Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Intellectual disability (ID) refers to a group of disorders that have in common deficits of adaptive and intellectual function and an age of onset before maturity is reached.
Contemporary conceptualizations of ID emphasize functioning and social interaction rather than test scores. The definitions of ID by the World Health Organization (WHO) International Classification of Diseases, Tenth Edition (ICD-10) , the U.S. Individuals with Disabilities Education Act (IDEA), the American Psychiatric Association (APA) Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5), and the American Association on Intellectual and Developmental Disabilities (AAIDD) all include significant impairment in general intellectual function (reasoning, learning, problem solving), social skills, and adaptive behavior. This focus on conceptual, social, and practical skills enables the development of individual treatment plans designed to enhance functioning. Consistent across these definitions is onset of symptoms before age 18 yr or adulthood.
Significant impairment in general intellectual function refers to performance on an individually administered test of intelligence that is approximately 2 standard deviations (SD) below the mean. Generally these tests provide a standard score that has a mean of 100 and SD of 15, so that intelligence quotient (IQ) scores <70 would meet these criteria. If the standard error of measurement is considered, the upper limits of significantly impaired intellectual function may extend to an IQ of 75. Using a score of 75 to delineate ID might double the number of children with this diagnosis, but the requirement for impairment of adaptive skills limits the false positives. Children with ID often show a variable pattern of strengths and weaknesses. Not all their subtest scores on IQ tests fall into the significantly impaired range.
Significant impairment in adaptive behavior reflects the degree that the cognitive dysfunction impairs daily function. Adaptive behavior refers to the skills required for people to function in their everyday lives. The AAIDD and DSM-5 classifications of adaptive behavior addresses three broad sets of skills: conceptual, social, and practical. Conceptual skills include language, reading, writing, time, number concepts, and self-direction. Social skills include interpersonal skills, personal and social responsibility, self-esteem, gullibility, naiveté, and ability to follow rules, obey laws, and avoid victimization. Representative practical skills are performance of activities of daily living (dressing, feeding, toileting/bathing, mobility), instrumental activities of daily living (e.g., housework, managing money, taking medication, shopping, preparing meals, using phone), occupational skills, and maintenance of a safe environment. For a deficit in adaptive behavior to be present, a significant delay in at least 1 of the 3 skill areas must be present. The rationale for requiring only 1 area is the empirically derived finding that people with ID can have varying patterns of ability and may not have deficits in all 3 areas.
The requirement for adaptive behavior deficits is the most controversial aspect of the diagnostic formulation. The controversy centers on two broad areas: whether impairments in adaptive behavior are necessary for the construct of ID, and what to measure. The adaptive behavior criterion may be irrelevant for many children; adaptive behavior is impaired in virtually all children who have IQ scores <50. The major utility of the adaptive behavior criterion is to confirm ID in children with IQ scores in the 65-75 range. It should be noted that deficits in adaptive behavior are often found in disorders such as autism spectrum disorder (ASD; see Chapter 54 ) and attention-deficit/hyperactivity disorder (ADHD; see Chapter 49 ) in the presence of typical intellectual function.
The issues of measurement are important as well. The independence of the 3 domains of adaptive behavior has not been validated. The relationship between adaptive behavior and IQ performance is insufficiently explored. Most adults with mild ID do not have significant impairments in practical skills. Adaptive behavior deficits also must be distinguished from maladaptive behavior (e.g., aggression, inappropriate sexual contact).
Onset before age 18 yr or adulthood distinguishes dysfunctions that originate during the developmental period. The diagnosis of ID may be made after 18 yr of age, but the cognitive and adaptive dysfunction must have been manifested before age 18.
The term “mental retardation” should not be used because it is stigmatizing, has been used to limit the achievements of the individual, and has not met its initial objective of assisting people with the disorder. The term intellectual disability is increasingly used in its place, but has not been adopted universally. In the United States, Rosa's law (Public Law 111-256) was passed in 2010 and now mandates that the term mental retardation be stripped from federal health, education, and labor policy. As of 2013, at least 9 states persist in using the outdated terminology. In Europe the term learning disability is often used to describe ID.
Global developmental delay (GDD) is a term often used to describe young children whose limitations have not yet resulted in a formal diagnosis of ID. In DSM-5, GDD is a diagnosis given to children <5 yr of age who display significant delay (>2 SD) in acquiring early childhood developmental milestones in 2 or more domains of development. These domains include receptive and expressive language, gross and fine motor function, cognition, social and personal development, and activities of daily living. Typically, it is assumed that delay in 2 domains will be associated with delay across all domains evaluated, but this is not always the case. Furthermore, not all children who meet criteria for a GDD diagnosis at a young age go on to meet criteria for ID after age 5 yr. Reasons for this might include maturational effects, a change in developmental trajectory (possibly from an intervention), reclassification to a different disability category, or imprecise use of the GDD diagnosis initially. Conversely, in patients with more severe delay, the GDD term is often inappropriately used beyond the point when the child clearly has ID, often by 3 yr of age.
