Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The development of the hypothalamus-pituitary-adrenal (HPA) axis begins early in gestation. Corticotropin-releasing hormone (CRH) is synthesized and secreted into the hypophyseal portal circulation by the paraventricular nucleus of the hypothalamus. CRH acts on corticotroph cells in the anterior pituitary gland to regulate the secretion of adrenocorticotropic hormone (ACTH). ACTH, in turn, stimulates cortisol secretion from the zona fasciculata of the adrenal cortex. ACTH is also an important stimulus for adrenal gland development and proliferation. The regulation of the secretion of these hormones is not fully established until late gestation, as demonstrated by the hormone levels seen in preterm neonates. This chapter reviews the basic development and regulation of the HPA axis, its interplay with the maternal HPA axis during fetal development, and the impact of fetal programming on the risk of chronic illnesses later in life.
Throughout pregnancy, significant changes in both the maternal and fetal HPA axis play crucial roles in the development and maturation of the fetus.
The hypothalamus develops from the ventral diencephalon and is differentiated by 9 to 10 weeks of gestation in the human fetus. The anterior pituitary derives from the Rathke pouch, an invagination of oral ectoderm, and is present at approximately 5 weeks gestation. The neurovascular link between the hypothalamus and the pituitary is evident as early as 11 weeks of gestation. By 12 to 13 weeks of gestation, the fetal hypothalamus shows immunoactivity and bioactivity of CRH. Therefore the fetal hypothalamus can provide the stimulus for ACTH secretion from the anterior pituitary starting from early in the second trimester. However, based on the hormone levels seen in preterm infants, the regulated axis of hypothalamic CRH, ACTH, and cortisol secretion (HPA) may not be fully developed until late gestation.
CRH is a 41–amino acid neuropeptide synthesized in the paraventricular nucleus of the hypothalamus. , CRH is secreted, along with arginine vasopressin (AVP), into the hypophyseal portal blood and regulates the release of ACTH from the anterior pituitary gland. CRH acts by binding G protein–coupled receptors located on corticotroph cells of the anterior pituitary gland, leading to the activation of adenylate cyclase resulting in increased levels of intracellular cyclic adenine monophosphate (cAMP). The induction of cAMP activates protein kinase A, which ultimately stimulates the secretion of ACTH from the corticotroph cells.
Studies in fetal sheep reveal that there is a progressive elevation in CRH that is particularly notable during the second half of gestation. The expression of hypothalamic CRH messenger RNA (mRNA) during this time appears to be regulated by a glucocorticoid-mediated negative feedback signal, as is the case postnatally. However, the sensitivity of this feedback may decrease with increasing gestational age. This could explain how, despite the presence of high levels of cortisol that stimulate parturition, CRH-stimulated ACTH secretion persists in late-gestation fetuses.
In addition to the central nervous system, CRH is produced in peripheral sites, including the adrenal medulla, ovaries, uterus, and placenta. Placental expression of CRH is unique to primates and, as a source of both maternal and fetal serum CRH, has an important role in the development of the placenta and fetus and the progression to parturition. , Placental CRH increases exponentially during pregnancy, with expression increasing 100-fold during the last 6 to 8 weeks of pregnancy. CRH from the placenta stimulates the production of fetal ACTH, leading to the production of dehydroepiandrosterone (DHEA) and cortisol by the fetal adrenal gland. , Fetal DHEA serves as the predominant precursor for the synthesis of estradiol by the placenta, which increases throughout the course of pregnancy up to the time of birth, when the rise in estrogen, particularly in amniotic fluid, promotes myometrial contractility. This is essential because the primate placenta does not express significant levels of 17α-hydroxylase-17,20-lyase, which is required for conversion of progesterone to estrogen, as is seen in the placenta of other mammals. In human pregnancies, the rise in estrogen comes from the conversion of DHEA secreted from the fetal adrenal.
