Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The urinary system maintains the electrolyte and water balance of the body fluids that bathe the tissues in a salty, aqueous environment. The development of this system involves the transient formation and subsequent regression or remodeling of vestigial primitive systems, thereby providing a glimpse of evolutionary history (another glimpse is provided by the development of the pharyngeal apparatus, covered in Chapter 17) . The development of the reproductive system is closely integrated with the primitive urinary organs in both males and females, as they share similar common tubular structures enabling both uresis and gamete transport. Therefore, this chapter has some overlap with Chapter 16 , which covers the development of the reproductive system.
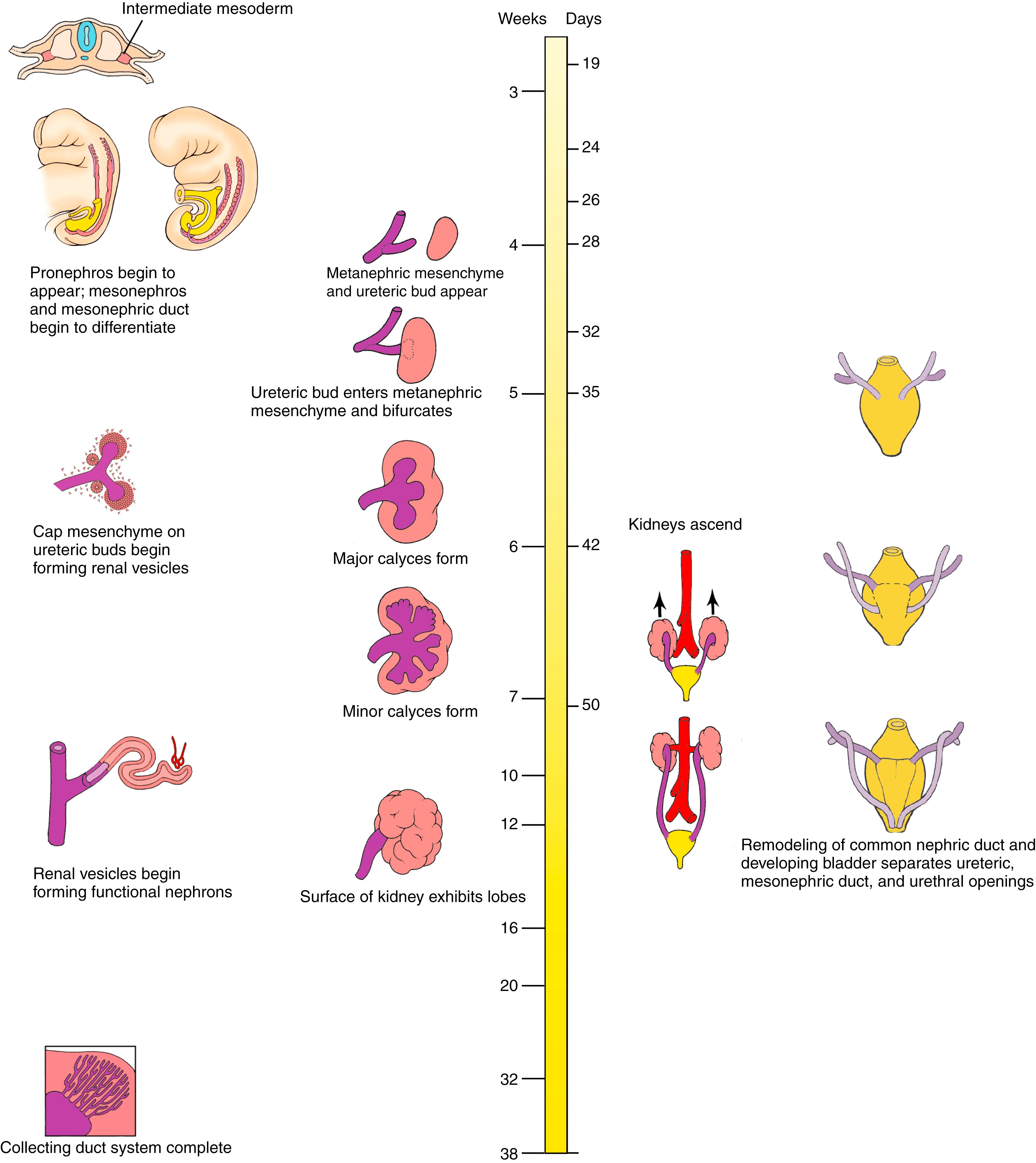
The intermediate mesoderm on either side of the dorsal body wall gives rise to three successive nephric structures of increasingly advanced design. The intermediate mesoderm, also known as the nephrotome , forms a segmental series of epithelial buds. In the cervical region, these structures presumably represent a vestige of the pronephroi , or primitive kidneys, which develop in some lower vertebrates. As these cranial pronephroi regress in the fourth week, a pair of elongated mesonephroi succeeds them, developing in the thoracic and lumbar regions. The mesonephroi become the first kidneys to function in the embryo, having complete, although simple, nephrons . A pair of mesonephric (Wolffian) ducts drains the mesonephroi; these grow caudally to open into the posterior wall of the primitive urogenital sinus. By the fifth week, a pair of ureteric buds sprouts from the distal mesonephric ducts, and tissue-tissue interactions with the overlying sacral intermediate mesoderm and developing blood vessels and nerves collectively generate the metanephroi , or definitive kidneys.
As described in Chapter 14 , the cloaca (the distal expansion of the hindgut) is partitioned into a dorsal anorectal canal and a ventral urogenital sinus. The latter is continuous with the allantois, which projects toward the umbilical cord. The expanded superior portion of the urogenital sinus becomes the bladder, whereas its inferior portion gives rise, in males, to the pelvic urethra (membranous and prostatic) and penile urethra and, in females, to the pelvic urethra (membranous) and vestibule of the vagina . During this period, the openings of the mesonephric ducts are translocated down onto the pelvic urethra by a process that also emplaces the openings of ureters on the bladder wall. Several urachal anomalies, fistulas, and anorectal anomalies can occur as a result of abnormal urorectal septal and cloacal membrane development.