It is important to distinguish the medical diagnosis of GDD from the federal disability classification of “developmental delay” that may be used by education agencies under IDEA. This classification requires that a child have delays in only 1 domain of development with subsequent need for special education. Each state determines its own precise definition and terms of eligibility under the broader definition outline by IDEA, and many states use the label for children up to age 9 yr.
Numerous identified causes of ID may occur prenatally, during delivery, postnatally, or later in childhood. These include infection, trauma, prematurity, hypoxia-ischemia, toxic exposures, metabolic dysfunction, endocrine abnormalities, malnutrition, and genetic abnormalities. However, more than two thirds of persons with ID will not have a readily identifiable underlying diagnosis that can be linked to their clinical presentation, meriting further medical evaluation. For those who then undergo further genetic and metabolic workup, about two thirds will have an etiology that is subsequently discovered. There does appear to be 2 overlapping populations of children with ID with differing corresponding etiologies. Mild ID (IQ 50-70) is associated more with environmental influences, with the highest risk among children of low socioeconomic status. Severe ID (IQ <50) is more frequently linked to biologic and genetic causes. Accordingly, diagnostic yield is generally higher among persons with more severe disability (>75%) than among those with mild disability (<50%). With continued advancement of technologic standards and expansion of our knowledge base, the number of identified biologic and genetic causes is expected to increase.
Nongenetic risk factors that are often associated with mild ID include low socioeconomic status, residence in a developing country, low maternal education, malnutrition, and poor access to healthcare. The most common biologic causes of mild ID include genetic or chromosomal syndromes with multiple, major, or minor congenital anomalies (e.g., velocardiofacial, Williams, and Noonan syndromes), intrauterine growth restriction, prematurity, perinatal insults, intrauterine exposure to drugs of abuse (including alcohol), and sex chromosomal abnormalities. Familial clustering is common.
In children with severe ID, a biologic cause (usually prenatal) can be identified in about three fourths of all cases. Causes include chromosomal (e.g., Down, Wolf-Hirschhorn, and deletion 1p36 syndromes) and other genetic and epigenetic disorders (e.g., fragile X, Rett, Angelman, and Prader-Willi syndromes), abnormalities of brain development (e.g., lissencephaly), and inborn errors of metabolism or neurodegenerative disorders (e.g., mucopolysaccharidoses) ( Table 53.1 ). Nonsyndromic severe ID may be a result of inherited or de novo gene mutations, as well as microdeletions or microduplications not detected on standard chromosome analysis. Currently, >700 genes are associated with nonsyndromic ID. Inherited genetic abnormalities may be mendelian (autosomal dominant de novo, autosomal recessive, X-linked) or nonmendelian (imprinting, methylation, mitochondrial defects; see Chapter 97 ). De novo mutations may also cause other phenotypic features such as seizures or autism; the presence of these features suggests more pleotropic manifestations of genetic mutations. Consistent with the finding that disorders altering early embryogenesis are the most common and severe, the earlier the problem occurs in development, the more severe its consequences tend to be.
| CAUSE | EXAMPLES | % OF TOTAL |
|---|---|---|
| Chromosomal disorder | Trisomies 21, 18, 13 Deletions 1p36, 4p, 5p, 11p, 12q, 17p Microdeletions; 47,XXX Klinefelter and Turner syndromes |
~20 |
| Genetic syndrome | Fragile X, Prader-Willi, Angelman, and Rett syndromes | ~20 |
| Nonsyndromic autosomal mutations | Variations in copy number; de novo mutations in SYNGAP1 , GRIK2 , TUSC3, oligosaccharyl transferase, and others | ~10 |
| Developmental brain abnormality | Hydrocephalus ± meningomyelocele; schizencephaly, lissencephaly | ~8 |
| Inborn errors of metabolism or neurodegenerative disorder | Phenylketonuria, Tay-Sachs disease, various storage diseases | ~7 |
| Congenital infections | HIV, toxoplasmosis, rubella, cytomegalovirus, syphilis, herpes simplex | ~3 |
| Familial intellectual disability | Environment, syndromic, or genetic | ~5 |
| Perinatal causes | Hypoxic-ischemic encephalopathy, meningitis, intraventricular hemorrhage, periventricular leukomalacia, fetal alcohol syndrome | 4 |
| Postnatal causes | Trauma (abuse), meningitis, hypothyroidism | ~4 |
| Unknown | 20 |
Etiologic workup is recommended in all cases of GDD or ID. Although there are only about 80 disorders (all of which are metabolic in nature) for which treatment may ameliorate the core symptoms of ID, several reasons beyond disease modification should prompt providers to seek etiologic answers in patients with ID. These include insight into possible associated medical or behavioral comorbidities; information on prognosis and life expectancy; estimation of recurrence risk for family planning counseling, potential validation, and closure for the family; increased access to services or specific supports; and better understanding of underlying pathology with the hope of new eventual treatment options. When surveyed, families of children with ID with no identified underlying etiology almost universally report that they would want to know of an etiologic diagnosis if given the choice.