The rise in placental CRH during pregnancy increases cortisol from both the fetal and maternal adrenal glands. In turn, placental CRH is paradoxically stimulated by cortisol such that a positive feedback system is created ( Fig. 143.1 ). The surge in fetal cortisol secretion that precipitates parturition is crucial in fetal organ maturation, preparing the fetus for extrauterine life. Cortisol is of particular importance to maturation of the fetal lungs, as indicated by the frequent occurrence of respiratory distress syndrome in preterm infants and the risk of which is reduced by antenatal corticosteroids. Placental CRH stimulation of both cortisol and DHEA tethers the effects of cortisol on fetal organ maturation to the timing of parturition. ,
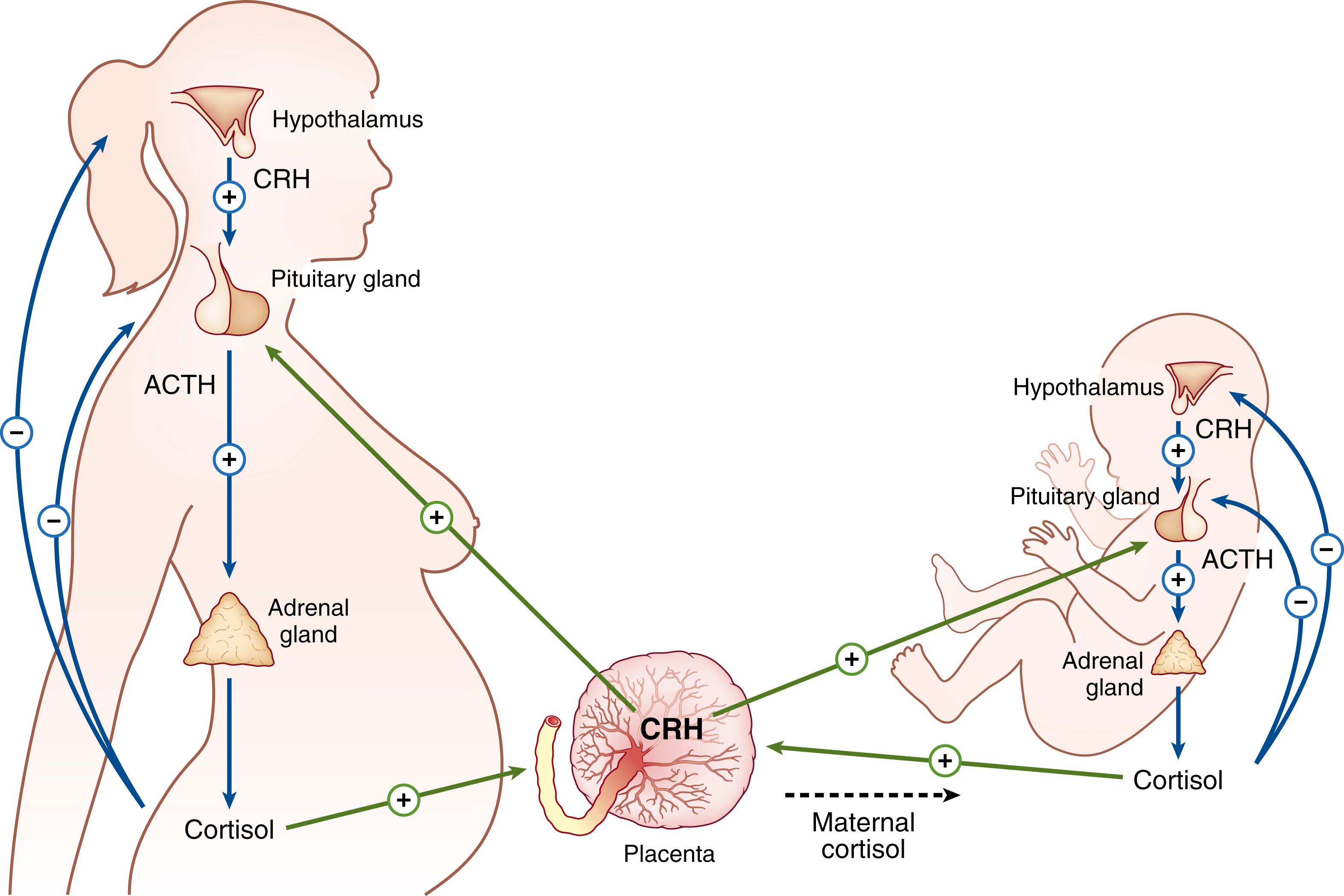
Increasing placental CRH contributes to the rise in maternal serum CRH. , Maternal plasma levels of CRH increase steadily in the second trimester, reaching up to 1000-fold above the levels of nonpregnant women. , However, abnormally elevated maternal CRH levels are associated with complications during pregnancy, including preterm labor, preeclampsia, intrauterine growth restriction (IUGR), and postpartum depressive symptoms. , , , High levels of cortisol from prenatal maternal stress can stimulate placental CRH secretion, leading to premature birth. Together, these clinical findings suggest that the timing of birth can be altered by modulation of the HPA axis.
