Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Since the dawn of plastic surgery, the lower abdominal region has always been a loyal provider of abundant well-perfused tissue. First used in pedicled and tubed flaps for distant transfers, the lower abdomen skin and fat was discovered to be an ideal material for breast reconstruction. That specific use of the rectus abdominis musculocutaneous flap was pioneered by Drever in 1977 and, a few years later, Hartrampf et al. showed that the skin island could be harvested transversally across the lower abdomen. The transverse rectus abdominis muscle (TRAM) flap was born and it eventually became the gold standard in autogenous breast reconstruction. Interestingly, the free TRAM was performed first in 1979 by Holmström but was not recognized for several years. The free TRAM eventually demonstrated better vascularity over its pedicled counterpart due to the vigorous blood supply brought in by the deep inferior epigastric system.
In the mid-1980s, following Taylor's landmark work on the vascular territory of the deep inferior epigastric artery, it became apparent that the lower abdominal flap could probably be perfused solely by a large periumbilical perforating vessel. That assumption was confirmed in 1989 when Koshima and Soeda published two cases of “inferior epigastric skin flaps without rectus abdominis muscle”. Allen and Treece and Blondeel et al. expanded the use of the deep inferior epigastric artery perforator (DIEAP) flap to breast reconstruction and raised the operation to a high level of technical refinement. The DIEAP flap quickly gained popularity, as it allowed the same advantageous reconstructive results as a TRAM flap but without the added morbidity of sacrificing an important muscle.
In the meantime, other surgeons transferred the same lower abdomen anatomic unit but instead used the superficial inferior epigastric vessels to vascularize the flap. Back in 1971, this was the basis for the first free flap ever reported in the literature. In 1976, Boeckx published his experience with six superficial inferior epigastric artery (SIEA) free flaps, most of them for facial reconstruction. Hester et al. demonstrated successful polyvalent use of the flap in 1984 and Grotting was the first to use the free abdominoplasty flap for breast reconstruction in 1991. The SIEA flap can provide ample skin and subcutaneous fat with minimal donor site morbidity as the abdominal musculature is not breached.
In this time of rapidly evolving science, perforator flaps constitute the latest milestones in the never-ending quest for the best possible result with the least cost to the patient. The DIEAP and SIEA flaps can provide excellent-quality autogenous reconstruction with minimum morbidity and should be considered as the golden standard by the contemporary surgeon.
The lower abdominal flap can be vascularized by either the deep or the superficial inferior epigastric artery. The superficial circumflex iliac artery only vascularizes the lateral part of the lower abdomen. By definition, the DIEAP flap is perfused only via one or more perforators of the DIEA.
The blood supply of the anterior trunk is founded on the presence of two epigastric arcades, each linking the external iliac artery and the subclavian artery through the rectus abdominis muscle. Caudally, the deep inferior epigastric artery (DIEA) originates from the external iliac artery just proximal to the inguinal ligament. After running superomedially for a short distance, it approaches the posterior aspect of the rectus abdominis muscle and divides into two main branches. These branches enter the rectus muscle and follow a cranial intramuscular course to feed the muscle and overlying skin. The cephalad portion of the epigastric arcade is made of the internal mammary artery, which arises from the subclavian artery. The internal mammary artery continues in the abdomen as the superior epigastric artery (SEA) to finally coalesce in a watershed area midway between the xiphoid process and the umbilicus. In this region, multiple anastomotic channels exist between the superior and inferior epigastric systems. Laterally, the epigastric arcade anastomoses with the terminal branches of the lower six intercostal arteries as well as with the ascending branch of the deep circumflex iliac artery.
It is evident that the DIEA is relatively more important than the SEA. First, the DIEA is larger, with an external diameter of 3.4 mm at its point of origin as compared with 1.6 mm for the SEA. Second and more importantly, the vast majority of cutaneous perforators are located in the periumbilical region, inferior to the watershed anastomotic zone, and therefore in the DIEA territory. The branching pattern of the DIEA is summarized in Figure 57.2 . There are muscular branches to the rectus abdominis, myocutaneous perforators with major contribution to the muscle and minor supply to the skin, and, conversely, perforators that are mainly directed at the skin. Obviously, the latter constitute the target vessels to vascularize a DIEAP flap. Occasionally, one can see perforators going around the edge of the rectus abdominis and not through the muscle itself. These pararectal fasciocutaneous perforators pass either medially just next to the linea alba or laterally from the lateral segmental vessels through the external oblique aponeurosis.