The prevalence of ID depends on the definition, method of ascertainment, and population studied, both in terms of geography and age. According to the statistics of a normal distribution, 2.5% of the population should have ID (based on IQ alone), and 75% of these individuals should fall into the mild to moderate range. Variability in rates across populations likely results from the heavy influence of external environmental factors on the prevalence of mild ID. The prevalence of severe ID is relatively stable. Globally, the prevalence of ID has been estimated to be approximately 16.4 per 1,000 persons in low-income countries, approximately 15.9/1,000 for middle-income countries, and approximately 9.2/1,000 in high-income countries. A meta-analysis of worldwide studies from 1980–2009 yielded an overall prevalence of 10.4/1000. ID occurs more in boys than in girls, at 2 : 1 in mild ID and 1.5 : 1 in severe ID. In part this may be a consequence of the many X-linked disorders associated with ID, the most prominent being fragile X syndrome (see Chapter 98.5 ).
In 2014–2015 in the United States, approximately 12/1000 students 3-5 yr old and 6.2/1000 students 6-21 yr old received services for ID in federally supported school programs. In 2012 the National Survey of Children's Health reported an estimated prevalence of ID among American children (age 2-17 yr) of 1.1%. For several reasons, fewer children than predicted are identified as having mild ID. Because it is more difficult to diagnose mild ID than the more severe forms, professionals might defer the diagnosis and give the benefit of the doubt to the child. Other reasons that contribute to the discrepancy are use of instruments that underidentify young children with mild ID, children diagnosed as having ASD without their ID being addressed, misdiagnosis as a language disorder or specific learning disability, and a disinclination to make the diagnosis in poor or minority students because of previous overdiagnosis. In some cases, behavioral disorders may divert the focus from the cognitive dysfunction.
Beyond potential underdiagnosis of mild ID, the number of children with mild ID may be decreasing as a result of public health and education measures to prevent prematurity and provide early intervention and Head Start programs. However, although the number of schoolchildren who receive services under a federal disability classification of ID has decreased since 1999, when developmental delay is included in analysis of the data, the numbers have not changed appreciably.
The prevalence of severe ID has not changed significantly since the 1940s, accounting for 0.3–0.5% of the population. Many of the causes of severe ID involve genetic or congenital brain malformations that can neither be anticipated nor treated at present. In addition, new populations with severe ID have offset the decreases in the prevalence of severe ID that have resulted from improved healthcare. Although prenatal diagnosis and subsequent pregnancy terminations could lead to a decreasing incidence of Down syndrome (see Chapter 98.2 ), and newborn screening with early treatment has virtually eliminated ID caused by phenylketonuria and congenital hypothyroidism, continued high prevalence of fetal exposure to illicit drugs and improved survival of very-low-birthweight premature infants has counterbalanced this effect.
The limitations in our knowledge of the neuropathology of ID are exemplified by 10–20% of brains of persons with severe ID appearing entirely normal on standard neuropathologic study. Most of these brains show only mild, nonspecific changes that correlate poorly with the degree of ID, including microcephaly, gray matter heterotopias in the subcortical white matter, unusually regular columnar arrangement of the cortex, and neurons that are more tightly packed than usual. Only a minority of the brain shows more specific changes in dendritic and synaptic organization, with dysgenesis of dendritic spines or cortical pyramidal neurons or impaired growth of dendritic trees. The programming of the central nervous system (CNS) involves a process of induction ; CNS maturation is defined in terms of genetic, molecular, autocrine, paracrine, and endocrine influences. Receptors, signaling molecules, and genes are critical to brain development. The maintenance of different neuronal phenotypes in the adult brain involves the same genetic transcripts that play a crucial role in fetal development, with activation of similar intracellular signal transduction mechanisms.
As the ability to identify genetic aberrations that correspond to particular phenotypes expands through the use of next-generation sequencing, more will be elucidated about the pathogenesis of ID at a genetic and molecular level. This expanding pathophysiologic knowledge base may serve as a framework with which to develop targeted therapies to bypass or correct newly identified defects. For example, use of histone deacetylase (HDAC) inhibitors has been shown to rescue structural and functional neural deficits in mouse models of Kabuki syndrome, a disorder of histone methylation that leads to variable levels of ID and characteristic facial features (see Chapter 100 ).
Early diagnosis of ID facilitates earlier intervention, identification of abilities, realistic goal setting, easing of parental anxiety, and greater acceptance of the child in the community. Most children with ID first come to the pediatrician's attention in infancy because of dysmorphisms, associated developmental disabilities, or failure to meet age-appropriate developmental milestones ( Tables 53.2 and 53.3 ). There are no specific physical characteristics of ID, but dysmorphisms may be the earliest signs that bring children to the attention of the pediatrician. They might fall within a genetic syndrome such as Down syndrome or might be isolated, as in microcephaly or failure to thrive. Associated developmental disabilities include seizure disorders, cerebral palsy, and ASD.
