Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The development of the mammalian kidney has been extensively studied for the past 60 to 70 years, and our understanding of renal development and molecular regulation is perhaps better understood than that of any other organ. This chapter provides an overview of the development of the three mammalian excretory organs (pronephroi, mesonephroi, and metanephroi) but explores metanephric development in detail. The processes of ureteric budding, ureteric branching morphogenesis, and nephrogenesis are also described in detail, as is our current understanding of the nature and roles of renal progenitor cells. In addition, the roles of the renal stroma in kidney development are considered, and renal vascular development is described. Finally, the effects of preterm birth on kidney development are addressed.
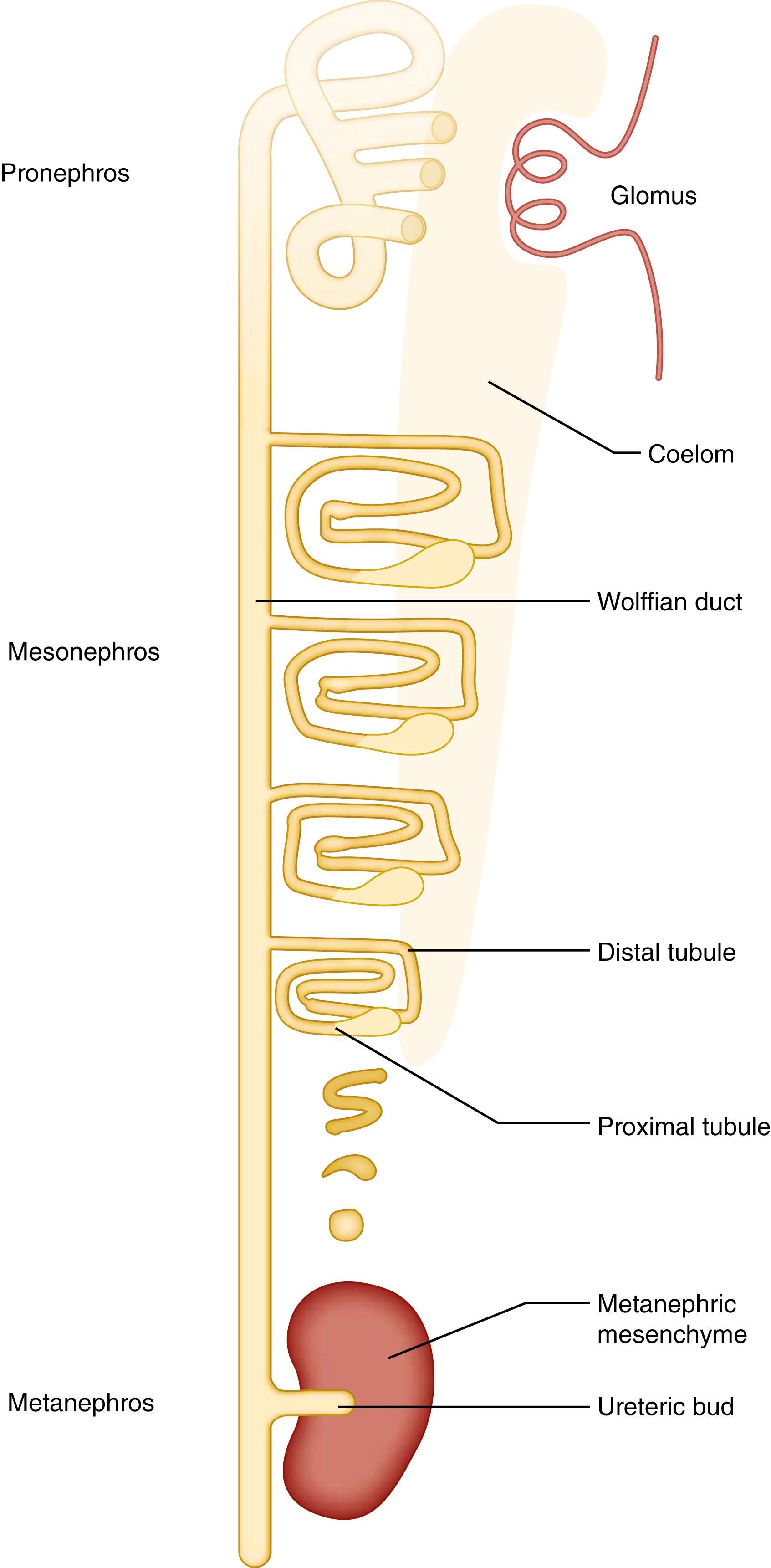
During mammalian embryogenesis, three pairs of excretory organs form from the intermediate mesoderm, which lies between the developing somites and the lateral plate on the flanks of the developing embryo. These are the pronephroi, mesonephroi, and metanephroi, which develop in a cranial to caudal fashion, respectively ( Fig. 93.1 ). The development of the permanent mammalian kidneys, or metanephroi, takes place after the successive formation and regression of the pronephroi and mesonephroi. All three pairs of kidneys are induced to develop from an epithelial tube, the nephric duct that migrates caudally through the nephrogenic cord along the anterior-posterior axis of the embryo and fuses with the cloaca.