A pregnant woman is seen at 20-weeks of gestation for her second transabdominal ultrasound. The imaging reveals a severe paucity of amniotic fluid ( oligohydramnios ) with no evidence of amniotic fluid leakage. Kidney profiles cannot be found within the renal fossae or in ectopic locations. She is referred to a high-risk perinatologist, and a 2-week follow-up ultrasound confirms the suspected diagnosis of bilateral renal agenesis based on the absence of kidneys, ureters, and bladder and the accompanying severe oligohydramnios.
The perinatologist explains the diagnosis to the parents and that the condition is incompatible with extrauterine life. The parents are informed about their options. They can decide to terminate the pregnancy now or allow the pregnancy to proceed, knowing the neonate may be stillborn or will survive only several hours. Although it is not urgent to decide immediately, the ethical and legal difficulties of termination of pregnancy after 24 weeks are discussed.
The incidence of bilateral renal agenesis is approximately 1 in 4000 to 4500 births, and the condition is more common in males. Because the main source of amniotic fluid by 16 weeks of gestation is urine production, the most prominent prenatal diagnostic feature of bilateral renal agenesis is reduced or absent amniotic fluid. As a consequence, neonates are characterized by a constellation of anomalies ( Potter sequence ), including a flat face, limb contractures, low-set ears, and dry, wrinkled skin. Neonatal mortality is due to the accompanying pulmonary hypoplasia caused by the altered dynamics of lung liquid movement during development. Bilateral renal agenesis is associated with other structural anomalies in more than 50% of cases, including an absent bladder, anal atresia, esophageal atresia, and genital anomalies.
Renal agenesis is often a component feature of syndromes caused by single gene mutations (e.g., EYA1, RET, GDNF, SIX1, WT1 genes) or is associated with chromosomal anomalies such as trisomy 13, 18, and 21 or the 22q11.2 deletion syndrome. About one-third of cases are associated with non-syndromic, autosomal dominant inheritance having variable penetrance. It is estimated that 9% to 14% of first-degree relatives of neonates with bilateral renal agenesis or dysgenesis have renal anomalies themselves. Therefore, it is recommended that mothers be evaluated for possible renal defects at the time of the prenatal ultrasound and that both parents and siblings undergo genetic evaluation and counseling. The risk of recurrence in subsequent pregnancies is estimated to be 3% to 6%.
Potter sequence can have multiple causes. Other clinical scenarios that result in this syndrome are given in the “Embryology in Practice” for this chapter and in the “Clinical Taster” for Chapter 6 .
![]()
Animations are available online at StudentConsult.
As covered in Chapter 3 , the intraembryonic mesoderm formed on either side of the midline during gastrulation differentiates into
three subdivisions: paraxial mesoderm, intermediate mesoderm (also called the nephrotome ), and lateral plate mesoderm ( Fig. 15.1 ). The fates of the paraxial and lateral plate mesoderm are discussed in other chapters. The intermediate mesoderm gives rise to the nephric structures of the embryo, portions of the suprarenal glands, the gonads, and the genital duct system. During embryonic development, three sets of nephric systems develop in craniocaudal succession from the intermediate mesoderm. These are called pronephros, mesonephros , and metanephros (or definitive kidneys). Formation of the pronephric kidney (i.e., pronephros) lays the foundation for induction of the mesonephros, and it in turn lays the foundation for induction of the metanephros. Hence, formation of a pronephros is really the start of a developmental cascade leading to the formation of the definitive kidney.

Early in the fourth week, intermediate mesoderm along the fifth to seventh cervical axial levels gives rise to a small duct generated by epithelialization of some of the intermediate mesoderm. This duct is called the mesonephric (Wolffian) duct . The mesonephric duct first appears as a solid longitudinal rod that condenses within the intermediate mesoderm beginning in the pronephric region ( Figs. 15.2A–C , 15.3 ). These rods develop in a caudal direction, driven through inductive mechanisms during ongoing mesenchymal-to-epithelial conversion of cells at their caudal tips. Meanwhile, intermediate mesoderm ventromedial and adjacent to the mesonephric duct condenses and reorganizes into a series of epithelial buds (see Fig. 15.2 ). These buds, which quickly become hollow, constitute the pronephros (plural, pronephroi; derived from the Greek for “first kidney”) because they resemble the functional embryonic pronephroi of some lower vertebrates. In humans, these units do not differentiate into functional excretory structures but instead cease to develop and disappear by around day 24 or 25.