| ITEM | POSSIBLE SIGNIFICANCE |
|---|---|
| General appearance | May indicate significant delay in development or obvious syndrome |
| Stature | |
| Short stature | Malnutrition, many genetic syndromes are associated with short stature (e.g., Turner, Noonan) |
| Obesity | Prader-Willi syndrome |
| Large stature | Sotos syndrome |
| Head | |
| Macrocephaly | Alexander syndrome, Canavan disease, Sotos syndrome, gangliosidosis, hydrocephalus, mucopolysaccharidosis, subdural effusion |
| Microcephaly | Virtually any condition that can restrict brain growth (e.g., malnutrition, Angelman syndrome, Cornelia de Lange syndrome, fetal alcohol effects) |
| Face | |
| Coarse, triangular, round, or flat face; hypotelorism or hypertelorism; slanted or short palpebral fissure; unusual nose, maxilla, and mandible | Specific measurements may provide clues to inherited, metabolic, or other diseases such as fetal alcohol syndrome, cri du chat (5p−) syndrome, or Williams syndrome. |
| Eyes | |
| Prominent | Crouzon, Seckel, and fragile X syndromes |
| Cataract | Galactosemia, Lowe syndrome, prenatal rubella, hypothyroidism |
| Cherry-red spot in macula | Gangliosidosis (GM 1 ), metachromatic leukodystrophy, mucolipidosis, Tay-Sachs disease, Niemann-Pick disease, Farber lipogranulomatosis, sialidosis type III |
| Chorioretinitis | Congenital infection with cytomegalovirus, toxoplasmosis, Zika virus, or rubella |
| Corneal cloudiness | Mucopolysaccharidosis types I and II, Lowe syndrome, congenital syphilis |
| Ears | |
| Low-set or malformed pinnae | Trisomies such as Down syndrome, Rubinstein-Taybi syndrome, CHARGE syndrome, cerebrooculofacioskeletal syndrome, fetal phenytoin effects |
| Hearing | Loss of acuity in mucopolysaccharidosis; hyperacusis in many encephalopathies |
| Heart | |
| Structural anomaly or hypertrophy | CHARGE syndrome, velocardiofacial syndrome, glycogenosis type II, fetal alcohol effects, mucopolysaccharidosis type I; chromosomal anomalies such as Down syndrome; maternal PKU; chronic cyanosis may impair cognitive development. |
| Liver | |
| Hepatomegaly | Fructose intolerance, galactosemia, glycogenosis types I-IV, mucopolysaccharidosis types I and II, Niemann-Pick disease, Tay-Sachs disease, Zellweger syndrome, Gaucher disease, ceroid lipofuscinosis, gangliosidosis |
| Genitalia | |
| Macroorchidism | Fragile X syndrome |
| Hypogenitalism | Prader-Willi, Klinefelter, and CHARGE syndromes |
| Extremities | |
| Hands, feet; dermatoglyphics, creases | May indicate a specific entity such as Rubinstein-Taybi syndrome or may be associated with chromosomal anomaly |
| Joint contractures | Signs of muscle imbalance around the joints; e.g., with meningomyelocele, cerebral palsy, arthrogryposis, muscular dystrophy; also occurs with cartilaginous problems such as mucopolysaccharidosis |
| Skin | |
| Café au lait spots | Neurofibromatosis, tuberous sclerosis, chromosomal aneuploidy, ataxia-telangiectasia, multiple endocrine neoplasia type 2b Fanconi anemia, Gaucher disease Syndromes: basal cell nevus, McCune-Albright, Silver-Russell, Bloom, Chediak-Higashi, Hunter, Bannayan-Riley-Ruvalcaba, Maffucci |
| Seborrheic or eczematoid rash | PKU, histiocytosis |
| Hemangiomas and telangiectasia | Sturge-Weber syndrome, Bloom syndrome, ataxia-telangiectasia |
| Hypopigmented macules, streaks, adenoma sebaceum | Tuberous sclerosis, hypomelanosis of Ito |
| Hair | |
| Hirsutism | De Lange syndrome, mucopolysaccharidosis, fetal phenytoin effects, cerebrooculofacioskeletal syndrome, trisomy 18, Wiedemann-Steiner syndrome (hypertrichosis cubiti) |
| Neurologic | |
| Asymmetry of strength and tone | Focal lesion, hemiplegic cerebral palsy |
| Hypotonia | Prader-Willi, Down, and Angelman syndromes; gangliosidosis; early cerebral palsy; muscle disorders (dystrophy or myopathy) |
| Hypertonia | Neurodegenerative conditions involving white matter, cerebral palsy, trisomy 18 |
| Ataxia | Ataxia-telangiectasia, metachromatic leukodystrophy, Angelman syndrome |
| AREA | ANOMALY/SYNDROME |
|---|---|
| Head | Flat occiput: Down syndrome, Zellweger syndrome; prominent occiput: trisomy 18 Delayed closure of sutures: hypothyroidism, hydrocephalus Craniosynostosis: Crouzon syndrome, Pfeiffer syndrome Delayed fontanel closure: hypothyroidism, Down syndrome, hydrocephalus, skeletal dysplasias |
| Face | Midface hypoplasia: fetal alcohol syndrome, Down syndrome Triangular facies: Russell-Silver syndrome, Turner syndrome Coarse facies: mucopolysaccharidoses, Sotos syndrome Prominent nose and chin: fragile X syndrome Flat facies: Apert syndrome, Stickler syndrome Round facies: Prader-Willi syndrome |
| Eyes | Hypertelorism: fetal hydantoin syndrome, Waardenburg syndrome Hypotelorism: holoprosencephaly sequence, maternal phenylketonuria effect Inner canthal folds/Brushfield spots: Down syndrome; slanted palpebral fissures: trisomies Prominent eyes: Apert syndrome, Beckwith-Wiedemann syndrome Lisch nodules: neurofibromatosis Blue sclera: osteogenesis imperfecta, Turner syndrome, hereditary connective tissue disorders |