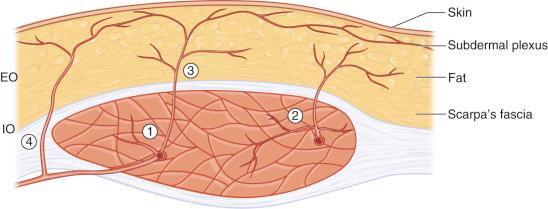
After piercing the anterior rectus sheath, the different perforators take on a variable course through the subcutaneous fat. Some ascend vertically to the overlying skin, whereas others will have a more horizontal trajectory. Perforators usually arborize in a fractal manner until they join the subdermal plexus to supply it. The subdermal plexus effectively constitutes an intricate network of microvessels spanning the entire abdominal skin and allowing innumerable redundant connections. Through the subdermal plexus, we find the second level of anastomotic channels between the source arteries. This is where perforators from the DIEA will meet cutaneous branches of the SEA, lower intercostal arteries, deep and superficial circumflex iliac arteries, superficial inferior epigastric artery, superficial external pudendal artery, and contralateral DIEA perforators. The subdermal plexus is the supporting vascular framework of lower abdominal skin flaps and the reason why the same skin/fat island can be perfused by either the deep or the superficial inferior epigastric system. In fact, the subdermal plexus can be conceptualized as an interface between the relatively scarce vessels originating from the deep fascia and the dense network of terminal dermal microvessels. It may act as a random equilibrating system that distributes blood evenly across surrounding cutaneous areas. If all vessels feeding into the subdermal plexus are ligated except for one perforator, the area of irrigated skin would be determined by the capacity of the perforator to provide pressure into the subdermal system. Therefore, the diameter and branching pattern of the perforator become key elements in defining the clinical territory of perforator flaps. Of course, further anatomic and physiologic studies are warranted to compare this theory to the angiosome and choke vessel concept in order to help elucidate the intricacies of skin perfusion.
The superficial inferior epigastric artery (SIEA) is the second important blood supply provider to the lower abdominal skin. The SIEA is a direct cutaneous vessel, originating from the common femoral artery 2–3 cm below the inguinal ligament in 17% of cases or from a common trunk with the superficial circumflex iliac artery in 48% of patients. The artery is absent or hypoplastic in 35% of specimens, in which case it is often substituted by a large ascending branch from the superficial circumflex iliac artery. The SIEA courses in the subcutaneous fat towards the umbilicus where it often anastomoses with large periumbilical perforating arteries.
deep inferior epigastric artery
Length: 16 cm (range 14–18 cm)
Diameter: 3.5 mm (range 2–4 mm)
After taking off from the external iliac artery, the DIEA travels superomedially in between the peritoneum and a very thin fibrous layer that is an extension of transverse fascia. In its lower portion, it gives off its first branches directed to the pubic bone, the lower rectus abdominis, and the pyramidalis muscle. A variable amount of peritoneal branches can emerge, the most cranial one providing vascularization to the umbilical stalk. The DIEA then enters the rectus muscle by passing anterior to the arcuate line. As it penetrates the muscle, the DIEA generally divides into two major branches. The lateral branch is dominant in 50% of cases and the medial branch in 7%. There is equal caliber of both branches in 15% and one central axis with multiple side branches in 28%. During their cranial course, the medial and lateral divisions of the DIEA give off several muscular and myocutaneous side branches. Overall, although small-caliber cutaneous perforators are numerous, there is an average of five perforators with a diameter of 0.5 mm originating from each DIEA. Sometimes, perforators as large as 1.5 mm can be encountered. Box 57.1 presents key points on the typical location of perforators.
More than 90% of the major perforators are located within a 6 cm radius lateral and inferior to the umbilicus.
There is a higher concentration of perforators in the middle and medial thirds of the muscle.
The largest periumbilical perforators take origin from the terminal branches of the DIEA and are often located within the lowest tendinous intersection of the rectus abdominis.
The lowest fifth of the muscle contains only sparse perforators and the veins are rarely of adequate size.
Medial and lateral pararectal fasciocutaneous perforators are often of small caliber.
The distribution of perforators on both sides of the abdomen is rarely symmetrical.
