Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Assessing therapeutic safety and efficacy of a drug/device or technology requires carefully designed trials that are sufficiently powered to detect differences in outcomes.
International trials can help reduce the time required for the study and improve generalizability of the findings.
Variations in regulations for clinical trials between jurisdictions can significantly prolong the time required to conduct the trial.
Anticipating challenges by obtaining local expertise and buy-in from collaborators are keys to the success of international trials.
In the past decades, the development and the study of drugs, technology, and interventions has moved from national and regionally centered activities to a global collaborative effort. This shift has occurred in all areas of medicine, including neonatology. Assessing therapeutic safety and efficacy of a drug/device or technology requires carefully designed trials that are sufficiently powered to detect differences in outcomes. This can be a particular challenge when studying interventions for a rare genetic disease, when the disease prevalence is relatively low (such as neonatal hypoxic ischemic encephalopathy), or when outcomes are rare. To complete a trial in a timely matter and recruit sufficient patients in a shorter time frame, multicenter, international trials have increased in the past 2 decades.
Although international trials enhance external validity of the findings, there are several issues that need to be considered before embarking on such trials. These include variations in population demographics, racial and ethnic variation in propensity for complications, genetic differences between populations, and country-specific variations in health services organization. , Moreover, variations in care practice between countries can be a significant issue when it comes to evaluating therapeutic interventions, because differences in co-interventions could be extremely challenging. The desire to enhance the generalizability of a trial's findings and the need for larger sample sizes recruited over short periods of time have led to an increase in international collaborations. An international trial is a clinical trial that is conducted in two or more countries and uses a common study protocol. International trials can be completed faster and more efficiently than even a multicenter trial in a single country. International trials can also contribute to our knowledge base from a global health perspective when certain types of trials are conducted in countries that would not otherwise have access to a specific intervention or health program. Whether an international trial involves low- or high-income countries, it requires careful consideration and planning. This chapter focuses on specific opportunities and challenges that arise when conducting research in more than one country and how to address them ( Fig. 99.1 ).
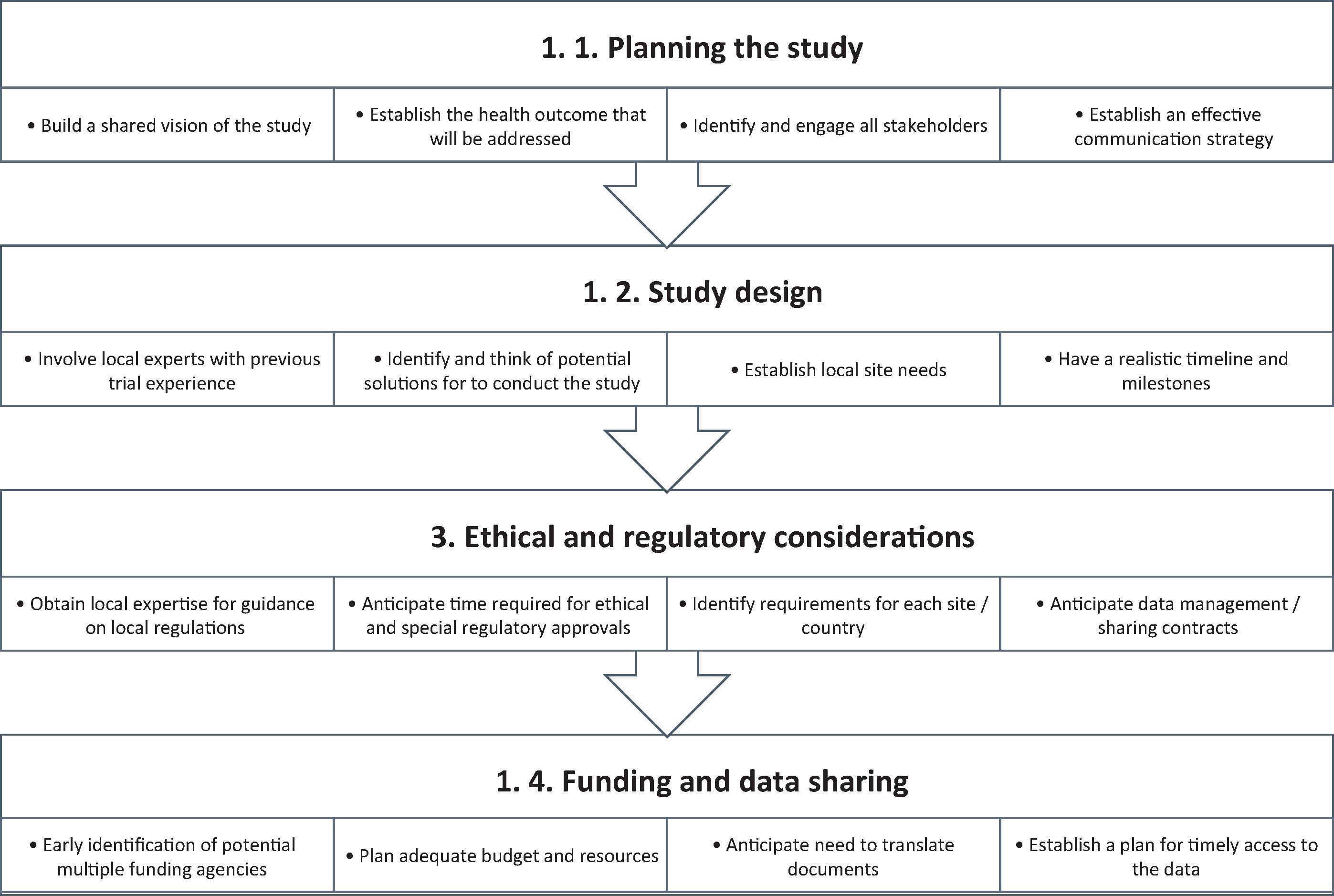
Careful planning of the trial is the most crucial initial step. Establishing a common understanding with potential collaborators about the health impacts of the disease and how the intervention can potentially improve a process or outcome or the health of participants is an essential first step to obtain buy-in to move forward. During the planning phase, careful attention must be paid to having clearly predefined study objectives that address a primary scientific question, the selection of appropriate subjects, the ability of the study design to appropriately address the research question, and having measurable and clinically important outcomes.
