Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Hematopoiesis is regulated by cytokine-cell and cell-cell interactions. Cytokines are polypeptides secreted by many cell types either constitutively or after induction and are usually classified based on primary structures associated with their receptor extracellular domains. They regulate a large range of biologic functions, including hematopoiesis, immunity, and inflammation. In context of hematopoiesis, they have effects on proliferation, differentiation, survival/cell death (apoptosis), and cell movement/migration/induced mobilization. They can act in a paracrine fashion on closely adjacent cells, cells at a distance in the same tissue area, or other tissue areas sometimes by their movement from one tissue/organ to another tissue/organ. Cytokines can also work in an autocrine fashion. A rigorous definition of autocrine regulation is that the cytokine works within the cell that produces it, and either maintains the cytokine for internal cellular signaling/activity or can release it, upon which it can then act on the same cell that produced and released it. Autocrine regulation is a much-discussed mode of action, especially in leukemia and other cancers, but in reality it is very difficult to prove this concept of a cell. Many papers that have referred to autocrine production/action have not rigorously proven this concept, even in the case of leukemia or other cancers. Paracrine modes of action refer to the situation when proteins are secreted/released by a cell and then act on another cell, which is more easily proven and verified.
To modify an old proverb: “What is forgotten is doomed to be repeated without gain of new knowledge.” Many current investigators do not cite or even know of crucial past efforts in the field of how cytokines, chemokines, and other growth-modulating factors influence hematopoietic stem cell (HSC) and hematopoietic progenitor cell (HPC) cell biology and of hematopoietic cell regulation. Thus they unfortunately lose out on knowledge that could help their research. For this reason, this review references the older, as well as more recent, literature on cytokines/interleukins (ILs)/chemokines, other growth factors, and their effects on cell regulation. Although this includes an extensive list of references, it is not an exhaustive list.
After many years of intensive research going back to at least the 1960s, it is recognized that cytokines are widely pleiotropic, with numerous cases of redundancy in action and with many overlapping functions. In fact, there are few cytokines that have been shown to have only one cell and mode of action, including production/release of other growth modulating factors, and it is likely even these few such cytokines will eventually be shown to be pleiotropic in action/activity. Individual cytokines can act as agents that induce either positive and/or negative effects or, when used in combination, additive to synergistically positive and/or negative effects. These different actions are sometimes dependent on the target cells(s) of action and depend on how they are combined for in vitro and in vivo assessments. It is highly likely that combined effects of cytokines occur with great frequency during in vivo regulatory events and in context of the microenvironment niche in which they are produced/released (e.g., in the bone marrow [BM]). Moreover, a cytokine can induce the production/release of other cytokines that can then produce effects that were originally ascribed to the cytokine that started this cascading set of regulatory events. Only by studying effects of cytokines at the single isolated target cell level, with and without serum/plasma (to eliminate effects of growth modulating factors present in serum/plasma) can one begin to truly understand the initial target cell(s) and the action(s) of the cytokine(s) of interest. Thus reductionist approaches of unraveling cytokine cell action(s) are more frequently used, as is single-cell ribonucleic acid (RNA) sequencing (RNA-seq), which is used to assess intracellular events. However, ultimately, to fully and clearly understand hematopoiesis and its regulation, it is necessary to put data together in a total context of interacting cytokines and target cells to get a more accurate picture of what is happening at a physiologic and pathologic level. This is necessary to better use our knowledge for eventual enhanced clinical efficacy for treatment of both malignant (e.g., leukemia, lymphoma) and nonmalignant (e.g., preleukemia, myelodysplastic syndromes [MDS], and myeloproliferative neoplasms [MPNs]) diseases.
Cytokines act through specific cell surface receptors to activate intracellular events, with some receptors acting in concert with other receptors to elicit their regulatory effects. It is notable that as late as the mid-1970s not much was known about specific growth factors, other than that for erythropoietin (EPO), the red blood cell hormone, which was the first growth factor to be purified. Recombinant growth factors were not yet produced or known. Growth factors were identified by their purported actions present in crude preparations of cell or tissue extracts or in media conditioned by cells or tissues. The biologic activities attributed to these cell/tissue extracts or conditioned media could have been and, in fact, were in many cases due to combinations of proteins, and one had to rule out effects of confounding and contaminating agents such as bacterial lipopolysaccharide (LPS). In fact, this contamination of even “purified” protein products is still a confounding problem because LPS can stimulate release of cytokines. An example of these growth regulatory factors were the terms for these activities: colony-stimulating activity (CSA), colony-inhibiting activity (CIA), or colony-stimulating factor (CSF), even before it was known what these proteins were. Presently, through biochemical purification procedures and then later through recombinant deoxyribonucleic acid (DNA) technology, the proteins from these crude preparations responsible for these biologic activities were found to include, in addition to EPO, the CSFs: granulocyte-macrophage (GM)-CSF (also known as CSF-2), granulocyte (G)-CSF (also known as CSF-3), interleukin-3 (IL-3) (previously termed multi-CSF), macrophage (M)-CSF (also known as CSF-1), and thrombopoietin (TPO). The CIA molecules were later identified as lactoferrin (LF), H-subunit (H)-ferritin, and some of the ILs, interferons (IFNs), tumor necrosis factors (TNFs), transforming growth factors (TGFs), and a large number of other, now well-defined and characterized, proteins. We now know that GM-CSF, G-CSF, IL-3, M-CSF, EPO, TPO, and two potent costimulating molecules, stem cell factor (SCF) and Flt3-ligand (FL), transmit their cascade of intracellular signaling molecules after binding to specific receptors (R). GM-CSFR is: GM-CSF-alpha (α), subunit cluster density (CD)116; G-CSFR is: CSF3R/CD114; IL-3R is: IL-3RA/CD123; M-CSFR is: tyrosine kinase CSF1R/c-FMS/CD115; EPO has two receptors, those on hematopoietic cells and those on other cells ; the TPOR is: myeloproliferative leukemia protein (MPL)/CD110; SCFR is: the tyrosine kinase c-kit/CD117 and is found as both a cell membrane and soluble protein; and FLR is: the tyrosine kinase, Flt3.