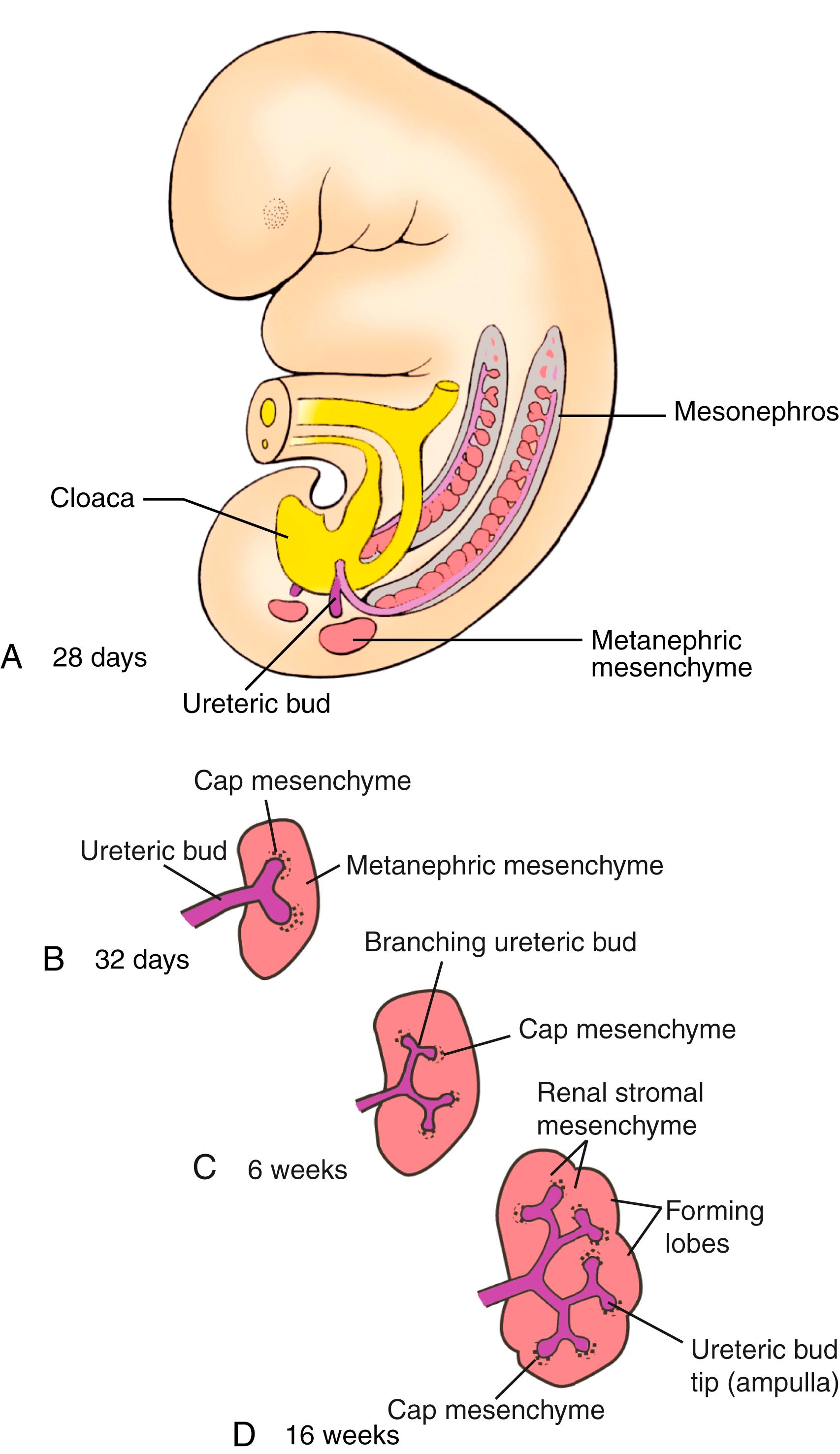
As the mesonephric ducts develop and extend caudally, they induce the formation of mesonephric buds from mesenchyme in the more caudal intermediate mesoderm, thereby initiating mesonephros formation (see Fig. 15.2B,C ). As the ducts grow into the lower lumbar region, they diverge from the intermediate mesodermal mesenchyme and grow toward and fuse with the ventrolateral walls of the cloaca on day 26 (see Figs. 15.2, 15.4A ). This region of fusion will become a part of the posterior wall of the future bladder. As the rods fuse with the cloaca, they begin to cavitate at their caudal ends, forming a lumen, and this canalization progresses cranially. The caudal end of each mesonephric duct induces the evagination of a ureteric bud (see Fig. 15.4 ).
Early in the fourth week, mesonephric tubules begin to develop within mesonephric buds adjacent to the mesonephric duct on either side of the vertebral column, from the upper thoracic region to the third lumbar level (see Fig. 15.2B–D ). About 40 mesonephric tubules are produced in craniocaudal succession within this mesonephric mesenchyme. Because the gonads begin to develop just medial to the mesonephric ridge, this region is sometimes collectively referred to as the urogenital ridge . As more caudal tubules differentiate, the more cranial ones regress, so there are never more than about 30 pairs in the mesonephroi. By the end of the fifth week, the cranial regions of the mesonephroi undergo massive regression, leaving only about 20 pairs of tubules occupying the first three lumbar levels. The mesonephric tubules differentiate into excretory units resembling an abbreviated version of the adult metanephric nephron (see Fig. 15.2D ; discussed later) with the medial end of the tubule forming a cup-shaped sac, called Bowman’s capsule , which wraps around a knot of capillaries, called a glomerulus , to form a renal corpuscle .
The lateral tips of the cranial six to seven mesonephric tubules fuse with the mesonephric duct, thus opening a passage from the excretory units to the cloaca. These mesonephric excretory units are functional between about the 6th and 10th weeks and produce small amounts of urine. After 10 weeks, they cease to function; they regress in the female, whereas in the male, they are thought to give rise to the efferent ductules. As covered in Chapter 16 , the mesonephric ducts also regress in the female. However, in the male, the mesonephric ducts persist and form important elements of the male genital duct system.
One of the earliest genes expressed in the nephrogenic intermediate mesoderm is odd-stripped-related-1 (Osr1) transcription factor. This transcription factor drives subsequent Pax2 and Lim homeobox-1 (Lhx1) transcription factors. Mice deficient in Pax2 form a mesonephric duct in the pronephric and mesonephric regions, but the mesonephric duct fails to extend into the metanephric region. Therefore, the metanephros does not develop (because it is dependent on branching from the caudal mesonephric duct). When double knockout mice for Pax2 and Pax8 (another Pax family member expressed in the intermediate mesoderm) are generated, the intermediate mesoderm does not form any portion of the mesonephric duct nor express the nephric markers Lhx1 and Ret (both required for subsequent metanephric kidney development). One downstream target of Pax2 and Pax8 activity is Gata3, which is necessary for Ret activation and specification of the nephric lineage. Pax2 is a particularly potent initiator of nephron development: ectopic nephric structures can be induced almost anywhere within the intermediate mesoderm, including the gonadal ridge of chick embryos, when Pax2 is ectopically expressed by viral transfection of mesoderm at the mid-primitive streak stage.
The tissue interactions responsible for specifying the nephric lineage within intermediate mesoderm are unclear, but this lineage seems to depend on the developing somites because Pax2 and Lhx1 expression is lost in chick embryos if the intermediate mesoderm is separated from the somites. Moreover, one can induce ectopic pronephric tissue within the intermediate and lateral plate mesoderm by grafting somites into ectopic locations. Ectoderm may also have a role in specifying or maintaining nephric capacity within intermediate mesoderm, as removal of the overlying ectoderm decreases expression of Pax2 and Lhx1 within the intermediate mesoderm, and this mesoderm loses its nephrogenic capacity. Therefore, secreted factors from adjacent tissues are needed to induce and maintain nephrogenic mesoderm.