| Ears | Large pinnae/simple helices: fragile X syndrome Malformed pinnae/atretic canal: Treacher Collins syndrome, CHARGE syndrome Low-set ears: Treacher Collins syndrome, trisomies, multiple disorders |
| Nose | Anteverted nares/synophrys: Cornelia de Lange syndrome; broad nasal bridge: fetal drug effects, fragile X syndrome Low nasal bridge: achondroplasia, Down syndrome Prominent nose: Coffin-Lowry syndrome, Smith-Lemli-Opitz syndrome |
| Mouth | Long philtrum/thin vermilion border: fetal alcohol effects Cleft lip and palate: isolated or part of a syndrome Micrognathia: Pierre Robin sequence, trisomies, Stickler syndrome Macroglossia: hypothyroidism, Beckwith-Wiedemann syndrome |
| Teeth | Anodontia: ectodermal dysplasia Notched incisors: congenital syphilis Late dental eruption: Hunter syndrome, hypothyroidism Talon cusps: Rubinstein-Taybi syndrome Wide-spaced teeth: Cornelia de Lange syndrome, Angelman syndrome |
| Hair | Hirsutism: Hurler syndrome Low hairline: Klippel-Feil sequence, Turner syndrome Sparse hair: Menkes disease, argininosuccinic acidemia Abnormal hair whorls/posterior whorl: chromosomal aneuploidy (e.g., Down syndrome) Abnormal eyebrow patterning: Cornelia de Lange syndrome |
| Neck | Webbed neck/low posterior hairline: Turner syndrome, Noonan syndrome |
| Chest | Shield-shaped chest: Turner syndrome |
| Genitalia | Macroorchidism: fragile X syndrome Hypogonadism: Prader-Willi syndrome |
| Extremities | Short limbs: achondroplasia, rhizomelic chondrodysplasia Small hands: Prader-Willi syndrome Clinodactyly: trisomies, including Down syndrome Polydactyly: trisomy 13, ciliopathies Broad thumb: Rubinstein-Taybi syndrome Syndactyly: de Lange syndrome Transverse palmar crease: Down syndrome Joint laxity: Down syndrome, fragile X syndrome, Ehlers-Danlos syndrome Phocomelia: Cornelia de Lange syndrome |
| Spine | Sacral dimple/hairy patch: spina bifida |
| Skin | Hypopigmented macules/adenoma sebaceum: tuberous sclerosis Café au lait spots and neurofibromas: neurofibromatosis Linear depigmented nevi: hypomelanosis of Ito Facial port-wine hemangioma: Sturge-Weber syndrome Nail hypoplasia or dysplasia: fetal alcohol syndrome, trisomies |
* Increased incidence of minor anomalies have been reported in cerebral palsy, intellectual disability, learning disabilities, and autism.
† The presence of 3 or more minor anomalies implies a greater chance that the child has a major anomaly and a diagnosis of a specific syndrome.
Most children with ID do not keep up with their peers' developmental skills. In early infancy, failure to meet age-appropriate expectations can include a lack of visual or auditory responsiveness, unusual muscle tone (hypo- or hypertonia) or posture, and feeding difficulties. Between 6 and 18 mo of age, gross motor delay (lack of sitting, crawling, walking) is the most common complaint. Language delay and behavior problems are common concerns after 18 mo ( Table 53.4 ). For some children with mild ID, the diagnosis remains uncertain during the early school years. It is only after the demands of the school setting increase over the years, changing from “learning to read” to “reading to learn,” that the child's limitations are clarified. Adolescents with mild ID are typically up to date on current trends and are conversant as to “who,” “what,” and “where.” It is not until the “why” and “how” questions are asked that their limitations become apparent. If allowed to interact at a superficial level, their mild ID might not be appreciated, even by professionals, who may be their special education teachers or healthcare providers. Because of the stigma associated with ID, adolescents may use euphemisms to avoid being thought of as “stupid” or “retarded” and may refer to themselves as learning disabled, dyslexic, language disordered, or slow learners. Some people with ID emulate their social milieu to be accepted. They may be social chameleons and assume the morals of the group to whom they are attached. Some would rather be thought “bad” than “incompetent.”
| AGE | AREA OF CONCERN |
|---|---|
| Newborn | Dysmorphic syndromes, (multiple congenital anomalies), microcephaly Major organ system dysfunction (e.g., feeding, breathing) |
| Early infancy (2-4 mo) | Failure to interact with the environment Concerns about vision and hearing impairments |
| Later infancy (6-18 mo) | Gross motor delay |
| Toddlers (2-3 yr) | Language delays or difficulties |
| Preschool (3-5 yr) | Language difficulties or delays Behavior difficulties, including play Delays in fine motor skills: cutting, coloring, drawing |
| School age (>5 yr) | Academic underachievement Behavior difficulties (e.g., attention, anxiety, mood, conduct) |
Children with ID have a nonprogressive disorder; loss of developmental milestones or progressive symptoms suggest another disorder (see Chapter 53.1 ).