Class or type I cytokines (often referred to as hematopoietins) regulate development, differentiation, and activation of hematopoietic and immune cells. Their receptors are type I membrane proteins with an N-terminal extracellular and C-terminal intracellular orientation. Type I cytokine receptors include receptors for CSFs, ILs, EPO, TPO ( Fig. 11.1 ), and hormones such as growth hormone (GH) and leptin.
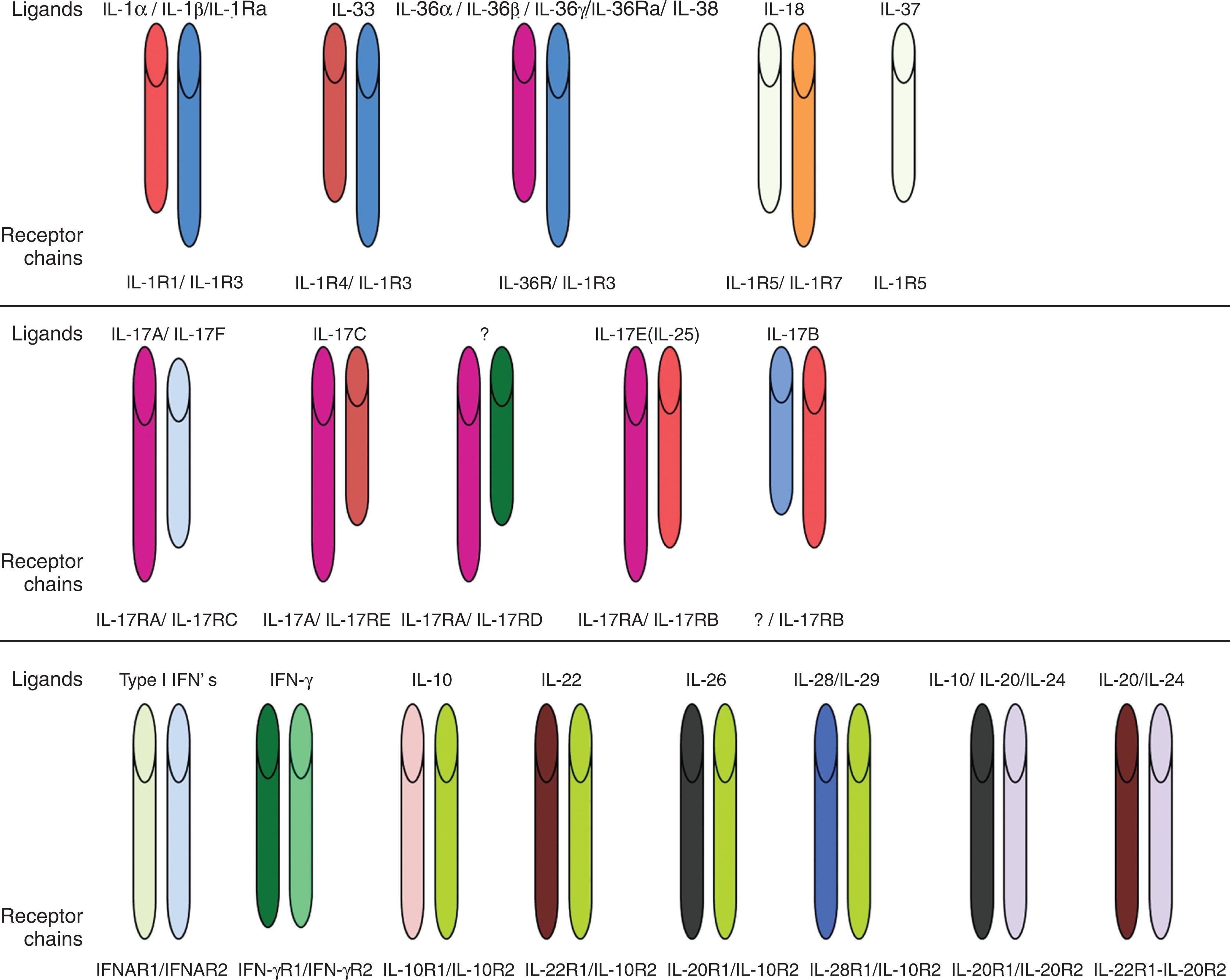
Class II cytokines consist of type I IFNs, which include 16 members that are produced by almost every nucleated cell, with approximately 20% to 60% sequence identity, including 12 subtypes of IFN-α, IFN-β, IFN-ε, IFN-κ, and IFN-ω. Type I IFNs initiate signaling by binding to the same receptor composed of two subunits called IFNAR1 and IFNAR2. Type II IFN consists of the single IFN-γ, which signals through a heterodimeric receptor composed of IFNGR1 and IFNGR2. Type III IFNs include IFN-λ1 (IL-29), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B). Some include the IL-10 family of cytokines (IL-10, IL-19, IL-20, IL-22, IL-24, IL-26) within the group of type III IFNs. The type III IFN receptor is composed of IL-10Rβ and IL-28R ( Fig. 11.2 ).
