Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Ruth Grunau’s research is supported by the Canadian Institute of Health Research (CIHR) and a Senior Scientist salary award from the BC Children’s Hospital Research Institute. Simon Beggs’ research is supported by the Medical Research Council (MRC, UK) and National Institute of Academic Anaesthesia (NIAA).
Pain is an intrinsic experience which is processed early in life by the maturing nervous system. However, for an individual to experience fully mature pain, sophisticated assimilation at higher cortical levels of sensory discriminatory and emotional or motivational dimensions is necessary, as is the involvement of neuronal activation of integrated central subcortical and cortical brain regions. However, in the very immature infant, pain can be considered as a more primitive experience involving the processing of nociceptive activity at the lower level of the central nervous system (CNS) (i.e., spinal cord and brain stem). Although thalamocortical connections begin around 24 weeks gestation, these are functionally immature. , Thus as the nervous system develops, the nociceptive signal reaches higher-order processing and can be discriminated from other types of sensory inputs. Moreover, understanding the development of ascending nociceptive and descending modulatory pain pathways, as well as these systems’ plasticity, is essential to appreciate the lasting impact early exposure to pain and stress may have on the immature neurologic system of the neonate.
Evidence in rodents suggests that early sensory experience can influence the development of nociceptive pathways, and tissue injury during this critical time of development may prime adult pain perception. Exposure to painful and stressful stimuli is inherent to high-technology neonatal intensive care for infants born very sick and/or prematurely. Infants born very preterm (<33 weeks gestational age) are exposed to an average of 7 to 17 invasive procedures per day during a period of rapid brain development and programming of the hypothalamic-pituitary-adrenal (HPA) axis. Notably, throughout this critical phase of immaturity, the nervous system shows heightened neuronal activation to sensation and less descending inhibitory input. The negative effects of pain on brain development and behavioral outcomes have been reported in both rodents , and humans (for a review see Ranger and Grunau )—for example, in two Canadian cohorts, short- and long-term adverse effects on brain development, the functional trajectory of activity of the HPA axis, and neurodevelopment.
This chapter reviews the development of pain sensation and processing, as well as long-term effects of early pain exposure on the developing infant. Because of the physiologic immaturity of the nervous system and extensive brain development and programming of the HPA axis in the preterm infant hospitalized in the neonatal intensive care unit (NICU), these infants are particularly vulnerable to pain exposure early in life. Research in rodents is especially relevant because rats and mice are born neurologically immature; the first week of life provides a good model for human prematurity and is physiologically “similar” to late second trimester preterm human neonates. Thus, both animal and clinical studies will be considered, with a particular focus on pain in the developing very preterm neonate.
Noxious stimuli are detected by sensory neurons throughout the body, and these nociceptive signals transmit via the spinal cord to the brain where the perception of pain is generated. Modulation of these signals can occur at any point on this journey involving interactions between neurons, glia, and the immune system. Physical interaction with the external world is detected by free nerve endings and specific peripheral end organs of primary sensory afferent fibers that respond to particular stimulus modalities. They transform mechanical, thermal, or chemical stimuli into electrical signals to be transmitted to the CNS. These primary afferent fibers arise from neurons within the sensory (dorsal root and trigeminal) ganglia during embryonic development. This gives rise to four main classes of sensory afferent based on their threshold and conduction velocity: thickly myelinated Aα and Aβ fibers that are low-threshold and transmit proprioception and touch, thinly myelinated Aδ and unmyelinated C fibers that are primarily high-threshold and are associated with transmitting noxious stimuli. These different classes of sensory neurons not only transmit distinct sensory modalities, but also undergo unique developmental trajectories.