When the pedicle is dissected down to the lower lateral rectus border, a typical pedicle length of 10–14 cm is obtained, depending on the position of the perforator. Although this length is generally sufficient for the purpose of free tissue transfer, it can be increased by up to 4 cm if the pedicle is dissected to its origin. At this point, the diameter of the artery is 3–3.5 mm.
superficial inferior epigastric artery
Length: 5 cm (range 4–7 cm)
Diameter: 1.6 mm (range 0.75–3.5 mm)
After piercing the deep fascia, the SIEA passes superiorly and laterally in the femoral triangle, crossing the inguinal ligament at its midpoint and lying deep to Scarpa's fascia. The vessel continues on superomedially towards the umbilicus, penetrating Scarpa's fascia, well above the inguinal ligament to lie in the superficial subcutaneous tissue ( Fig. 57.3 ).
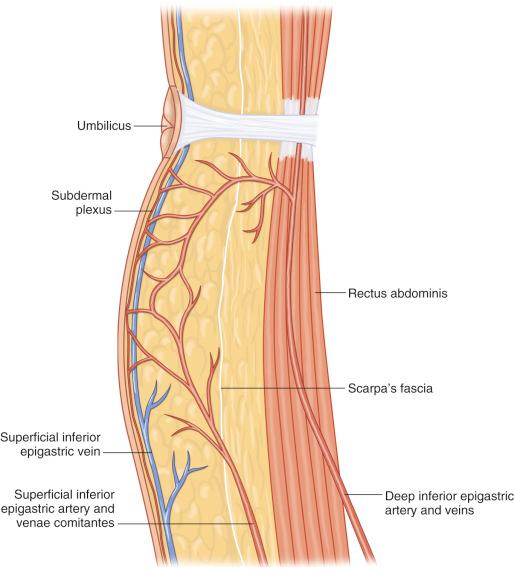
A recent anatomic study showed that the SIEA was identified in 72% of specimens, with an average diameter of 1.6 mm (0.75–3.5 mm) at the level of the inguinal ligament. In 58% of cases, the artery was present bilaterally. The length of the pedicle ranged from 4 cm to 7 cm.
superior epigastric artery
Length: 6 cm (range 4–8 cm)
Diameter: 2 mm (range 0.5–2.5 mm)
Secondary arterial inputs to the lower abdominal flap come from the SEA, lower intercostal arteries, superficial and deep circumflex iliac arteries, and superficial external pudendal artery. Since the DIEAP and SIEA flaps are elevated as skin islands isolated on their respective dominant pedicle, the minor contributors are ligated during harvesting of the flap. If indicated, however, a minor pedicle can be dissected and anastomosed in addition to the dominant pedicle to extend the territory of the flap.
The venous anatomy of the lower abdominal area is similar to the arterial anatomy in that the different venae comitantes closely follow their paired arteries to the proximal vessels. Indeed, the small veins perforating the anterior rectus sheath drain into the deep inferior epigastric veins (DIEV) and then on to the external iliac vein. Similarly, the SIEA possesses two venae comitantes closely related to it, which drain into the common femoral vein or occasionally into the saphenous bulb. However, these veins are often small and can even be absent. On the other hand, there is a constant and often large superficial inferior epigastric vein (SIEV) located medial to the SIEA and more superficial ( Fig. 57.3 ). The SIEV is not accompanied by an artery and is a tributary of the greater saphenous vein close to the fossa ovale. It actually drains an extensive polygonal network of subcutaneous veins in the anterior abdominal wall. There exist anastomotic connections between this superficial system and perforating veins from the DIEV. The SIEV is considered by certain authors to be the preferential drainage path of the abdominal panniculus in the normal, non-surgical condition.
deep inferior epigastric veins
Length: 16 cm (range 14–18 cm)
Diameter: 4 mm (range 3.5–4.5 mm)
superficial inferior epigastric veins
Length: 5 cm (range 4–7 cm)
Diameter: 4 mm (range 3–5 mm)
The lower abdominal skin has two primary outflow pathways: the deep and the superficial inferior epigastric veins. In most cases, the DIEAP flap is primarily drained via the DIEV. These paired veins have multiple ladder-like connections (H-bridges) and often unite just prior to their junction with the external iliac vein. However, there exists an inverse size relationship between veins from the deep system and the superficial veins. The SIEV network might actually be dominant over the deep system in some cases. When the SIEV is important in diameter, it should be preserved because the selected perforating vein(s) from the DIEV could be insufficient to drain the flap. Conversely, if the superficial veins have been interrupted by a previous Pfannenstiel incision, a delay-type phenomenon can occur, resulting in enlargement of the deep system veins. Of course, in the case of an SIEA flap, the SIEV constitutes the primary venous drainage system. In 18% of the cases, a large venous connection exists between the left and right SIEV over the midline and below the umbilicus.
the secondary venous drainage of the flap is composed of the venae comitantes of the SIEA, SEA, circumflex iliac arteries, and lower intercostals.