Intellectual disability is one of the most frequent reasons for referral to pediatric genetic providers, with separate but similar diagnostic evaluation guidelines put forth by the American College of Medical Genetics, the American Academy of Neurology, the American Academy of Pediatrics (AAP), and the American Academy of Child and Adolescent Psychiatry. ID is a diagnosis of great clinical heterogeneity, with only a subset of syndromic etiologies identifiable through classic dysmorphology. If diagnosis is not made after conducting an appropriate history and physical examination, chromosomal microarray is the recommended first step in the diagnostic evaluation of ID. Next-generation sequencing represents the new diagnostic frontier, with extensive gene panels (exome or whole genome) that increase the diagnostic yield and usefulness of genetic testing in ID. Other commonly used medical diagnostic testing for children with ID includes neuroimaging, metabolic testing, and electroencephalography ( Fig. 53.1 ).
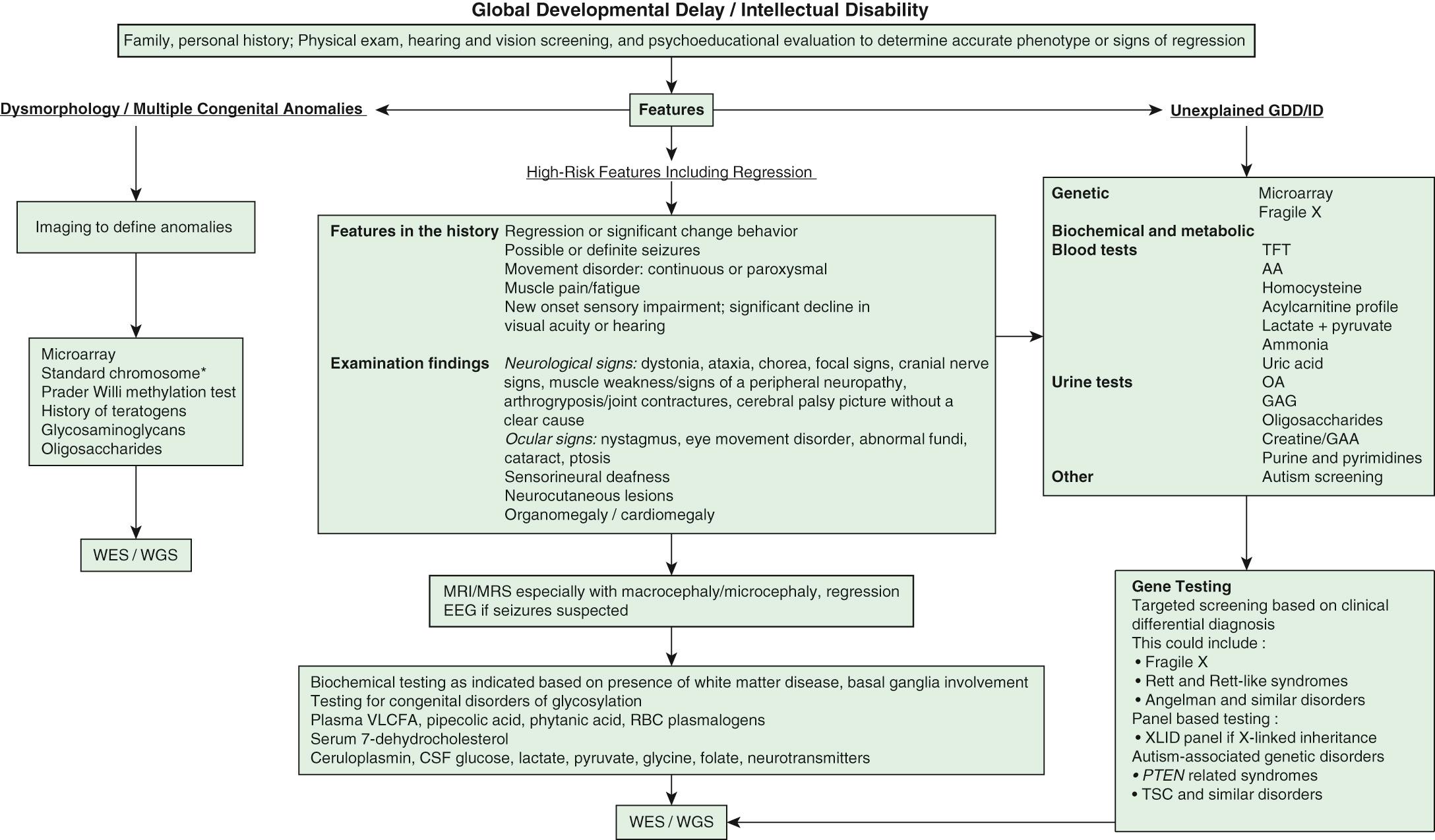
Decisions to pursue an etiologic diagnosis should be based on the medical and family history, physical examination, and the family's wishes. Table 53.5 summarizes clinical practice guidelines and the yields of testing to assist in decisions about evaluating the child with GDD or ID. Yield of testing tends increase with worsening severity of delays.
