Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The stomach plays a number of roles in the digestive process and in host defense. Not only does the stomach serve as a reservoir for ingested foods and an important site of digestion; it is also exposed to a wide variety of swallowed bacteria, fungi, viruses, and parasites, and as such, it has a role in both innate and adaptive immunity. These functions are performed, in part, by a number of secretory processes, and the production of gastric acid is unique among them. The acidic environment created in the stomach also provides protective functions, such as gastric mucus and trefoil production, which require additional secretory activities.
The transition from the fetus to the newborn is accompanied by a rapid maturation of the secretory capacity of the stomach, but these processes develop in an asynchronous manner. In this chapter, we review the developmental biology and the developmental physiology of gastric secretory activity. Much of this information is derived from the experimental examination of either nonmammalian or lower mammalian species, so how this relates to human gastric development is still not entirely known.
The stomach begins as an outpouching of the foregut at 4 weeks’ gestation. It undergoes a 90-degree rotation at 8 weeks that establishes the left-sided greater curvature. Stomach elongation occurs at variable rates, with the greater curvature forming from more rapid growth along the dorsum of the stomach. The combined effects of rotation and differential growth result in a transverse orientation from the upper-left quadrant to the midline. The fixation of the stomach is at two points, the gastroesophageal and gastroduodenal junctions, which provides considerable mobility. The final J-shaped appearance of the stomach can be seen at 22 weeks’ gestation. The innervation of the stomach by the vagus nerve reflects the rotational event, with the right vagus innervating the dorsal (right) wall and the left vagus innervating the anterior (left) wall.
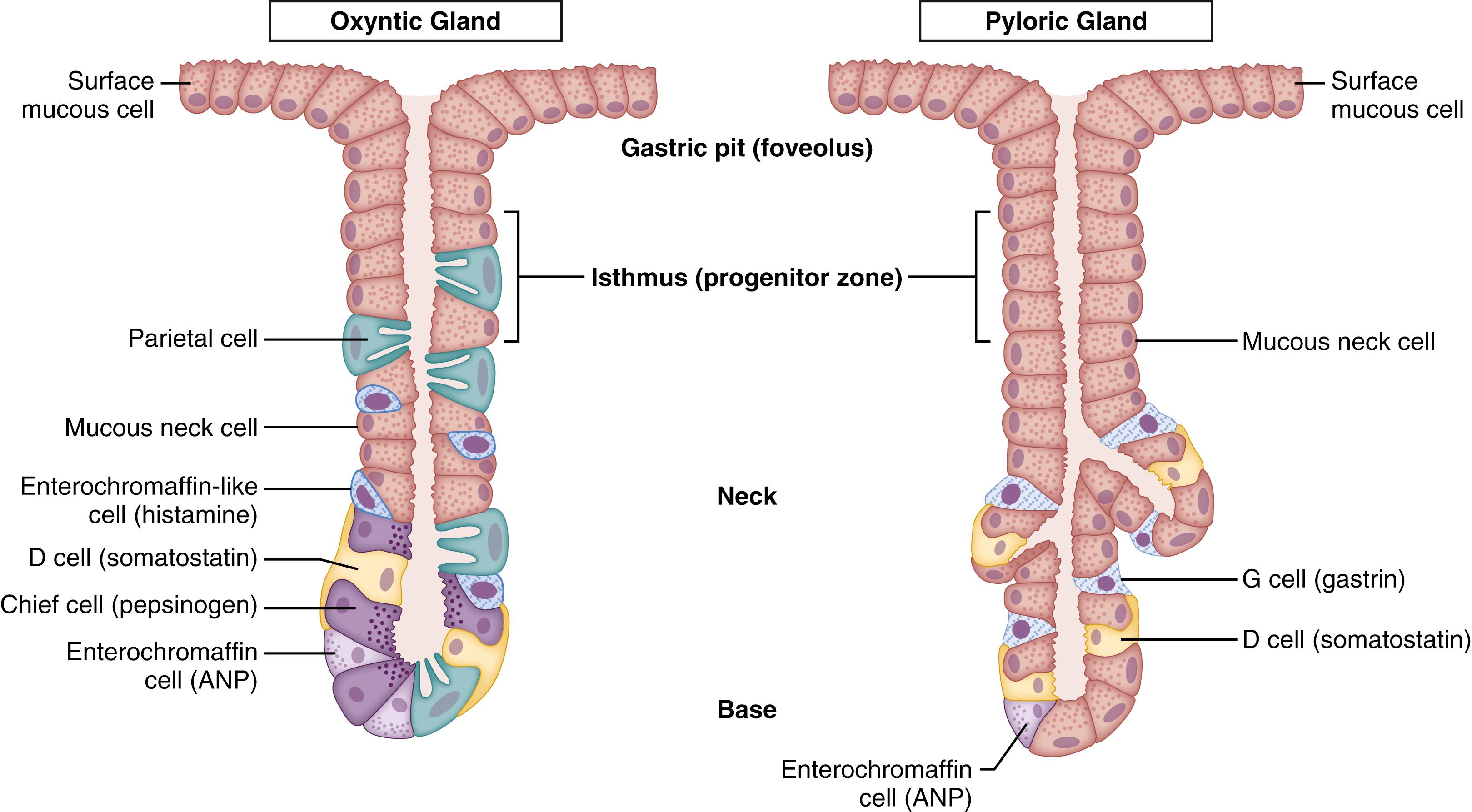
The stomach can be divided into three anatomic regions or two functional zones. From an anatomic perspective, the proximal two-thirds of the stomach consists of the fundus and body, whereas the distal third is referred to as the antrum. The two functional zones refer to the oxyntic (acid) glands located primarily in the fundus and body (which constitute 80% of the glandular stomach) and pyloric glands, which make up the remaining 20% in the distal part of the stomach (antrum). The hallmark of the pyloric gland is the G or gastrin cell ( Fig. 83.1 ). Glandular development is seen with the short gastric pits identifiable at 6 to 8 weeks’ gestation. Definitive gastric glands are evident by the fourth month of gestation, with maturation continuing to the fifth and sixth months.