The definitive kidneys, or metanephroi , are composed of two functional components: the excretory portion and the collecting portion. These two portions are derived from different sources of intermediate mesoderm ( Table 15.1 ). Development of the metanephros involves mesenchymal-to-epithelial conversion, epithelial tube formation and elongation, tubular branching, cell aggregation, angiogenesis, and specification and differentiation of numerous specialized cell types.
| Collecting Portion (Ureteric Bud) | Excretory Portion (or Nephron) (Metanephric Mesenchyme) |
| Ureter | Bowman’s capsule |
| Renal pelvis | Proximal convoluted tubule |
| Major and minor calyces | Loop of Henle |
| Collecting ducts | Distal convoluted tubule |
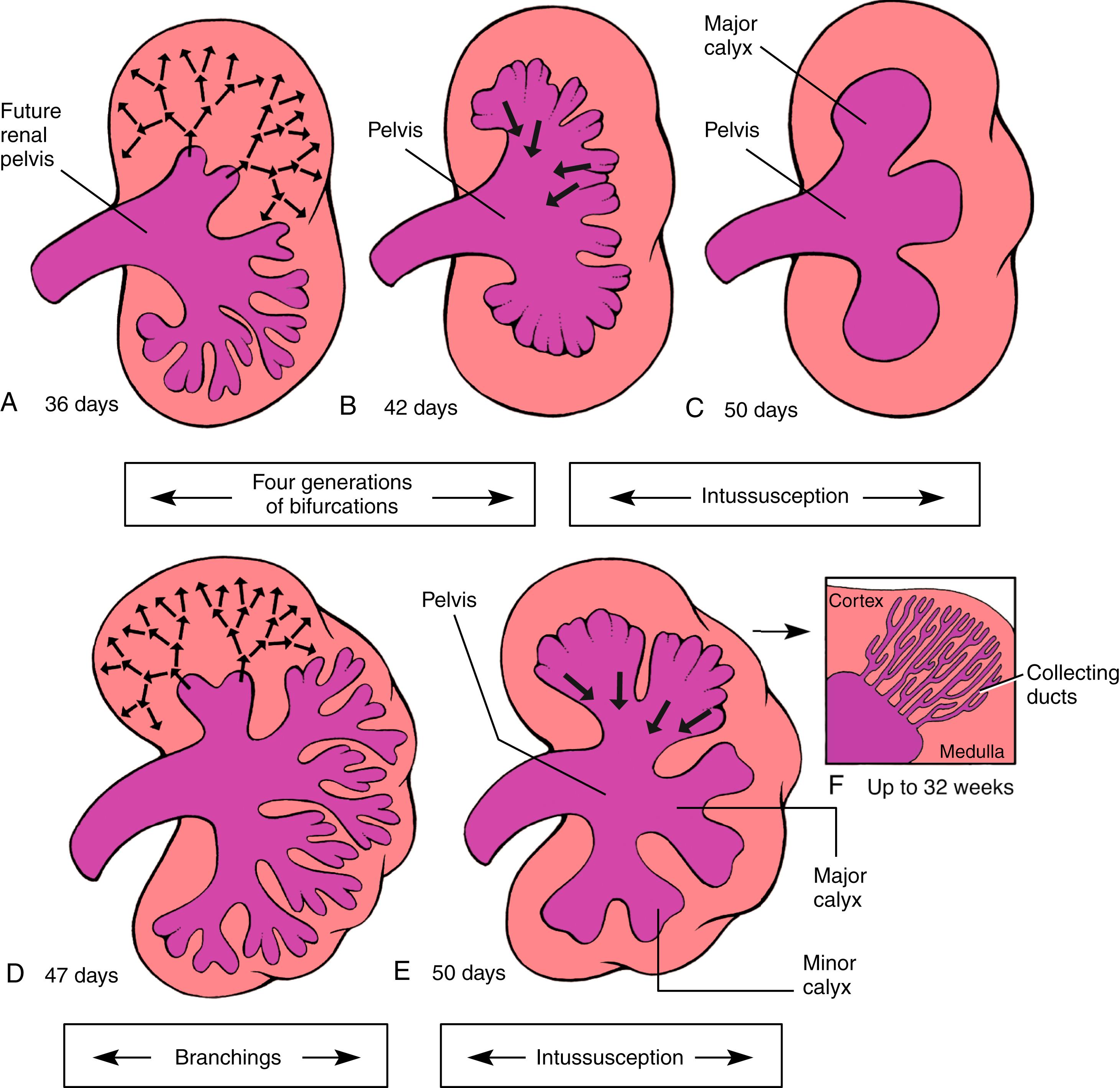
Formation of the metanephros begins with the induction and formation of a pair of new structures, the ureteric buds , within the intermediate mesoderm of the sacral region. A ureteric bud sprouts from the caudal end of each mesonephric duct on about day 28 (see Fig. 15.4 ). By day 32, each ureteric bud penetrates a portion of the sacral intermediate mesoderm called the metanephric mesenchyme , and the bud begins to bifurcate (see Figs. 15.4B, 15.5 ). As the ureteric buds continue to branch, each new growing ureteric tip (ureteric ampulla) acquires a cap-like aggregate of metanephric mesenchymal tissue referred to as cap mesenchyme . By the end of the 16th week, 14 to 16 lobes have formed, giving the metanephros a lobulated appearance.