If the venae comitantes of the SIEA are large, they can be used to drain the flap. However, they are almost always <1 mm in diameter or even non-existent.
The motor and sensory innervation to the abdominal wall is provided mainly by the intercostal nerves 7–12 with some contribution from the iliohypogastric and ilio-inguinal nerves. As shown in Table 57.1 , the lateral muscles are supplied by all eight nerves, whereas the more medial rectus abdominis and pyramidalis are innervated solely by the intercostals. The sensory innervation to the abdominal skin is derived from the lateral and anterior cutaneous branches of the intercostal nerves giving rise to segmental dermatomes. A small skin area immediately superior to the pubis is supplied by the anterior cutaneous branch of the iliohypogastric nerve.
| Rectus Abdominis | Pyramidalis | EO/IO/TA | Cutaneous Innervation | |
|---|---|---|---|---|
| Intercostal nerves (T7–T11) | + | + | + | |
| Subcostal nerve (T12) | + | + | + | + |
| Iliohypogastric nerve (L1) | + | + | ||
| Ilio-inguinal nerve (L1) | + |
The DIEAP flap can be innervated using one of the sensory intercostal branches entering the panniculus on its way to the skin, usually in company with a perforator from the DIEA (see Fig 11.2, Fig 11.9, Fig 11.10 ).
After traveling in the plane between the internal oblique and the transversus abdominis muscles along with their respective intercostal arteries and veins, the mixed intercostal nerves T7–T12 pierce the lateral edge of the rectus sheath and then run on the posterior surface of the muscle for a short distance. Less frequent the mixed nerves enter the muscle at the lateral border or its anterior surface. Posteriorly, they enter the rectus abdominis usually at the junction of the middle and lateral thirds ( Fig. 57.4 ). At that point, they arborize into muscular motor branches to the medial and lateral muscle and a pure sensory branch that perforates the anterior rectus sheath together with the perforator vessels to supply the overlying skin. The SIEA flap is not used as an innervated flap because harvesting a sensory nerve entails an intramuscular dissection.
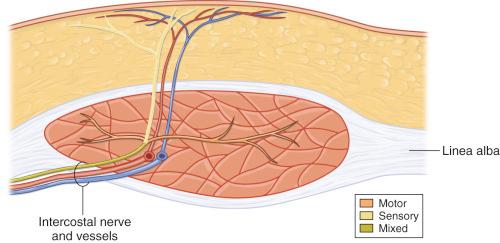
No muscle is included with these flaps.
The DIEAP and SIEA flaps consist solely of skin and fat from the lower abdomen. No muscle or fascia is harvested.
The DIEAP/SIEA flap essentially has all the advantages of the TRAM flap with few of its disadvantages. It provides generous amounts of well-perfused soft tissue and its complication rate is similar to other free tissue transfers.
The first and foremost advantage of the DIEAP/SIEA over the TRAM is its markedly decreased donor site morbidity. Since no muscle or fascia is sacrificed, the incidence of late hernias and abdominal bulges is minimized.
The problem of abdominal and umbilical asymmetry seen with the TRAM flap is also eliminated.
Postoperative pain is minimal and the hospitalization time is reduced, resulting in lower healthcare costs. Furthermore, patients can return more quickly to work and physical activities.
Because of the intramuscular dissection, the pedicle length obtained with a DIEAP flap is longer by several centimeters, translating into superior flexibility in the way the flap can be positioned at the recipient site, resulting in easier and better flap shaping.
Patients undergoing a DIEAP/SIEA flap reconstruction also benefit from an abdominal contour-improving procedure with a well-concealed scar.
The DIEAP/SIEA flap can be harvested with an additional contralateral SIEA pedicle and lymph nodes enabling a simultaneous lymph node transfer to the axilla.
As with other perforator flaps, the operation involves a certain learning curve and demands delicate dissection. The operation duration can be marginally longer than a comparable free TRAM flap, especially if the surgeon is not familiar with the technique.
On rare occasions, a state of venous congestion can occur after flap harvest as a result of the limited diameter of the veins. However, this situation is successfully treated with an extra venous anastomosis of the SIEV.
With a SIEA flap, the volume of tissue safely perfused can be significantly less than that of a DIEAP or TRAM flap.