The processes involved in stomach development are complex and still incompletely understood. There is exquisite spatial and temporal control of gene expression during development, and cell-to-cell contact (epithelial-to-mesenchymal transition) also plays a role in regionalization and elongation. , Many of the genes identified during development are shared between the stomach and the small intestine, with a small subset that is specific to the stomach. Some of the more important signaling pathways involved in organ development and regionalization include sonic hedgehog, Indian hedgehog, bone morphogenetic protein, Wnt signaling, and fibroblast growth factor pathways. This represents only a fraction of the genes and transcription factors that are expressed during organ development ; the relative importance of each remains to be defined. There does not appear to be transcription factors that function solely in stomach development, thus a “master” regulator has not been discovered. The expression of genes is a critical factor in organogenesis, but what is also clear is that there is a precisely timed and orchestrated procession of gene expression and gene repression that has to occur for both the stomach and the intestinal tract to develop normally.
All gastric epithelial cells are thought to arise from a common progenitor cell located in the gastric gland, , which is supported by studies in murine species. , Originally, gastric progenitor cells were thought to be located only in the isthmus, but cell marker studies reveal that they may be located in different areas of the gland and that these cells can be mobile. These studies also suggest that some progenitor cells may give rise to all the various types of gastric glandular cells, whereas others may be directed to more restricted lineages. The various mucosal cell types of the stomach express different transcription factors that control genes for cell type differentiation and function.
The gastric mucosa contains glands that are composed of secretory epithelial cells (see Fig. 83.1 ). The major cell types in the oxyntic mucosa are parietal cells, chief cells, and mucous neck cells. Parietal cells contain the machinery for acid secretion and are the most abundant secretory cell in the stomach. Mucous neck cells secrete a viscous glycoprotein to help maintain the integrity of the gastric epithelium, whereas chief cells are responsible for the production of pepsinogen, which is the inactive form of pepsin. Additional secretory cells in the glands include histamine-producing enterochromaffin-like (ECL) cells, somatostatin-producing D cells, and enterochromaffin cells that produce a variety of regulatory peptides.
In fetal mice, gastric glands are present in the gastric epithelium at 18 days’ gestation without evidence of the acid-producing pump, H + , K + –ATPase. On day 19 of gestation, approximately 1 to 2 days before birth, parietal cells are observed along with α and β subunits of H + ,K + –ATPase. In humans, parietal cells are present, marked with positive immunostaining of H + ,K + –ATPase, as early as 13 weeks’ gestation. At this point in development, parietal cells are found throughout the entire stomach, but by the time of birth, parietal cells will disappear from the antrum, creating a transition zone from the body to the antrum. However, in approximately 20% of adults, parietal cells can be found throughout the stomach extending to the pylorus.
Gastrin-producing G cells in the pyloric mucosa are detected in the fetus as early as 12 weeks’ gestation and are located only in the antrum, , and their numbers increase with gestational age. Neonates exhibit higher serum gastrin levels than what is considered normal in adults, and this “physiologic hypergastrinemia” continues in the first 2 months of life. , This is thought to be due to the relative unresponsiveness of parietal cells to gastrin stimulation during this period of time (as discussed later).
In rats, histamine-producing mature ECL cells do not appear until after birth. However, histamine immunoreactivity is observed in the oxyntic mucosa at day 18 of gestation. On postnatal day 21, the number of histamine-immunoreactive ECL cells approaches adult levels. In humans, ECL cells are first observed at week 13 of gestation. D cells first appear in humans at 10 weeks’ gestation, although somatostatin-immunoreactive cells are noted as early as 8 weeks’ gestation. ,
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here