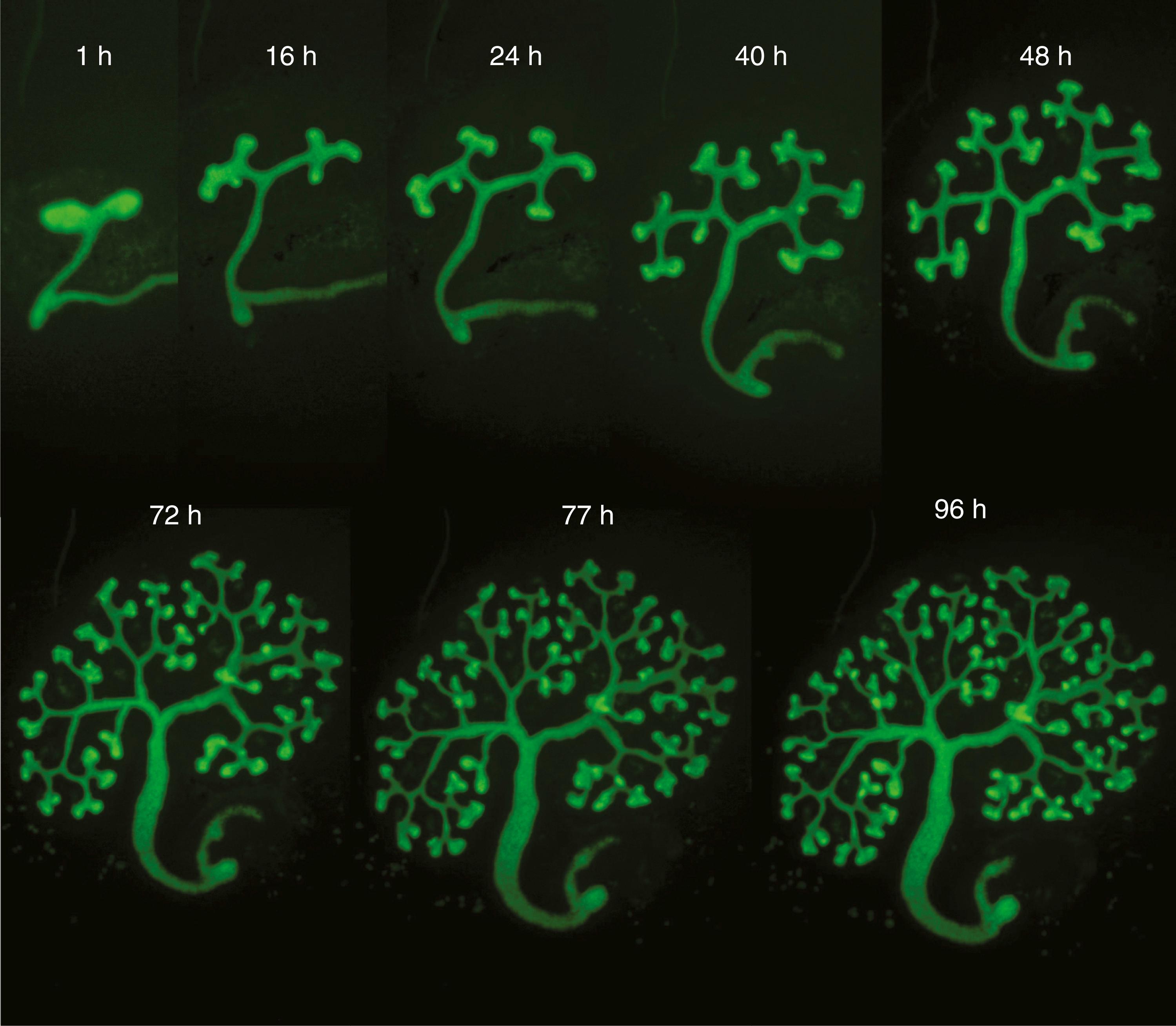
The ureters and the collecting duct system of the kidneys differentiate from the ureteric bud; the nephrons (the definitive urine-forming units of the kidneys) differentiate from the metanephric mesenchyme. In the mature kidney, urine produced by the nephrons flows through a collecting system consisting of collecting ducts, minor calyces, major calyces, the renal pelvis, and, finally, the ureter. This system is entirely the product of the ureteric bud. The ureteric bud undergoes an exact sequence of bifurcations ( Fig. 15.6 ), and the expanded major and minor calyces arise through phases whereby previously formed branches coalesce. When the ureteric bud first expands into the metanephric mesenchyme, its tip expands to form an initial ampulla that will give rise to the renal pelvis . During the sixth week, the ureteric bud bifurcates four times, yielding 16 branches. These branches then coalesce to form two to four major calyces extending from the renal pelvis. By the seventh week, the next four generations of branches also coalesce, forming the minor calyces . By 32 weeks, approximately 11 additional generations of bifurcation have formed 1 to 3 million branches, which will become the future collecting ducts of the kidney (see Fig. 15.6F ). The definitive morphology of the collecting ducts is created by variations in the pattern of branching and by a tendency for distal branches to elongate.
Like the nephrons and mesonephric duct of the mesonephric kidney, the differentiation of metanephric nephrons depends on inductive signals between the ureteric bud and the cap mesenchyme (see the following “In the Research Lab” entitled “Factors Expressed in Metanephric Mesoderm Regulate Induction and Branching of Ureteric Bud”). As the buds continue to grow into the future renal cortex, some of the cap mesenchymal cells come to lie adjacent to the ureteric stalk (the portion of the ureteric bud found immediately adjacent to the ureteric tip; Fig. 15.7A,B ), where the cells migrate, aggregate, and undergo mesenchymal-to-epithelial conversion to form a renal vesicle . Several hours of direct contact between the ureteric stalk and the forming renal vesicle are required to induce subsequent nephron differentiation within the renal vesicle. If the ureteric bud is abnormal or missing, the nephron does not develop. Conversely, reciprocal inductive signals from the cap mesenchyme regulate the orderly branching and growth of the bifurcating tips of the ureteric buds. The number of nephrons formed ultimately depends on growth and branching of the ureteric bud and cap mesenchyme proliferation, as well as renal vesicle formation and conversion into epithelial tubules.