Cutaneous receptive fields are large in the newborn rat and preterm neonate, and peripheral sensory fibers have a heightened sensitivity to tissue injury. Moreover, discrimination between noxious and non-noxious stimuli is imperfect for the neonate due to a combination of the transient overlapping between axon terminals in the superficial laminae of the spinal cord and low-threshold tactile inputs and an underdeveloped local and descending inhibitory influence. ,
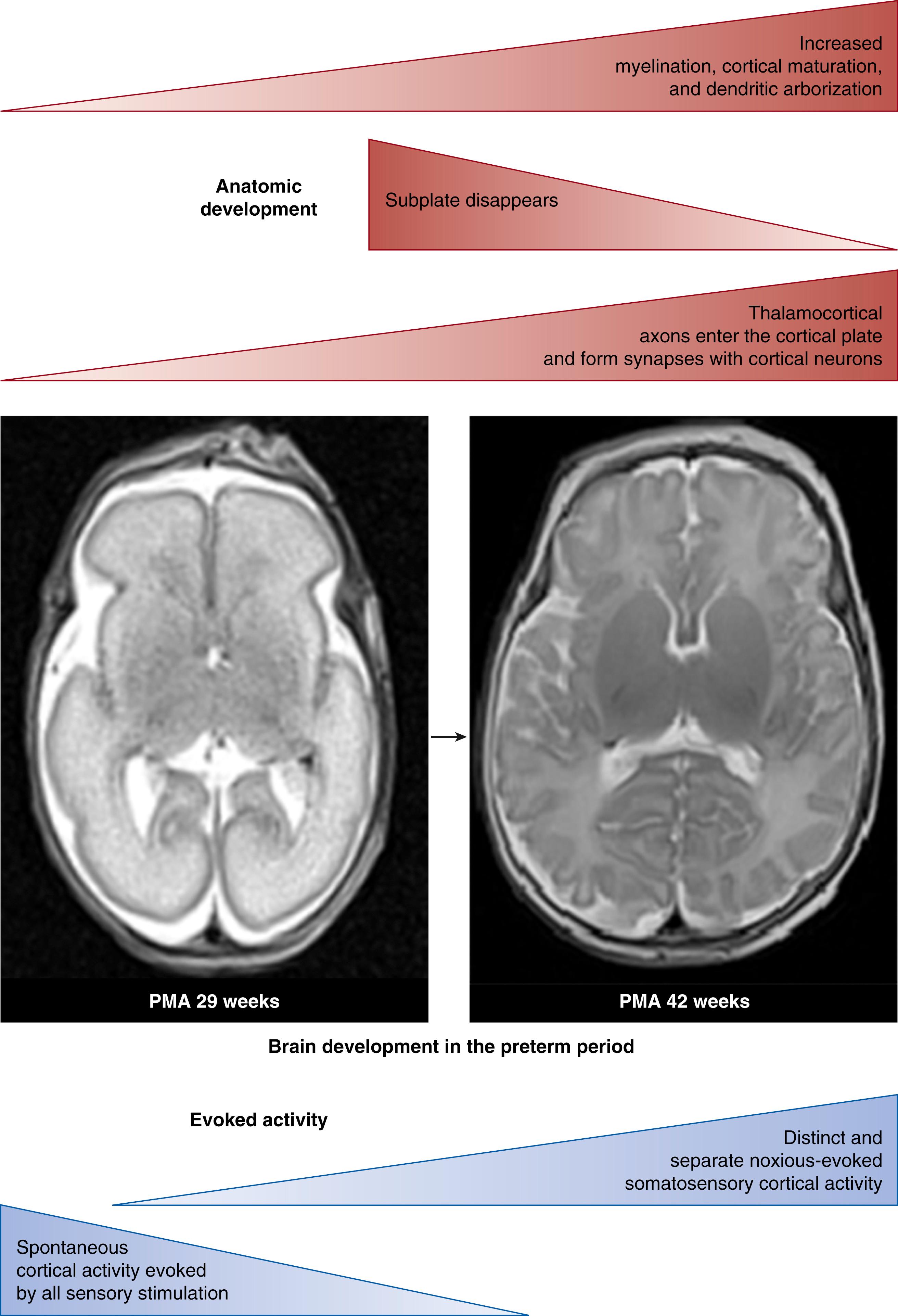
Although reflex behaviors and autonomic and hormonal responses to pain exist early in development due to nociceptive transmission through the spinal cord, brain stem, and subcortical midbrain regions, this is not enough to generate a comprehensive perception and awareness of pain. The development of thalamocortical afferents in the human is characterized by colossal connection growth and discrimination: in the infant younger than 24 weeks gestational age, thalamocortical afferents are temporarily in “standby” in the subplate (SP) zone; between 24 and 26 weeks gestational age, axons start to enter the cortical plate and begin to form functional synapses with cortical neurons in layer IV; from 33 to 35 weeks gestational age, the SP region commences to wane, followed by a significant development of massive associative connections with the cortex after 36 weeks gestation. Fig. 128.1 illustrates this critical stage of maturation. During fetal life, general development and myelination of pain pathways occur in parallel with cortical maturation, dendritic arborization, and thalamocortical fiber synaptogenesis.