| TEST | COMMENT |
|---|---|
| In-depth history | Includes pre-, peri-, and postnatal events (including seizures); developmental attainments; and 3-generation pedigree in family history (focusing on neurologic or developmental abnormalities, miscarriages, consanguinity, etc.) |
| Physical examination | Particular attention to minor or subtle dysmorphisms; growth issues; neurocutaneous findings; eye and skull abnormalities; hepatosplenomegaly; and neurologic examination for focality Behavioral phenotype |
| Vision and hearing evaluation | Essential to detect and treat; can mask as developmental delay |
| Gene microarray analysis | A 15% yield overall Better resolution than with karyotype; may identify up to twice as many abnormalities as karyotyping |
| Karyotype | Yield of 4% in ID/GDD (18.6% if syndromic features, 3% excluding trisomy 21) Best for inversions and balanced insertions, reciprocal translocations, and polyploidy |
| Fragile X screen | Combined yield of 2% Preselection on clinical grounds can increase yield to 7.6% |
| Next-generation gene sequencing | Detects inherited and de novo point mutations, especially in nonsyndromic severe intellectual disability Whole exome sequencing (WES, introduced in 2010) gives an additional yield of about 30–40%. Although not yet used clinically, pilot studies of whole genome sequencing (WGS) reveal additional yield of about 15%. |
| Neuroimaging | MRI preferred; positive findings increased by abnormalities of skull contour or microcephaly and macrocephaly, or focal neurologic examination (30–40% if indicated, 10–14% if screening). Identification of specific etiologies is rare; most conditions that are found do not alter the treatment plan; need to weigh risk of sedation against possible yield. |
| Thyroid (T 4 , TSH) | Near 0% in settings with universal newborn screening program |
| Serum lead | If there are identifiable risk factors for excessive environmental lead exposure (e.g., low socioeconomic status, home built before 1950) |
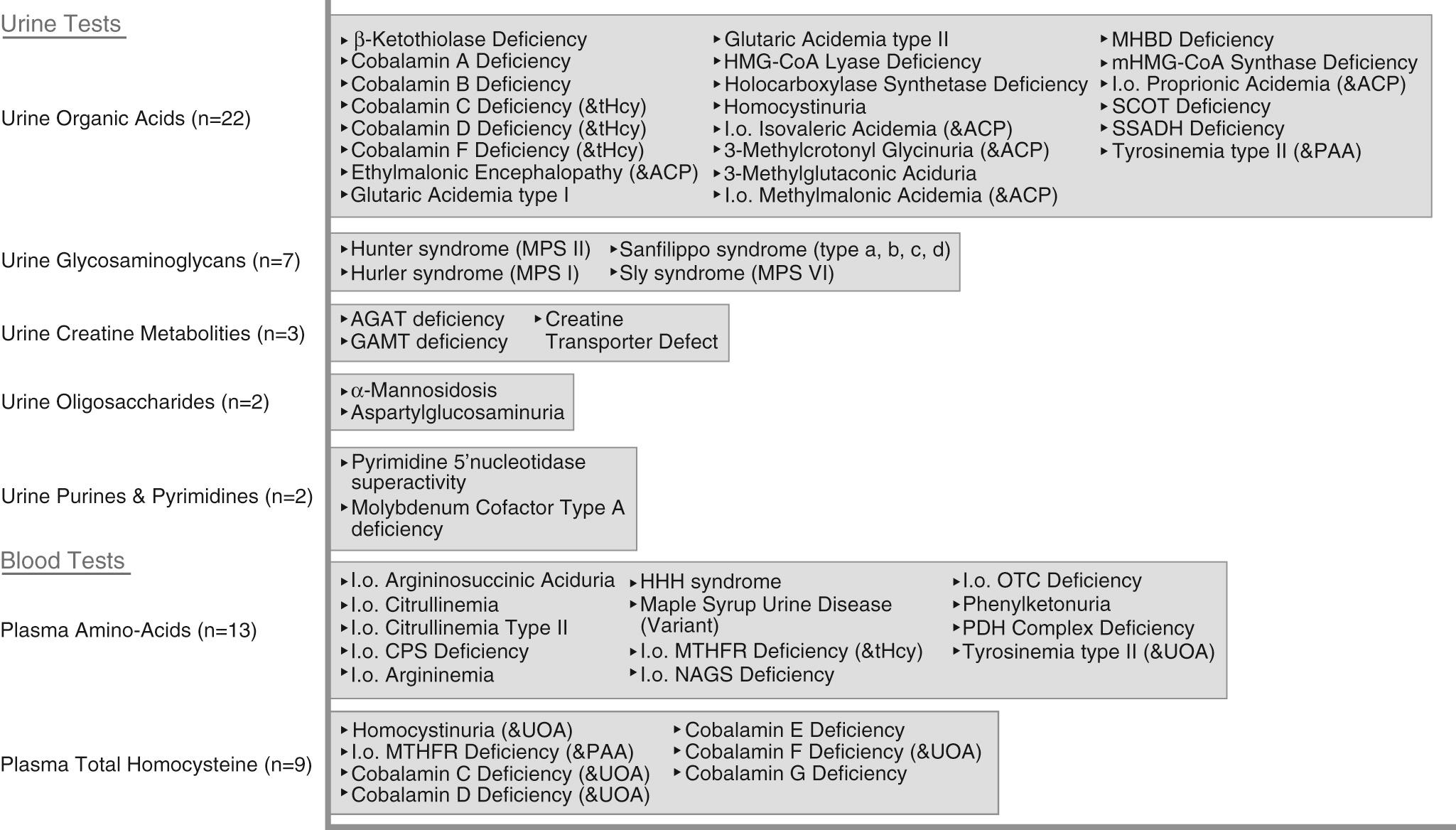
| Metabolic testing | Yield of 0.2–4.6% based on clinical indicators and tests performed Urine organic acids, plasma amino acids, ammonia, lactate, and capillary blood gas Focused testing based on clinical findings is warranted if lack of newborn screen results or suggestive history/exam (e.g., regression, consanguinity, hepatosplenomegaly, course facies). Tandem mass spectrometry newborn screening has allowed for identification of many disorders in perinatal period and have decreased yield in older children; other disorders have emerged, such as congenital disorders of glycosylation (yield 1.4%) and disorders of creatine synthesis and transport (yield 2.8%). |
| MECP2 for Rett syndrome | 1.5% of females with criteria suggestive of Rett (e.g., acquired microcephaly, loss of skills) 0.5% of males |
| EEG | May be deferred in absence of history of seizures |
| Repeated history and physical examination | Can give time for maturation of physical and behavioral phenotype; new technology may be available for evaluation. |