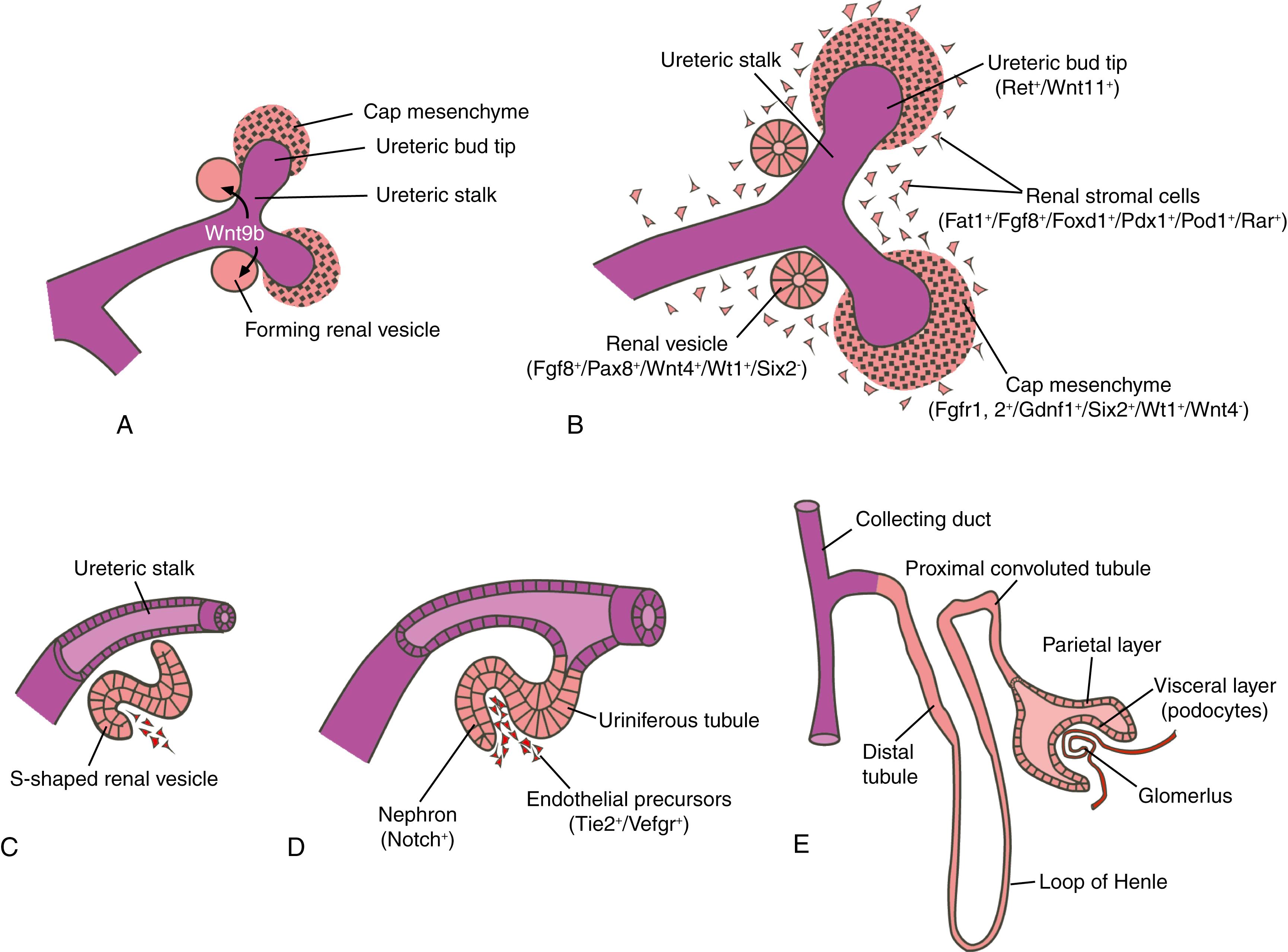
Each nephron originates from a renal vesicle derived from proliferating cap mesenchyme. Formation of a nephron from this vesicle involves several stages. First, the nephric vesicle develops into a comma-shaped structure, which then forms an S-shaped tubule (see Fig. 15.7C,D ). The S-shaped tubule fuses with the ureteric stalk, and eventually the two lumens become continuous, forming a uriniferous tubule . Meanwhile, the future renal corpuscle segment (proximal portion) of the S-shaped tubule forms the outer (parietal) layer of Bowman’s capsule and the glomerular epithelial cells ( podocytes ) that surround the glomerular tuft of capillaries developing within the adjacent stroma. While the renal corpuscle is forming, the lengthening uriniferous tubule forms the remaining elements of the nephron: the proximal convoluted tubule, descending and ascending limbs of the loop of Henle, and the distal convoluted tubule (see Fig. 15.7E ). The medulla of the kidney also begins to take shape as the growing nephron tubules and interstitial tissue develop. Nephrogenesis is complete by birth in humans.
Morphogenesis of the renal vascular supply during development of the nephron and collecting systems is poorly understood. Organ cultures and interspecies grafting experiments show that angiogenesis is likely the major mechanism responsible for the development of the renal vasculature, including the glomerular capillaries. However, the prevascular metanephric mesenchyme expresses vasculogenic markers (e.g., Vegf, Vegfr, Tie2) and, if fetal mouse kidney tissue is grafted into the mouse anterior eye chamber (an area devoid of blood vessels), grafted tissue can form capillaries, suggesting that it has intrinsic vasculogenic capacity.
During the 10th week, the metanephroi become functional. Blood plasma from the glomerular capillaries is filtered within the renal corpuscle to produce a dilute glomerular filtrate, which is concentrated and converted to urine by the activities of the convoluted tubules and the loop of Henle. The urine passes down the collecting system into the ureters and thence into the bladder. Even though the fetal kidneys produce urine throughout the remainder of gestation, their main function is not to clear waste products out of the blood; that task is handled principally by the placenta. Instead, the production of fetal urine is important because urine contributes to the amniotic fluid. Fetuses with bilateral renal agenesis (complete absence of both kidneys) do not have enough amniotic fluid ( oligohydramnios ) and hence are confined to an abnormally small amniotic space. This leads to a condition called Potter sequence (covered in the following “In the Clinic” entitled “Renal Agenesis and Dysplasia,” and in the “Clinical Taster” and “Embryology in Practice” for this chapter, as well as in the “Clinical Taster” for Chapter 6 ).