Noxious information is not processed in the brain in the same way as other sensory modalities. There is no dedicated primary “pain” cortex analogous to the primary somatosensory or visual cortices; rather, noxious stimulation evokes a diffuse pattern of activity in many brain areas. The current view of pain is that it arises from a distributed network of brain activity, and that the conscious experience of pain arises from a dynamic change in a distributed network of brain activity, , but how does this network develop? Sensory networks in the visual and auditory systems require modality-specific input for their functional maturation, but nociceptive circuits do not require noxious sensory experience in order to develop normally. Instead their development occurs through a cross-modality mechanism requiring spontaneous tactile input during a critical period of early life. ,
How and when this complex brain network develops to encode noxious stimuli distinct from tactile stimuli and create the experience of pain is an important and ongoing area of research. This information has clear clinical implications for the developing infants and also how noxious input at an early stage of development might affect the maturation of the nociceptive system. Sensory system connectivity and functioning are established during specific developmental time windows called critical periods , during which deprivation of that normal modality-specific input or disruption of neuronal activity causes long-lasting disruption of sensory cortical maps and consequent sensory impairment. While this phenomenon has been well characterized in animal models for the visual, auditory, and somatosensory systems, it is difficult to define for nociception because nociceptive stimuli are normally absent during development. However, both pre-clinical animal models and clinical studies have shown that early exposure to noxious procedures causes long-term alterations of pain perception, and brain structure and function. ,
The nervous and immune systems are inextricably linked and the degree of complexity of their interactions continues to be discovered. Mechanisms involving elements of the immune system are crucial throughout normal nervous system development. Dysfunction of these interactions has been implicated in many neurodevelopmental and psychiatric disorders. Any exploration of neuronal function and development in health or disease requires consideration of the impact of the immune system.
For nociception, the spinal cord dorsal horn is the first site of processing within the CNS. Transmission of the nociceptive information from the periphery to the spinal cord is via sensory primary afferent neurons as previously discussed; subsequent transmission to the brain is also a neuronally mediated process. However, the processing of the nociceptive signal within the spinal cord is more complex. It is here that the immune system of the CNS becomes important, specifically the actions of microglia.
It is now well documented that microglia are intrinsically active in the normal development of the CNS. Not simply reactive to injury or disease, microglia have an instructive role in synaptic maturation and the refinement of neuronal circuitry perinatally. Microglia sculpt immature neuronal circuits, engulfing and eliminating excessive synaptic structures, all under the control of the classical complement cascade. This function of microglia is important in the development of spinal nociceptive circuitry where considerable postnatal refinement of connectivity occurs, as well as the strengthening and maturation of developmentally appropriate synaptic connectivity (Beggs, unpublished observations). An intriguing prospect is that microglia may be driving these developmental processes in preference to the defined immune role they play in the adult, preventing the risk of an autoinflammatory response to the clearance of axonal debris. The complex molecular machinery underlying this process remains unclear, and it is likely that other molecules are working in tandem with complement to control spatial and temporal aspects of pruning. However, evidence is accumulating that suggests aberrant pruning during critical periods of postnatal development contributes to neurodevelopmental disorders.
Immune activation in early life has the potential to impact both neuronal maturation and the normal developmental influence of microglia on circuit formation, disrupting both basal nociceptive function and, as is discussed in the next section, responses to painful stimuli in later life. Neuroimmune responses occur not only to direct immune activation but also to tissue-damaging injury (e.g., surgical incision). If an injury occurs within the critical period of plasticity that spans the first postnatal week in the rodent (which likely maps onto preterm infancy in humans), the immune response appears muted in terms of microglial reactivity, in comparison to the adult, but there is a priming of microglia such that they mount a greater response to reinjury in later life, resulting in an amplified behavioral pain response. , A surprising discovery is that this microglial-mediated priming is specific to males, and while the same behavioral priming occurs in females, it is a microglia-independent phenomenon.
Sexual dimorphism is highly prevalent in pain epidemiology, and it is perhaps not surprising that the neuroimmune interactions that underlie the long-term consequences of early-life painful events is sexually dimorphic. The development of microglia is influenced by extrinsic factors including sex. Microglial responses to inflammation and microbiota-derived signals differ between males and females, as do pain responses, both to injury in adulthood and, as described, the long-term consequences of injury in early life. , Although the reason why this occurs is not clear, there are a few clues to where differences lie. Male and female microglia have a different transcriptomic profile in early life that persists into adulthood and is independent of sex hormonal control, suggesting the differences are established in early life. Following preterm birth, male sex is a risk factor for adverse neurodevelopmental outcome, while repeated exposure to potentially painful procedures has a greater impact on brain development in females. Both males and females born extremely preterm demonstrate altered but different somatosensory function and pain experience.
Neuroimmune interactions are central to CNS circuit formation and immune cell function, with long-term implications for CNS homeostasis. How microglia function impacts health across the lifespan and how their dysfunction contributes to disease is only just beginning to be understood. For the study of pain, what is becoming apparent is that the long-lasting effects of pain in early life are partly a consequence of a disruption in the interactions of developing nervous and immune systems. This is a fascinating development and opens new vistas of research opportunities to investigate the translational potential leading to a better understanding and treatment of pain.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here