece confirmed these studies and demonstrated that hyperglycemia-induced alterations in neural tube closure include disordered cells, decreased mitoses, and changes indicative of premature maturation. These investigators further demonstrated that hyperglycemia during organogenesis has a primary deleterious effect on yolk sac function with resultant embryopathy.
Altered oxidative metabolism from maternal diabetes may cause increased production of free oxygen radicals in the developing embryo, which are likely teratogenic. Supplementation of oxygen radical–scavenging enzymes, such as superoxide dismutase, to the culture medium of rat embryos protects against growth delay and excess malformations. It has been suggested that excess free oxygen radicals may have a direct effect on embryonic prostaglandin biosynthesis. Free oxygen radical excess may enhance lipid peroxidation, and in turn, generated hydroperoxides might stimulate thromboxane biosynthesis and might inhibit prostacyclin production, an imbalance that could have profound effects on embryonic development. Finally, oxidative stress in diabetic rats is associated with the accumulation of glycation products and altered vascular endothelial growth factor expression in cardiovascular regions of the developing heart associated with endocardial cushion defects.
Macrosomia has been variously defined as birthweight greater than 4000 to 4500 g, as well as LGA, in which birthweight is greater than the 90th percentile for population and sex-specific growth curves. Fetal macrosomia complicates as many as 50% of pregnancies in women with GDM and 40% of pregnancies complicated by type 1 and type 2 diabetes, which includes some women treated with intensive glycemic control ( Fig. 45.13 ). Maternal diabetes, independent of obesity, is a risk factor for macrosomia. Delivery of an infant weighing greater than 4500 g occurs 10 times more often in women with diabetes compared with a population of women with normal glucose tolerance. Vaginal deliveries of these large infants are at increased risk for shoulder dystocia and birth trauma.
According to the Pedersen hypothesis, maternal hyperglycemia results in fetal hyperglycemia and subsequent fetal hyperinsulinemia. Insulin is the most important fetal growth hormone, and fetal hyperinsulinemia results in excessive fetal growth. Increased fetal β-cell mass may be identified as early as the second trimester. Evidence in support of the Pedersen hypothesis has come from the studies of amniotic fluid and cord blood insulin and C-peptide concentrations. Both reflect fetal hyperinsulinemia and are increased at term in the amniotic fluid of women with diabetes, and they correlate with neonatal fat mass. Lipids and amino acids, which are elevated in pregnancies complicated by GDM, may also play a role in excessive fetal growth by either stimulating the release of insulin and other growth factors from the fetal pancreatic β-cells or by providing the necessary nutrients for excessive fetal growth. Infants of mothers with GDM have an increase in fat mass, compared with fat-free mass, in comparison with infants of women with normal glucose tolerance. In addition, the growth is disproportionate, with chest-to-head and shoulder-to-head ratios larger than those of infants of women with normal glucose tolerance. These anthropometric differences may contribute to the higher rate of shoulder dystocia and birth trauma observed in these infants.
The results of several clinical series have validated the Pedersen hypothesis inasmuch as tight maternal glycemic control has been associated with a reduction in macrosomia and, in particular, with a reduction in fat mass. Using daily capillary glucose values obtained during the second and third trimesters in women who required insulin, Landon and colleagues reported a rate of 9% for macrosomia when mean values were less than 110 mg/dL compared with 34% when less optimal control was achieved. Jovanovic and associates have suggested that 1-hour postprandial glucose measurements correlate best with the frequency of macrosomia. After controlling for other factors, these authors noted that the strongest predictor for birthweight in patients with diabetes was third-trimester nonfasting glucose measurements. More recently, the large, prospective Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study found that in normal pregnancies the maternal fasting glucose level at 28 weeks’ gestation was the most important predictor of neonatal birthweight.
In a series of metabolic studies, Catalano and associates estimated body composition in 186 neonates using anthropometry. Fat-free mass, which represented 86% of mean birthweight, accounted for 53% of the variance in birthweight, and fat mass, which made up only 14% of birthweight, accounted for 46% of the variance in birthweight, suggesting that neonatal fat mass accounts for the vast majority of variance in birthweight among infants of pregnancies complicated by diabetes. In addition, significantly greater fat-free mass was observed in male compared with female infants. Using independent variables such as maternal height, pregravid weight, weight gain during pregnancy, parity, paternal height and weight, neonatal sex, and gestational age, the authors accounted for 29% of the variance in birthweight, 30% of the variance in fat-free mass, and 17% of the variance in fat mass. Including estimates of maternal insulin sensitivity in 16 additional subjects, they were able to explain 48% of the variance in birthweight, 53% of the variance in fat-free mass, and 46% of the variance in fat mass. Studies by Caruso and colleagues have corroborated these findings, reporting that women with unexplained FGR had greater insulin sensitivity compared with a control group of women whose infants were an appropriate weight for their gestational age. The potential mechanisms for this relate to the possibility that maternal circulating nutrients (e.g., glucose, FFAs, and amino acids) available for placental transport to the fetus are decreased because of the relative increase in maternal insulin sensitivity.