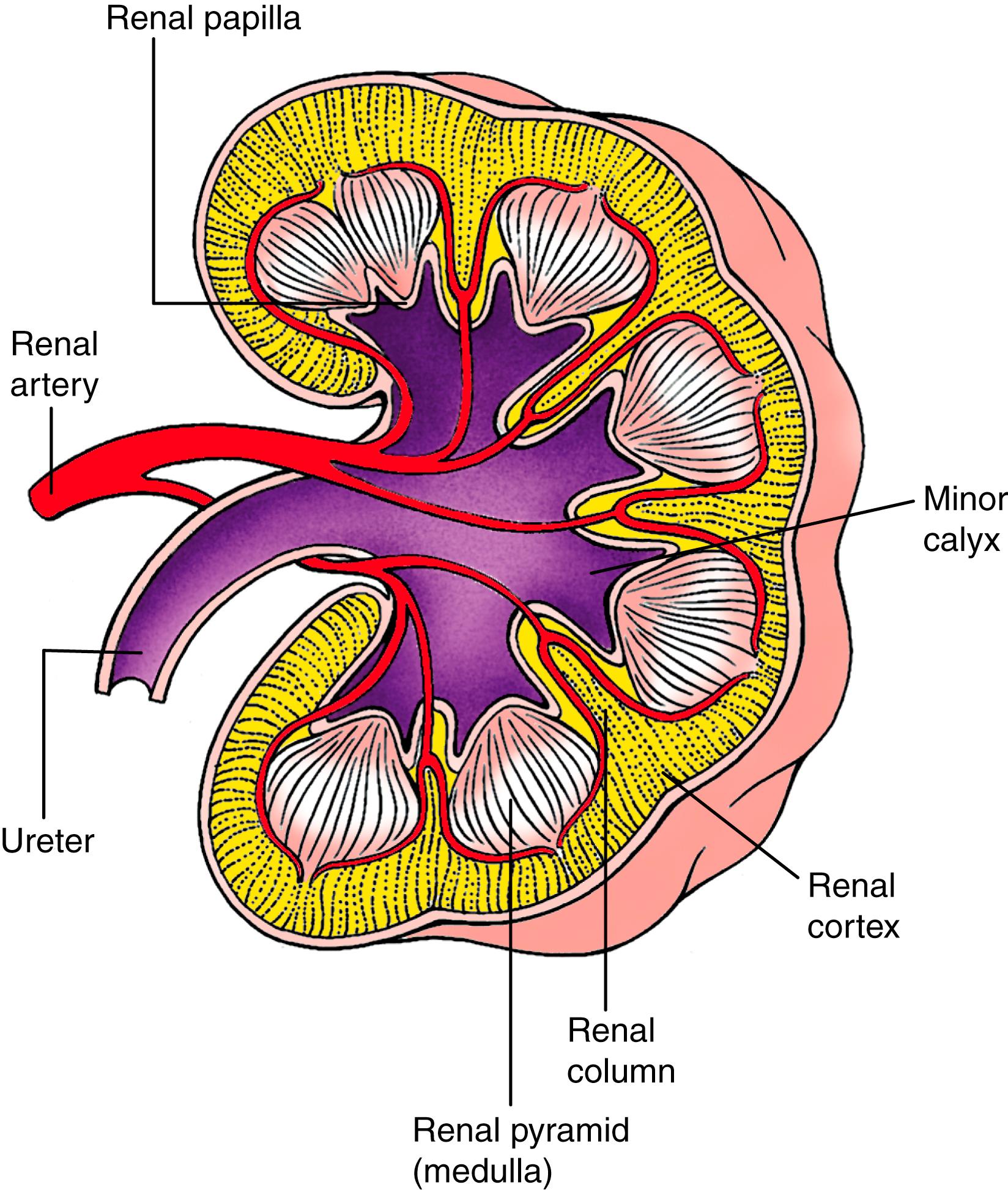
Fig. 15.8 shows the general structure of the definitive fetal kidney. This architecture reflects the events of the first 10 weeks of renal development, that is, weeks 5 to 15 of intrauterine development. The kidney is divided into an inner medulla and an outer cortex. The cortical tissue contains the nephrons and collecting ducts, whereas the medulla contains the collecting ducts and loops of Henle. Each minor calyx drains a tree of collecting ducts within a renal pyramid ; the collecting ducts converge to form the renal papilla . Zones of nephron-containing cortical tissue called renal columns separate the renal pyramids of the kidney. Thus, in the definitive kidney, the cortical tissue not only makes up the peripheral layer of the kidney, it also forms piers projecting toward the pelvis. Nevertheless, all nephrons in the cortical tissue arise from cortical regions of the metanephric mesenchyme.
The autonomic nervous system of the kidney, which regulates blood flow and secretory function, arises from neural crest cells that invade the metanephroi early in their development. Further aspects of development of the autonomic nervous system of the abdomen and pelvis are covered in Chapter 10 .
What induces the formation of the ureteric bud and specifies its location along the mesonephric duct? It seems that the induction and location of the ureteric bud largely depends on the nephrogenic mesenchyme of the intermediate mesoderm. Formation of the ureteric bud from the mesonephric duct is induced by signals emanating from the adjacent mesoderm and involves the Ret receptor, its co-receptor, Gfr1α, and its ligand, Gdnf. Ret and Gfr1α are expressed within the mesonephric duct, whereas the ligand, Gdnf (and also Gfr1α), is expressed within the metanephric mesenchyme ( Fig. 15.9 ). Misexpression of Gdnf elsewhere within the intermediate mesoderm is sufficient to induce ectopic ureteric buds, and mice deficient in Ret or Gdnf exhibit bilateral renal agenesis. Therefore, faults in tissue-tissue interactions between the metanephric mesoderm and the mesonephric duct mediated through Ret signaling may be responsible for the formation of duplex kidneys or for renal agenesis seen in humans. Among humans with renal agenesis, approximately 40% have RET mutations and 5% to 10% have mutations in GDNF.