Bone morphogenetic protein (Bmp) signaling plays a critical role in the specification of the intermediate mesoderm, wherein a gradient of signaling orchestrates the differentiation of the lateral plate (high), intermediate mesoderm (intermediate), and paraxial mesoderm and somites (low levels). Simultaneous deletion of the functionally redundant transcription factors paired box 2 ( Pax2, a Bmp target gene ) and paired box 8 (Pax8) results in a failure of nephric duct formation, without induction of associated renal lineage genes such as rearranged during transfection (Ret) and Lim1 . These actions appear to be complemented by nodal-like signaling (mediated by Vg1/Nodal) in a manner that intersects with Bmp signaling. Retinoic acid (RA), produced by paraxial mesoderm, is also required for initial specification of renal progenitor cells.
The first indication of renal development in humans is evident at approximately embryonic day 22 (E22) with the appearance of the nephric or wolffian duct (WD). In mice, this occurs at E8. The nephric duct forms as a consequence of a mesenchymal to epithelial transition. Both the surface ectoderm and somites appear to play an inductive role in triggering the formation of the nephric duct. As the embryo ages, the nephric duct extends caudally through a process of migration and changes in cell shape that, depending on the species concerned, involve further contribution from cells derived from the uncommitted intermediate mesoderm. As the duct elongates and development progresses, the pronephros, mesonephros, and finally the metanephros are sequentially formed. The last of these structures ultimately develops into the functional or permanent mammalian kidney.
The pronephros is the first of the excretory organs to form as the nephric duct differentiates. In humans, as with other amniotes, this is a transitory structure, which appears as 5 to 7 paired pronephroi that connect to the nephric duct around 3 weeks’ gestation. In mice, elements of this structure appear in a highly rudimentary form at E8.5 to E9, but by E9 the mesonephros, the “second” excretory structure in developing amniotes, is established in the intermediate mesoderm field adjacent to the nephric duct. In humans, the pronephros begins to develop around E22 in the cervical region of the embryo. At this time, segmentally arranged sets of epithelial tubules appear within the nephrogenic cord. These structures are known as nephrotomes, and they connect to the anterior region of the nephric duct (pronephric duct). The pronephroi are nonfunctional in mammals; however, amphibians and fish have well-developed and functional pronephroi that persist throughout life to regulate water and solute balance and blood pH. The mammalian pronephric tubules regress around 5 weeks’ gestation in humans, but the pronephric duct persists and becomes the mesonephric or WD. ,
The mesonephros is the second transient kidney and appears in humans at 3 to 4 weeks’ gestation immediately caudal to the last pronephric tubules. The WD induces the adjacent mesenchyme within the nephrogenic cord to undergo a mesenchymal to epithelial transition to induce a renal vesicle. This differentiates into an S -shaped structure that elongates and eventually forms a proximal tubule connected to the WD. These structures develop a vascularized, glomerulus-like filtering component connected to proximal and distal tubules draining into the WD and are the first definitive functional unit or nephron in the renal excretory system. The mesonephric nephrons are transient structures, with up to 40 present at any one time in humans. As with the pronephros, the mesonephros degenerates in a craniocaudal direction from 5 to 12 weeks’ gestation and ceases to function as an excretory organ. , , Remnant structures of the mesonephros are involved in genitourinary development. In females, the mesonephric duct completely disappears during the third embryonic month, whereas some mesonephric tubules persist as the epoöphoron and paroöphoron, which have no known function in humans. In males, mesonephric tubules in the area of the gonad form the efferent ductules, whereas the mesonephric duct gives rise to the epididymis and the ductus deferens.