Microarray analysis has replaced a karyotype as first-tier testing given that it discerns abnormalities that are far below the resolution of a karyotype. Microarray analysis may identify variants of unknown significance or benign variants and therefore should be used in conjunction with a genetic consultation. Karyotyping has a role when concerns for inversions, balanced insertions, and reciprocal translocations are present. Fluorescence in situ hybridization (FISH) and subtelomeric analysis have been largely replaced by microarray analysis but are occasionally used for specific indications. If microarray analysis is not diagnostic, whole exome sequencing increases the diagnostic yield in many children with nonsyndromic severe ID. Starting with whole exome sequencing may be more cost-effective and may substantially reduce time to diagnosis with higher ultimate yields compared with the traditional diagnostic pathway.
Molecular genetic testing for fragile X syndrome is recommended for all children presenting with GDD. Yields are highest in males with moderate ID, unusual physical features, and/or a family history of ID, or for females with more subtle cognitive deficits associated with severe shyness and a relevant family history, including premature ovarian failure or later-onset ataxia-tremor symptoms. For children with a strong history of X-linked ID, specific testing of genes or the entire chromosome may be revealing. Testing for Rett syndrome (MECP2, methyl CpG–binding protein 2) should be considered in girls with moderate to severe disability.
A child with a progressive neurologic disorder, developmental regression, or acute behavioral changes needs metabolic investigation as shown in Figure 53.1 . Some are advocating that metabolic testing should be done more frequently in children with ID because of the possibility of detecting a condition that could be treatable ( Fig. 53.2 and Table 53.6 ). A child with seizure-like episodes should have an electroencephalogram (EEG), although this testing is generally not helpful outside the scope of ruling out seizures. MRI of the brain may provide useful information in directing the care of a child with micro- or macrocephaly, change in head growth trajectory, asymmetric head shape, new or focal neurologic findings, or seizure. MRI can detect a significant number of subtle markers of cerebral dysgenesis in children with ID, but these markers do not usually suggest a specific etiologic diagnosis.
Blood
Plasma amino acids
Plasma total homocysteine
Acylcarnitine profile
Copper, ceruloplasmin
Urine
Organic acids
Purines and pyrimidines
Creatine metabolites
Oligosaccharides
Glycosaminoglycans
Amino acids (when indicated)
* Low threshold for ordering tests.
(1 or more of:)
Audiology
Ophthalmology
Cytogenetic testing (array CGH)
Thyroid studies
Complete blood count (CBC)
Lead
Metabolic testing
Brain MRI and 1H spectroscopy (where available)
Fragile X
Targeted gene sequencing/molecular panel
Other
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here