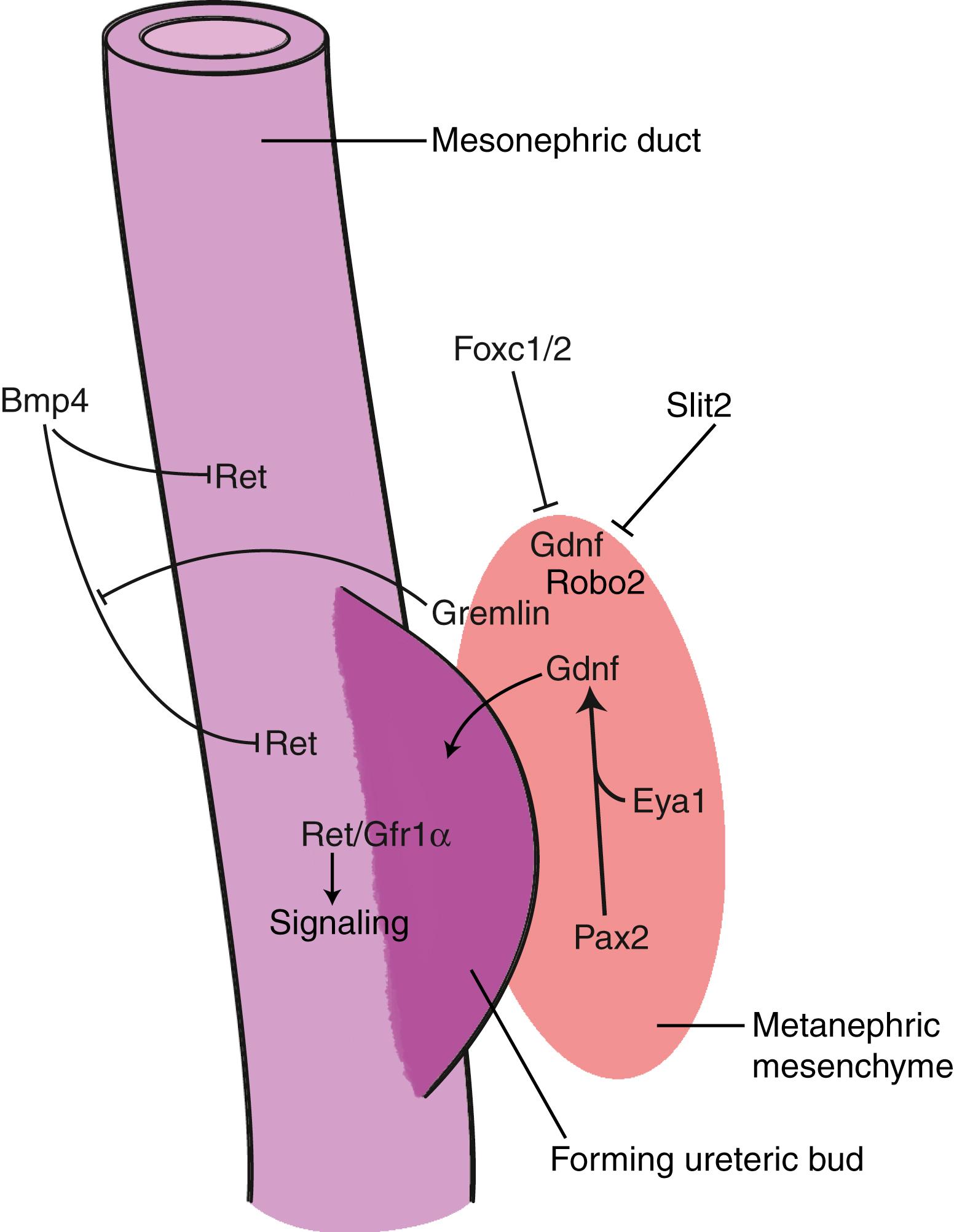
Experiments suggest that the cranial-caudal positioning of ureteric bud formation may be the result of repression of nephrogenic determinants within the more cranial regions of the intermediate mesoderm. Forkhead genes make up one group of transcription factors that seem to be involved. Foxc1/2 expression is normally restricted to the cranial end of the nephrogenic mesoderm. When Foxc1 is knocked out in mice or Foxc1/2 null heterozygotes are generated, ectopic ureteric buds form over a broad span of the mesonephric duct. A similar phenotype is seen in mice deficient in Slit2, or its receptor, Robo2 (pathfinding signaling molecules; covered further in Chapter 10 ). Foxc1/2 and Slit2/Robo2 may repress Gdnf expression in the more cranial regions, because Slit2 expression occurs in a cranial-caudal gradient within the mesenchyme that is the inverse of Gdnf expression.
Bmp4 is another extracellular signaling molecule implicated in restricting ureteric bud development. Bmp4-deficient mice develop ectopic ureteric buds and double ureters. Bmp4 is normally expressed in the mesoderm surrounding the mesonephric duct and ureteric buds but not in the mesonephric duct itself. Experiments suggest that Bmp4 inhibits Ret signaling within the mesonephric duct (see Fig. 15.9 ), rather than altering Gdnf levels released from the mesoderm, because Bmp4 can block the effect of ectopic Gdnf on ureteric bud formation in metanephric organ cultures. Metanephric mesenchyme also produces gremlin, a Bmp inhibitor, thereby blocking any potential Bmp inhibition of Ret signaling in this region.
Other factors important in the formation and budding of the ureteric bud are eyes absent 1 (Eya1) and the Hox11 group of homeotic genes. Mutants deficient in these genes fail to turn on Gdnf and do not develop ureteric buds. Hence, the induction and position of the ureteric bud seems to depend on a balance between mesodermally mediated activation and negative regulation of Ret signaling within the mesonephric duct. Several malformations can arise if the ureteric buds sprout from incorrect sites along the mesonephric duct, as the resulting ureters will be incorrectly emplaced into the dorsal wall of the developing bladder.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here