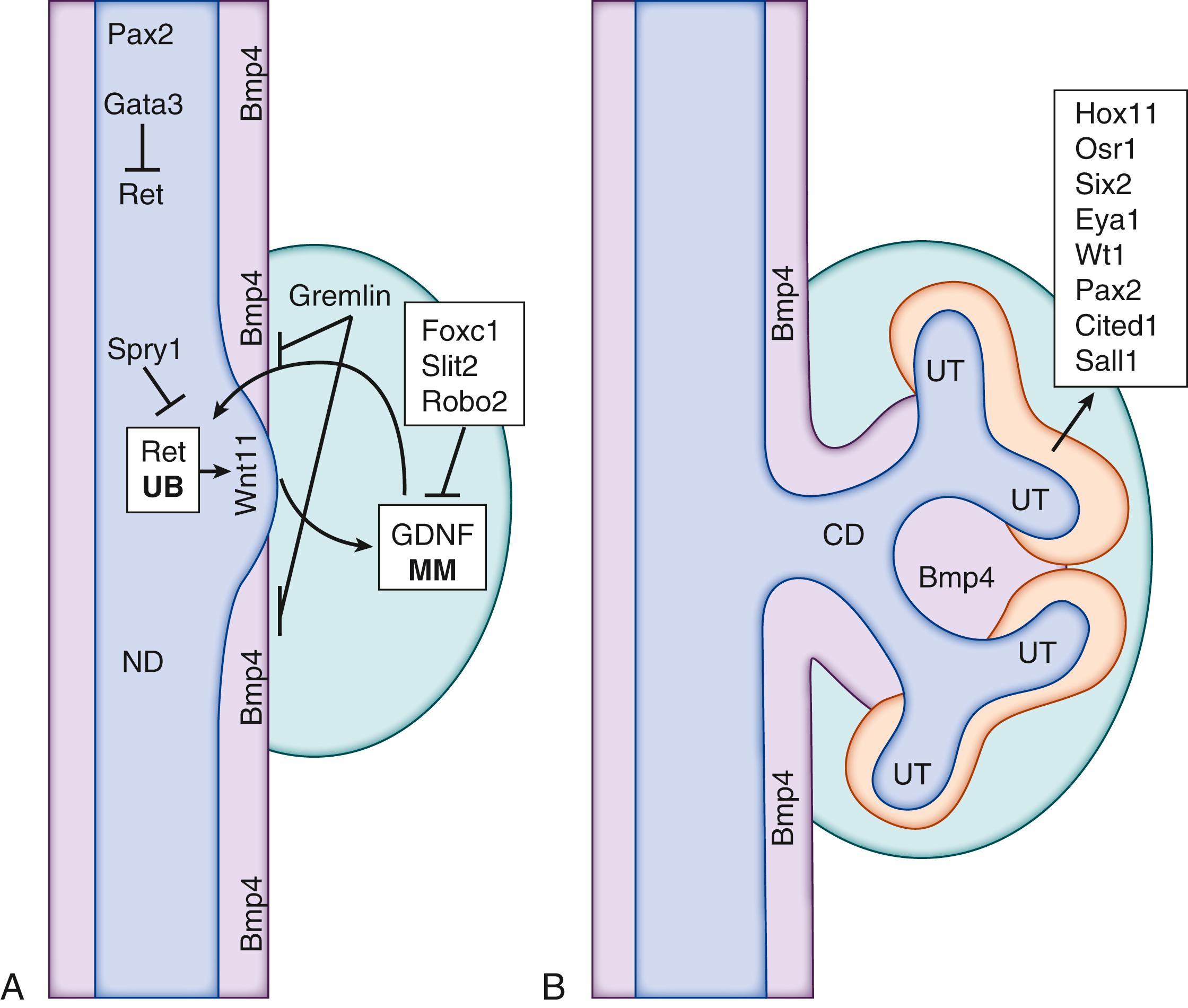
The formation of the metanephros gives rise to the permanent kidney in humans, as well as in other mammals, reptiles, and birds. The development of the metanephros is initiated by the outgrowth of the ureteric bud (UB) from the caudal end of the WD at 4 to 5 weeks’ gestation. The timing and site at which the UB emerges from the WD are well orchestrated such that it enters the adjacent metanephric mesenchyme. Reciprocal inductive signals occur between the UB and the metanephric mesenchyme ( Fig. 93.2A ). The metanephric mesenchyme induces the UB to grow and repetitively bifurcate to form the ureteric tree via branching morphogenesis. The ureteric tree subsequently forms the collecting ducts, calyces, and renal pelvis. The region of the UB that does not enter the metanephric mesenchyme becomes the ureter. Simultaneously, the tips of the ureteric tree induce subpopulations of committed metanephric mesenchyme cells to condense and undergo a mesenchymal to epithelial transition to form a renal vesicle that further differentiates into the functional unit of the kidney, the nephron (see Fig. 93.2B ). This process is known as nephrogenesis . Branching morphogenesis and nephrogenesis occur in the human embryo from 6 to 36 weeks’ gestation.