Maternal plasma ACTH levels increase moderately during pregnancy, with corresponding increases in cortisol concentrations. , By 16 weeks gestation, ACTH is detectable in fetal blood, and studies report that levels of ACTH increase slightly as gestation progresses. , However, the extent of the increase in ACTH during pregnancy is disproportionate to the massive increase in circulating placental-derived CRH. ACTH is synthesized and secreted by corticotroph cells in the anterior pituitary gland, and it is a hormone end product of its precursor, proopiomelanocortin (POMC) ( Fig. 143.2 ). Depending on the site of origin, POMC is converted, through a series of enzyme-induced cleavages, to various peptides. In the anterior pituitary, the enzyme prohormone convertase 1/3 acts on POMC to form pro-ACTH and β-lipotropin. Pro-ACTH is processed further to yield other peptides, including ACTH and α-melanocyte-stimulating hormone (αMSH). The action of αMSH, through the melanocortin-1 receptor (MC1R), stimulates production of melanin, resulting in darkening of skin cells. This explains why individuals with primary adrenal insufficiency, such as Addison disease, develop hyperpigmentation. The loss of negative feedback from cortisol results in excessive production of ACTH along with other POMC peptides, including αMSH. It is also likely that the precursors of αMSH, including POMC, at high enough concentrations can act at the MC1R to cause hyperpigmentation.
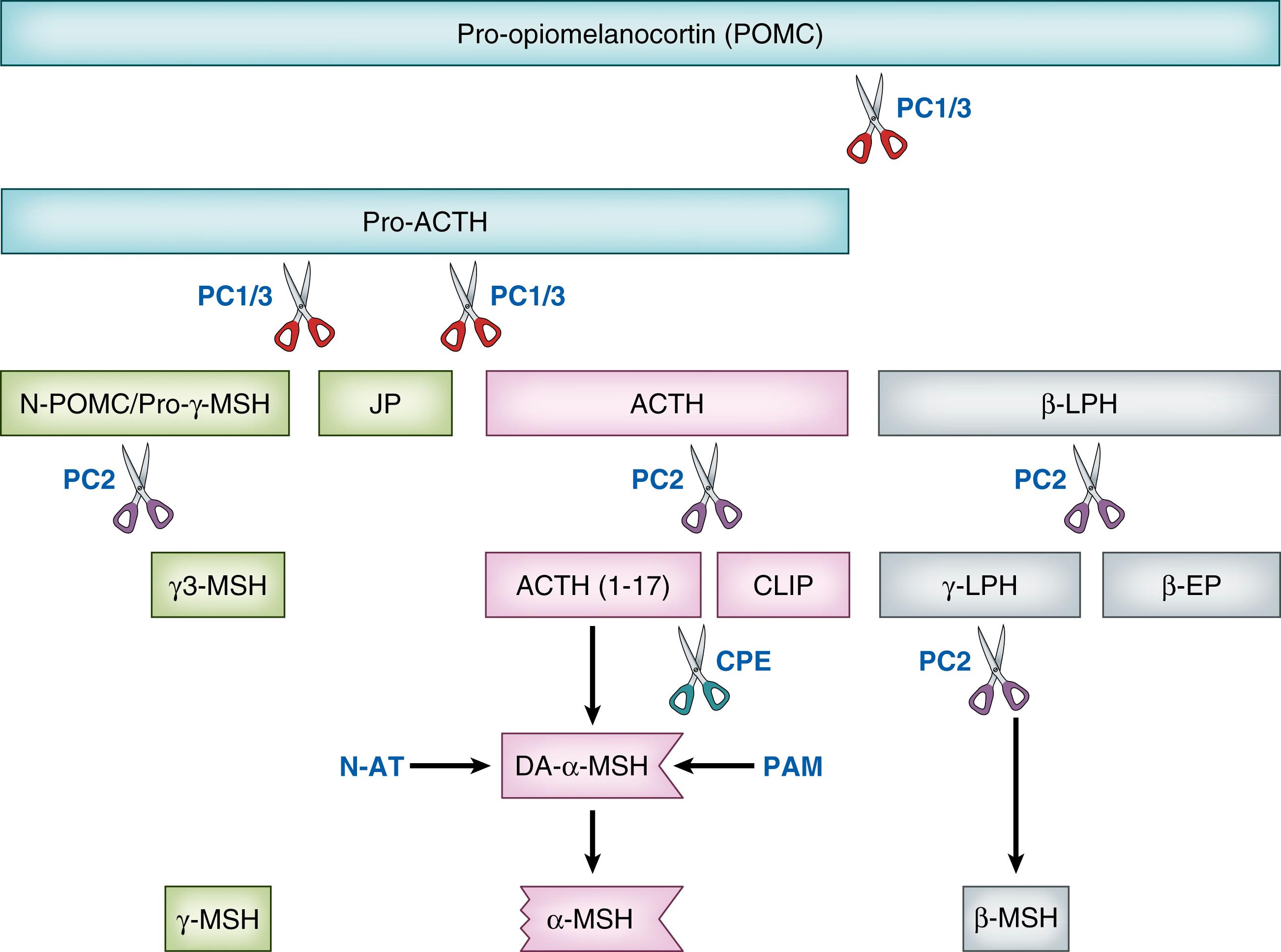
The action of ACTH occurs when the hormone binds to its receptor, the melanocortin-2 receptor (MC2R), located in all zones of the adrenal cortex. The primary site of ACTH action is in the zona fasciculata, where binding results in activation of the adenylate cyclase system, which induces the synthesis of cortisol. , Cortisol has negative feedback on its own secretion by fast inhibition of ACTH secretion and delayed inhibition of CRH and ACTH production. The stimulatory actions of ACTH