A positive correlation between birthweight and gestational weight gain has been observed in women with normal glucose tolerance. The correlation was strongest in women who were lean before conception, and the correlation became progressively weaker as pregravid weight for height increased. In women with GDM, no significant correlations were found between maternal weight gain and birthweight irrespective of pregravid weight for height. Although these studies emphasize the role of the maternal metabolic environment and fetal growth, Kim and colleagues have reported that GDM actually contributed the least (2.0% to 8.0%) to the increase in LGA infants in a general obstetric population, whereas excessive gestational weight gain contributed the most (33.3% to 37.7%).
However, normalization of birthweight in infants of women with GDM may in itself not help those infants achieve optimal growth. In a study of approximately 400 infants of women with normal glucose tolerance and GDM, Catalano and coworkers showed that the infants of women with GDM had increased fat mass, but not lean body mass or weight, compared with a control group, even after adjustment for potential confounding variables ( Table 45.3 ). Similarly, when only infants who were an appropriate size for their gestational age (i.e., between the 10th and 90th percentiles) were examined, the infants of the women with GDM had significantly greater fat mass and percentage of body fat but had less lean mass compared with the control group, and no difference was observed in birthweight. Of note, in the infants of the women with GDM, the strongest correlations with fat mass were fasting glucose and gestational age; this accounted for 17% of the variance in infant fat mass.
| GDM ( N = 195) | NGT ( N = 220) | P Value | |
|---|---|---|---|
| Weight (g) | 3398 ± 550 | 3337 ± 549 | .26 |
| FFM (g) | 2962 ± 405 | 2975 ± 408 | .74 |
| Fat mass (g) | 436 ± 206 | 362 ± 198 | .0002 |
| Body fat (%) | 12.4 ± 4.6 | 10.4 ± 4.6 | .0001 |
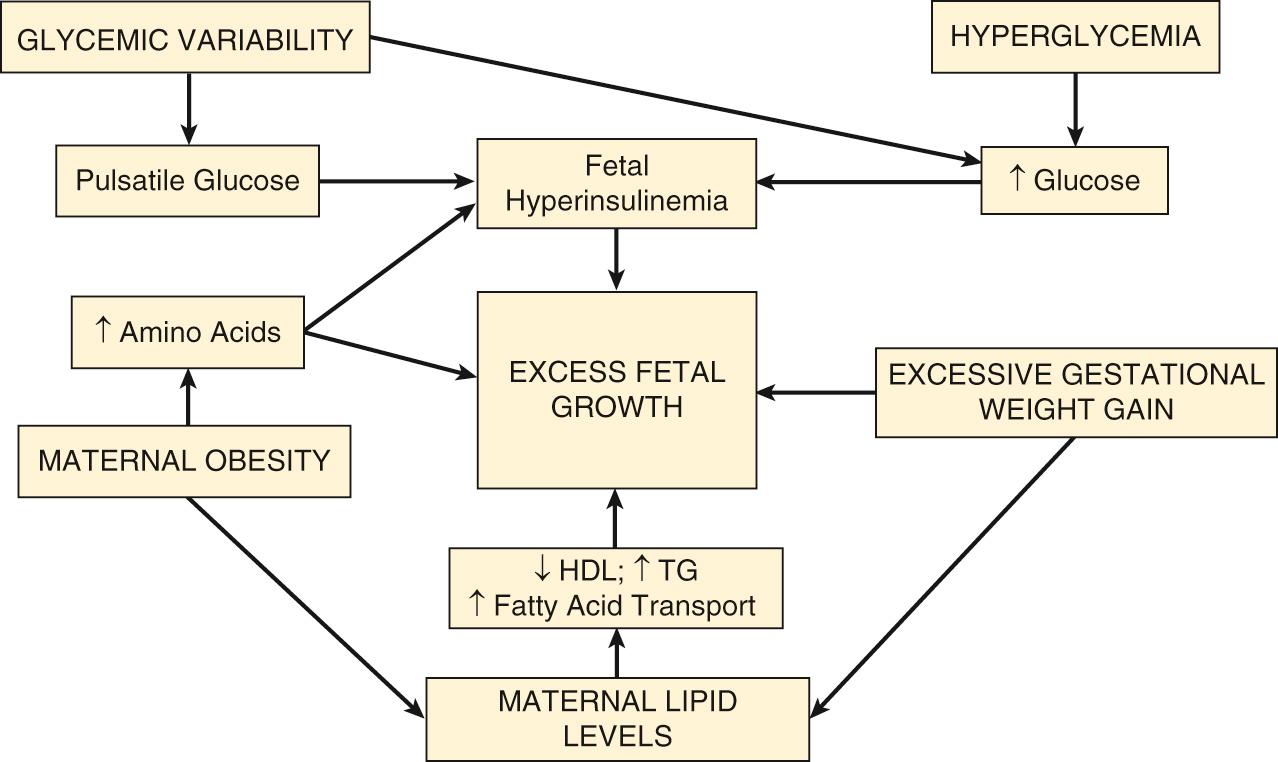
To summarize, excessive fetal growth in the pregnancies complicated by diabetes may be a reflection of several important factors including: maternal prepregnancy weight and weight gain, maternal hyperglycemia with frequent excursions in blood glucose levels, and increased fetal levels of amino acids, triglycerides, and fatty acids ( Fig. 45.14 ).