As the metanephros develops, it is drawn in a cranial direction. The upward movement of the metanephros from a pelvic position to its final lumbar position is complete by the eighth embryonic week. On emerging from the pelvis, the metanephros undergoes a 90-degree rotation so that the original ventral hilum takes its final medial position.
The principal feature presaging the development of the metanephros is the demarcation of the metanephric mesenchyme, which is marked by the expression of Wilms tumor-1 (Wt1) and Pax2 . , This occurs at E10 of development in the mouse and approximately E30 in humans. Even at this early stage, the metanephric mesenchyme contains progenitor cell populations, which will ultimately form nephrons, stromal cells, and some of the vascular elements of the adult kidney. The positional specification of the metanephric kidney relies on molecules expressed in both the differentiating nephric duct and in the adjacent metanephric mesenchyme. The ultimate product of these interactions is the initiation of an outgrowth of a single structure from the nephric duct—the UB. Unlike with the pronephros or mesonephros, it is critical that a single UB forms on each side of the metanephros and that it does so in a defined rostrocaudal position. Multiple or positionally inappropriate UBs contribute to the development of a number of diseases of the congenital abnormalities of the kidney and urinary tract (CAKUT) spectrum, including hypodysplasia, urinary outflow obstruction, and vesicoureteric reflux. Notably, mouse and human kidneys have both conserved and divergent features of their structure and molecular and cellular features although the contribution of these differences to development of pathology following gene mutation is poorly understood.
The poor structural outcomes due to inappropriate positional or numeric induction of UB formation are further reflected in altered communication between epithelial and mesenchymal cells, especially regarding signaling between the mesenchymally expressed glial cell–derived neurotrophic factor (Gdnf) and its co-receptors Ret and glial cell line derived neurotrophic factor family receptor alpha 1 (Gfra1) (see Fig. 93.2A ). These genes are all required for kidney development. In the duct, Pax2 and Gata3 are required to establish the position of the UB (see Fig. 93.2A ). , , In parallel, the formation and position of the metanephric mesenchyme is dictated by expression of a collection of transcription factors that includes members of the Hox11 gene family ( Hoxa11 , Hoxc11, and Hoxd11 ) and odd-skipped related 1 ( Osr1 ), a member of the odd-skipped family of zinc finger proteins. Wt1 is also expressed in the neighboring intermediate mesoderm and is required to maintain the competency of the mesenchyme for metanephric kidney growth. As the embryo develops, further cohorts of mesenchyme-expressed factors direct metanephric differentiation including Sall1, Eya1, Six1, Six4, and Gdf11, which themselves reinforce positionally appropriate Gdnf expression next to the site of UB outgrowth. A complementary program of repression in the more rostral mesenchyme serves to suppress Gdnf and includes Foxc1, Slit2, and Robo2 (see Fig. 93.2A ). The capacity of the rostral part of the nephric duct to form the metanephric kidney is also repressed by the action of Bmp4 (expressed in the metanephric mesenchyme) and Sprouty ( Spry1 ; expressed in the nephric duct downstream of Wt1). The actions of Bmp4 are overcome in the region of eventual metanephric growth by the expression of Gremlin (Grem1) (see Fig. 93.2A ). The relative importance of these different genes in metanephric specification is reflected in the findings of causative roles for BMP4 , SIX2 , PAX2 , EYA1 , SIX1 , and SIX5 in renal hypodysplasia. The end result of these complex interactions is the emergence of the UB.