Once the need for a trial has been established, designing the trial is the next opportunity to have co-investigators actively contribute and continue to build engagement. This inherently leads to changes in the various design elements of the study, but this should be viewed as a unique opportunity to consolidate buy-in and participation of site investigators. Multicenter trial planning can be complex due to organizational differences between units. Mapping out how an intervention will be implemented in each unit is critical to troubleshoot and anticipate hurdles. International trials have added complexity due to between-country variations in roles of healthcare professionals, standards of care, cultural practices, and regulations that may impact the implementation of the protocol. Having local experts in trial design in each country can provide anticipatory guidance.
During the study design, the selection of participating sites will usually depend on disease prevalence at the institute, local expertise, availability of research infrastructure including administrative staff and clinical staff, a track record of implementing similar trials, and the willingness of the center to participate. Practical aspects of such organization include a meeting of potential site investigators either face to face or via use of technologies (video/teleconferencing). During these meetings, the principal investigator(s) should explicitly list the time and resources required to participate to help potential sites assess their capacity to enroll.
Early involvement of all stakeholders in the study design and during each and every step of the preparation is the most important consideration. To achieve effective engagement, establishing effective communications is key. Successful communication strategies include regular tele/videoconferences, in-person meetings if possible, and regular electronic communications by e-mail or newsletters. , The essence of success will include incorporation of views from all stakeholders to the extent possible and feasible. Supporting and creating an environment for open dialogue will lead to development of a culture that values and encourages critical thinking about the quality of the trial. For the sites that are passive participants, actively seeking feedback would uncover some issues that would best be addressed in advance.
It is relatively easy to conduct a single-center trial, from the perspectives of ethical and regulatory oversight. However, when a trial extends to either multiple centers within one jurisdiction or country or internationally within multiple jurisdictions and regulatory organizations, the complexity increases immensely. A majority of countries have their own agencies for oversight and regulations and guidelines for conducting clinical trials (e.g., the U.S. Food and Drug Administration, Health Canada, the European Medicines Agency, the UK Medicines and Healthcare Products Regulatory Agency, etc.). Regulations for the conduct of clinical trials refer to the rules and requirements for ethical and safe conduct of clinical trials.
To conduct clinical trials, investigators must obtain approval from an ethical board, also known as an institutional review board, research and ethics board, independent ethics committee, or ethical review board. Ethics boards are meant to apply ethical and regulatory frameworks that aim to minimize risks, have an optimal risk-benefit ratio, ensure informed consent is obtained, ensure that patients are recruited and compensated fairly, protect privacy, and ensure a methodologically rigorous approach. , Three basic ethical principles guide ethics boards: respect for participants, beneficence, and justice. The ethics board is usually composed of a multidisciplinary panel of scientific peers, researchers, experts in bioethics and law, and representatives of the lay community. Typically, in the United States, a trial's coordinating center will obtain ethical board approval for the trial, and each participating center will need to obtain approval from their local ethical board before recruitment can start in their center. This process can vary based on the country.
Individual institutional ethics board review is based on the regulatory framework applied in the institution's jurisdiction. Some countries have attempted to harmonize these regulations to minimize variations. For example, in Europe, trials have to adhere to the European Union Clinical Trials Directive and European Union Good Clinical Practice Directive. However, interpretation and applications of these directives can vary. Several different aspects of regulations need to be considered in international trials, such as the ethics board review process, regulations regarding the intervention, data sharing, data monitoring, contracts, and consent for participants.
Despite similar frameworks and guiding ethical principles, each country, health region, and institution may have different ethics board requirements. This can mean having a single ethics board application for a single region or country or having to apply to the ethics board of each institution. For example, a single ethics board review is required for nationwide clinical trials in the United Kingdom, France, and Australia, whereas individual institution review is required in countries such as Canada and the United States.
There are currently efforts to streamline the approach in the United States with the proposed revisions to the Common Rule, which is the regulation that guides federally supported human research in the United States. Although the European Union Clinical Trials Regulation includes provisions for low-risk intervention trials and cluster randomized trials, the International Council for Harmonization of Good Clinical Practice guideline does not currently address these issues. , In Canada, attempts are underway to allow one ethics board to be the board of record; review from that ethics board will be acceptable to other ethics boards within the jurisdiction and would require only an administrative review. However, these are still local/regional initiatives, and national harmonization is lacking.
The variations in the ethics board review process can significantly affect the time required to obtain ethics board approval. For example, in a recent multicenter study in Canada involving 16 sites, the ethics board approval took a median time of 42 days (range, 4–443 days). The investigators reported that the main issues raised by the ethics boards were different interpretations of privacy rules and language of the consent.
Sufficient time must be allocated for the ethics board applications, considering the process will vary based on institutions despite being in the same country and operating under the same regulations. Variability in local requirements may include variations in assessment of the project itself, assessment of the level of risk of participation, privacy concerns, and the assessment of local resource availability to perform the research. , Strategies to mitigate delays include having collaborators with expertise in running clinical trials in each jurisdiction involved and consulting the ethics board at the time of trial design. It is often helpful to submit previous correspondence with the ethics board that has already granted approval along with a new ethics application for the same trial to another ethics board.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here