The SIEA pedicle is rather short, making the insetting and shaping of the flap more difficult.
The SIEA is anatomically inconstant and can be absent in up to a third of cases.
The SIEA is particularly prone to vessel spasm.
Due to the pedicle dissection in the area of inguinal nodes, the SIEA flap has a higher rate of donor site seroma than DIEAP or TRAM flaps.
When contemplating elective microsurgical reconstruction with perforator flaps, appropriate patient selection is mandatory in order to insure a satisfactory outcome. During the patient's first visit, a complete history is taken and physical examination performed. Comorbid conditions and abdominal scars are noted. A previous full abdominoplasty or abdominal lipectomy is an absolute contraindication for a DIEAP or SIEA flap. If the peri-umbilical perforators are still intact after a mini-abdominoplasty, a DIEAP flap can still be considered. A past history of abdominal liposuction is a relative contraindication but if a DIEAP/SIEA flap is considered nonetheless, a preoperative duplex or angio-CT scan investigation of the cutaneous vessels will be required. So as to withstand the prolonged anesthesia associated with free tissue transfer, the patient should be in a good general state of health. Age is usually not an important factor but we prefer to offer autogenous microsurgical reconstruction to patients under the age of 80. Although perforator flap surgery is not contraindicated in obese patients, they are advised to lose weight before the operation, to limit the incidence of peri- and postoperative complications. Smokers are asked to refrain from smoking for at least 3 months before surgery. Use of aspirin, non-steroidal anti-inflammatory drugs, and herbal medications should be avoided for 3 weeks before surgery. Tamoxifen and any other drugs increasing thrombosis risk is stopped at 1 preoperative week.
During the initial consultation, selected patients also receive thorough explanations on the procedure, including possible complications, at both the donor and recipient sites. The usual course of reconstruction is discussed and typical expected outcomes are presented with the use of photographs. All questions are answered and the patient's motivation is carefully assessed.
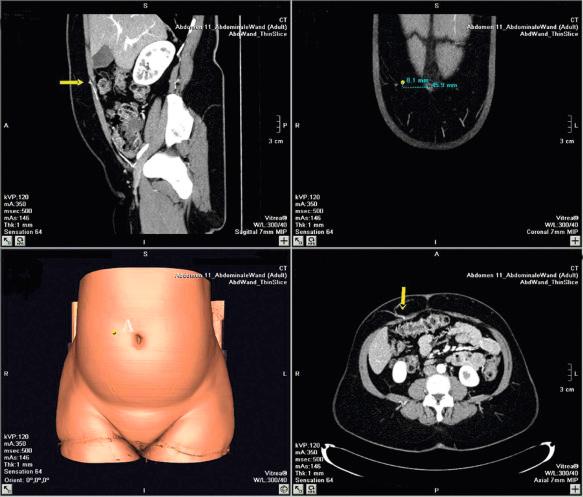
The preoperative investigation should include a study of the abdominal wall vascular anatomy. Although it is possible to harvest a DIEAP flap without prior knowledge of the exact position and size of the perforators, we believe it is much safer and faster to do so with a map of the perforators on hand. Therefore, angio-CT imaging is used routinely in our department in the planning of DIEAP flaps. This allows the creation of a 3-dimensional map of the perforating vessels with coordinates centered on the umbilicus. An alternative to angio-CT, duplex-Doppler ultrasound allows the location and diameter of the vessels to be evaluated plus the blood flow and arborization patterns. This provides the surgeon with invaluable information to help with the safe planning of a DIEAP flap in different individuals.
The status of the internal mammary vessels is also assessed at the same time, as this is our preferred recipient vessel in breast reconstruction. Alternatively, the perforator mapping can be done using a handheld Doppler; however, one should be aware that, although more accessible and less costly, this device generates more false-positive and false-negative signals and provides less detailed anatomic and functional vessel information.
The use of high-resolution multidetector spiral CT scan has replaced color duplex imaging in our practice. Contrast-enhanced CT scanning appears to be the modality of choice in preoperative investigation of perforating vessels. Although no flow information is obtained, it allows excellent three-dimensional understanding of the arterial and venous perforator morphology and the intramuscular course of the DIEA, DIEV and SEIV ( Fig. 57.5 ). A preoperative angio-CT can gain 60–90 min of operating time. More recently, the use of magnetic resonance angiography has been described for preoperative work-up of perforator vessels. Although great imaging can be obtained with this modality, routine use can be restricted because of accessibility reasons, cost and length of the procedure.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here