at the fetal adrenal are also inhibited by other POMC peptides that contain the ACTH sequence, namely, POMC itself and pro-ACTH. Consequently, the precursor peptide levels in the adrenal may be as important as levels of ACTH. Immunoreactive ACTH, as measured by some assays, may reflect levels of both ACTH and the precursors.
ACTH is required for normal structure and function of the adrenal cortex. In mice with complete absence of POMC, the adrenal glands fail to proliferate, resulting in atrophic adrenals. High-dose replacement of ACTH in these mice rescues the adrenal glands in terms of weight, morphology, and cortisol secretion, but this may be due to hypertrophy of the zona fasciculata, not full regeneration of the glands. Similarly, human babies born with agenesis of the pituitary gland or hypopituitarism often have atrophic or malformed and unresponsive adrenal glands. In addition, the presence of excess exogenous or endogenous glucocorticoids suppresses ACTH secretion and can also cause atrophic and hyporesponsive adrenal glands.
Pituitary responsiveness to CRH increases during development, resulting in stimulation of ACTH secretion. Interesting, CRH appears to be the major driver of ACTH secretion in early fetal development, whereas responsiveness to AVP increases later in gestation, corresponding to increasing fetal cortisol levels. This association suggests that cortisol levels feedback to influence the CRH-ACTH axis in the pituitary and brain during fetal development. Consistent with this, the receptor for cortisol (glucocorticoid receptor [GR]) is expressed in the fetal pituitary, hypothalamus, and hippocampus as early as midgestation. Immediately preceding parturition, there is an increase in ACTH levels. This surge is paradoxical to usual feedback mechanisms because cortisol levels are simultaneously elevated. Although unexplained, it is important to keep this process in mind when interpreting laboratory values in samples taken during this time.