Neonatal hypoglycemia, a blood glucose level less than 35 to 40 mg/dL during the first 12 hours of life, results from a rapid drop in plasma glucose concentrations following clamping of the umbilical cord. Hypoglycemia, a byproduct of hyperinsulinemia, is particularly common in macrosomic newborns, in whom rates exceed 50%. We have observed an overall rate of 27% in offspring of diabetic women delivered at our institution. The degree of hypoglycemia may be influenced by at least two factors: maternal glucose control during the latter half of pregnancy as well as during labor and delivery. Poor maternal glucose control can result in fetal β-cell hyperplasia, which leads to exaggerated insulin release following delivery. IDMs who exhibit hypoglycemia have elevated cord C-peptide and free insulin levels at birth and show an exaggerated pancreatic response to glucose loading. The hyperinsulinemia and resultant hypoglycemia typically persist for several days following birth.
Experimental animal studies have provided evidence that hyperglycemia and hyperinsulinemia can affect pulmonary surfactant biosynthesis, and in vitro studies have documented that insulin can interfere with substrate availability for surfactant biosynthesis. Insulin excess may delay the normal timing of glucocorticoid-induced pulmonary maturation in the fetus. Cortisol apparently acts on pulmonary fibroblasts to induce synthesis of fibroblast-pneumocyte factor, which then acts on type 2 cells to stimulate phospholipid synthesis.
Clinical studies to investigate the effect of maternal diabetes on fetal lung maturation have produced conflicting data. Respiratory distress syndrome (RDS) due to surfactant deficiency may occur more frequently in the IDM; however, several studies suggest that in women with well-controlled diabetes whose fetus is delivered at 38 to 39 weeks’ gestation, the risk for RDS is no higher than that observed in the general population . Mimouni and associates compared outcomes of 127 IDMs with matched controls and concluded that well-controlled diabetes in pregnancy is not a direct risk factor for the development of RDS. Yet cesarean delivery not preceded by labor and prematurity, both of which are increased in pregnancies complicated by diabetes, clearly increases the likelihood of neonatal respiratory disease. With cesarean delivery, many of these cases represent retained lung fluid or transient tachypnea of the newborn, which usually resolves within the first days of life.
Neonatal hypocalcemia, with serum levels less than 7 mg/dL or an ionized level less than 4 mg/dL, occurs at an increased rate in IDMs when controlling for predisposing factors such as prematurity and birth asphyxia. With modern management emphasizing good glycemic control, the frequency of neonatal hypocalcemia is currently less than 5% in IDMs. Hypocalcemia in IDMs has been associated with a failure to increase parathyroid hormone synthesis following birth. Decreased serum magnesium levels have also been documented in pregnant diabetic women, as well as in their infants. Mimouni and associates described reduced amniotic fluid magnesium concentrations in women with type 1 diabetes. These findings may be explained by a drop in fetal urinary magnesium excretion, which would accompany a relative magnesium-deficient state. Paradoxically, magnesium deficiency may then inhibit fetal parathyroid hormone secretion.
Hyperbilirubinemia is frequently observed in the IDM. Neonatal jaundice has been reported in as many as 25% to 53% of pregnancies complicated by pregestational diabetes and 38% of pregnancies in women with GDM. Jaundice observed in IDMs can be attributed largely to prematurity. However, jaundice is also increased in macrosomic IDMs.
Although severe hyperbilirubinemia may be observed independent of polycythemia, a common pathway for these complications most likely involves increased RBC production, which is stimulated by increased erythropoietin in the IDM. Presumably, the major stimulus for red cell production is a state of relative hypoxia in utero. Cord erythropoietin levels generally are normal in IDMs whose mothers demonstrate good glycemic control during gestation; however, hemoglobin A1c (HbA1c) values in late pregnancy are significantly elevated in mothers of hyperbilirubinemic infants.
A transient form of cardiomyopathy may occur in IDMs. Among symptomatic infants, septal hypertrophy may cause left ventricular outflow obstruction. Although most infants are asymptomatic, respiratory distress or signs of cardiac failure may arise. Cardiac hypertrophy likely results from fetal hyperinsulinemia, which leads to fat and glycogen deposition in the myocardium. Thus cardiomyopathy generally occurs in pregnancies complicated by macrosomia in which glucose levels are poorly controlled. Elevated levels of B-type natriuretic peptide produced during cardiac stress have been reported in umbilical blood of IDMs and have been correlated with maternal glycemic control. In most cases, symptoms of cardiomyopathy improve over several weeks with supportive care, and echocardiographic changes resolve as well.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here