Between E9.5 and E10.5 in mice the caudal end of the nephric duct undergoes a distinctive thickening that presages the emergence of the UB. This thickening is evidence of a pseudo-stratification of epithelial cells in this region, which is driven, at least in part, by signals from the metanephric mesenchyme. , It is from this thickened region that the UB is generated in a process mediated in large part by cell movements orchestrated by Ret / Gdnf signaling (see Fig. 93.2A ). In a series of elegant experiments analyzing chimeric mice composed of a mixture of Ret + and Ret − cells, it was found that the formed UBs were composed principally of Ret + cells, which assembled in the very tip of the emerging bud. Further evidence supporting a role for Ret signaling in this process was provided by complementary experiments in which cells with heightened Ret signaling (by loss of a downstream regulator Sprouty) were added and which preferentially occupied the UB tip in competition with wild-type cells. Even in wild-type nephric ducts there exists a considerable range in Ret activation, as assessed by surrogate markers of pathway activation, so it seems likely that a similar “sorting” mechanism is employed to generate the UB.
The downstream targets of Ret signaling are activated by a range of other receptor tyrosine kinases, raising the possibility that cell signaling pathways activated by growth factors other than GDNF might contribute to UB outgrowth. Evidence for such a pathway is provided by the observation that UB outgrowth and subsequent development in mice lacking Ret or Gdnf can be rescued by the deletion of Spry1 (see Fig. 93.2A ). A prime candidate for such an alternate signaling molecule is fibroblast growth factor 10 (FGF10), which is normally expressed in the metanephric mesenchyme and plays a role in branching morphogenesis in the kidney and other organs. Indeed, deletion of Fgf10 in a Spry1/Gdnf null background blocks UB outgrowth, indicating that FGF signaling most likely acts to facilitate UB outgrowth by impacting on common cell signaling pathways downstream of a number of different receptor tyrosine kinases. Whether there are further contributors to this process and which exact downstream signaling pathways are involved remain to be fully defined. Having initiated outgrowth, the UB then enters into a process of branching morphogenesis, largely driven by bifurcation events. Considerable evidence, particularly from live imaging in organ culture, suggests that this process is mediated by increased cell division in the tips. Some cells are then maintained in this domain, whereas others remain in the “trunk,” and the tip pushes into the surrounding metanephric mesenchyme. These choices in cell fate are reflected in heterogeneity in gene expression within the tip progenitor cell niches. Although the evidence for lateral branching from established ureteric trunks in the mouse kidney is relatively scarce, it is clear that, at least in culture, trunk cells do remain competent to initiate new branch formation in response to exogenous signals.
A number of members of the Wnt gene family are also dynamically expressed during metanephric kidney development. One of these in particular, Wnt11, seems to be intimately associated with UB outgrowth. Although it occurs in a number of other organ systems, Wnt11 expression in the kidney is notable because it is restricted to UB tips (see Fig. 93.2A ). When exogenous sources of GDNF are focally added in cultured kidney, corresponding increases in Wnt11 expression were detected, , suggesting that the gene is a downstream target of GDNF signaling. Engineered loss of Wnt11 results in renal hypoplasia and concomitant decreases in Gdnf expression, suggesting a positive, autoregulatory feedback loop between Ret/GDNF and Wnt11 that is required for maintaining the program of branching morphogenesis (see Fig. 93.2B ).
Having “invaded” the neighboring metanephric mesenchyme, the UB begins to undergo a process of branching, which essentially establishes the future collecting duct network of the adult kidney. None of this epithelium will contribute to the nephrons. In the mouse, the UB undergoes approximately 12 to 13 branching events, although its extent is determined to some degree by the position of the tip in the organ (the anterior and posterior poles have greater depth). In humans the situation is similar with a proposed 15 branch generations giving rise to the adult organ.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here