ACTH stimulates synthesis of glucocorticoids in the adrenal cortex that are then released into the circulation to reach most cells in the body. Cortisol is the primary glucocorticoid generated in humans. After the neonatal period, basal secretion of ACTH and cortisol occur with a circadian rhythm, rising prior to waking and peaking approximately half an hour after waking in most diurnal individuals living with wake/sleep patterns that are concordant with the approximately 24-hour light/dark cycles of the planet. The precise timing of when this circadian rhythm develops remains undefined, and there is variability between individuals that are likely driven by diversity in when consistent sleep patterns develop. However, it appears to take months to develop and sustain a consistent circadian HPA axis pattern, and therefore measuring morning ACTH and cortisol levels in newborns is unlikely to be informative of the status of the HPA axis, as it is when this is done later in life. Furthermore, secretion of cortisol into the circulation is pulsatile and rapidly responsive to changes in the systemic environment such as stress and hypoglycemia. The physician must synthesize all of these factors when interpreting test results of glucocorticoid levels.
Glucocorticoids are so termed because of their major actions to increase plasma concentrations of glucose. This occurs by their induction of the transcription of the genes encoding the enzymes of the Embden-Meyerhof glycolytic pathway and other hepatic enzymes that divert amino acids, such as alanine, to the production of glucose. Virtually all of these actions are mediated through GRs, which are found in most cells. However, despite the initial simple appearance of the hormone-receptor relationship, receptor bound glucocorticoids exert surprisingly diverse context and cell-specific activities. Recent advances in the field have identified a variety of mechanisms by which this is accomplished, including context-specific allosteric changes in the receptor conformation that drive the formation of distinct transcriptional regulatory complexes. This can be observed at the molecular level by comparing RNA expression profiles across different tissues and cell types in response to the same glucocorticoid signal. This plasticity has important physiologic implications, serving as the underpinning mechanism for the different tissue responses to glucocorticoids, including the promotion of visceral adipose tissue expansion while simultaneously inhibiting growth of muscle and bone.
The GR was the first nuclear hormone receptor to be cloned, which revealed a series of functional domains in the protein that turned out to be prototypical for the nuclear receptor family. In particular, the ligand-binding domain (LBD) is located at the C-terminus of the protein, which is also a region for interaction with co-activator proteins. The LBD of the GR is particularly highly homologous to the mineralocorticoid receptor (MR). Subtle structural differences between MR and GR confer specificity for aldosterone binding to MR, but cortisol is able to bind to both receptors. Sensitive tissues, such as the kidney, express the enzyme 11-beta-hydroxysteroid dehydrogenase type 2 (11βHSD2), which converts cortisol into inactive cortisone, protecting MRs from being activated by cortisol. Other domains identified in the GR that are conserved across nuclear receptors include the DNA-binding domain (DBD), which is centrally located in the GR protein. The DBD facilitates receptor dimerization and also contains two zinc-finger motifs that can bind to the major groove of DNA. The N-terminal domain of GR is the least conserved and enables interactions with coregulators.
Unbound GRs exist predominantly in the cytoplasm of cells. When glucocorticoid levels increase, more GRs in cells become bound by hormone and translocate into the nucleus. Rather than being a passive process, the binding of glucocorticoids induces conformational changes in GR that expose nuclear localization motifs, promoting facilitated trafficking of the hormone-receptor complex into the nucleus. Once in the nucleus, the action of glucocorticoids/GR occurs by (1) occupancy of defined sequences of DNA designated as glucocorticoid response elements (GREs), (2) context-specific interactions with cofactors and coregulators, and (3) engagement with transcriptional machinery. The combinatorial sum of these actions modulates the expression of target genes. Glucocorticoid/GR access to GREs in the DNA can be gated by a variety of factors, including chromatin structure, contributing to cell-specific activity of glucocorticoids. Similarly, posttranslational modifications of the GR further refine glucocorticoid action on target genes. Together, these variables enable context and cell-specific glucocorticoid activity as well as a capacity for fine-tuned modulation of the transcription levels of target genes in response to dynamic physiologic needs.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here