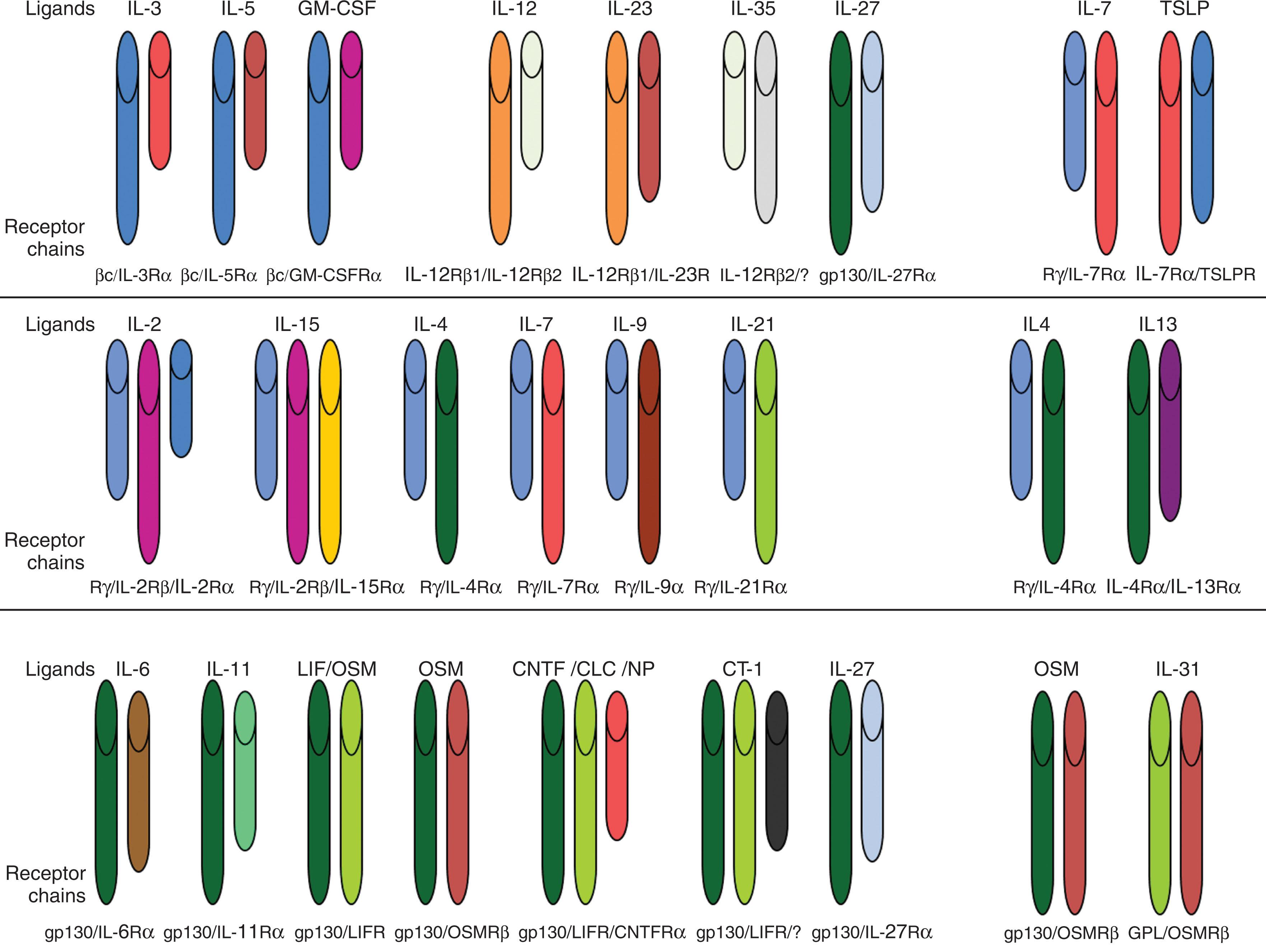
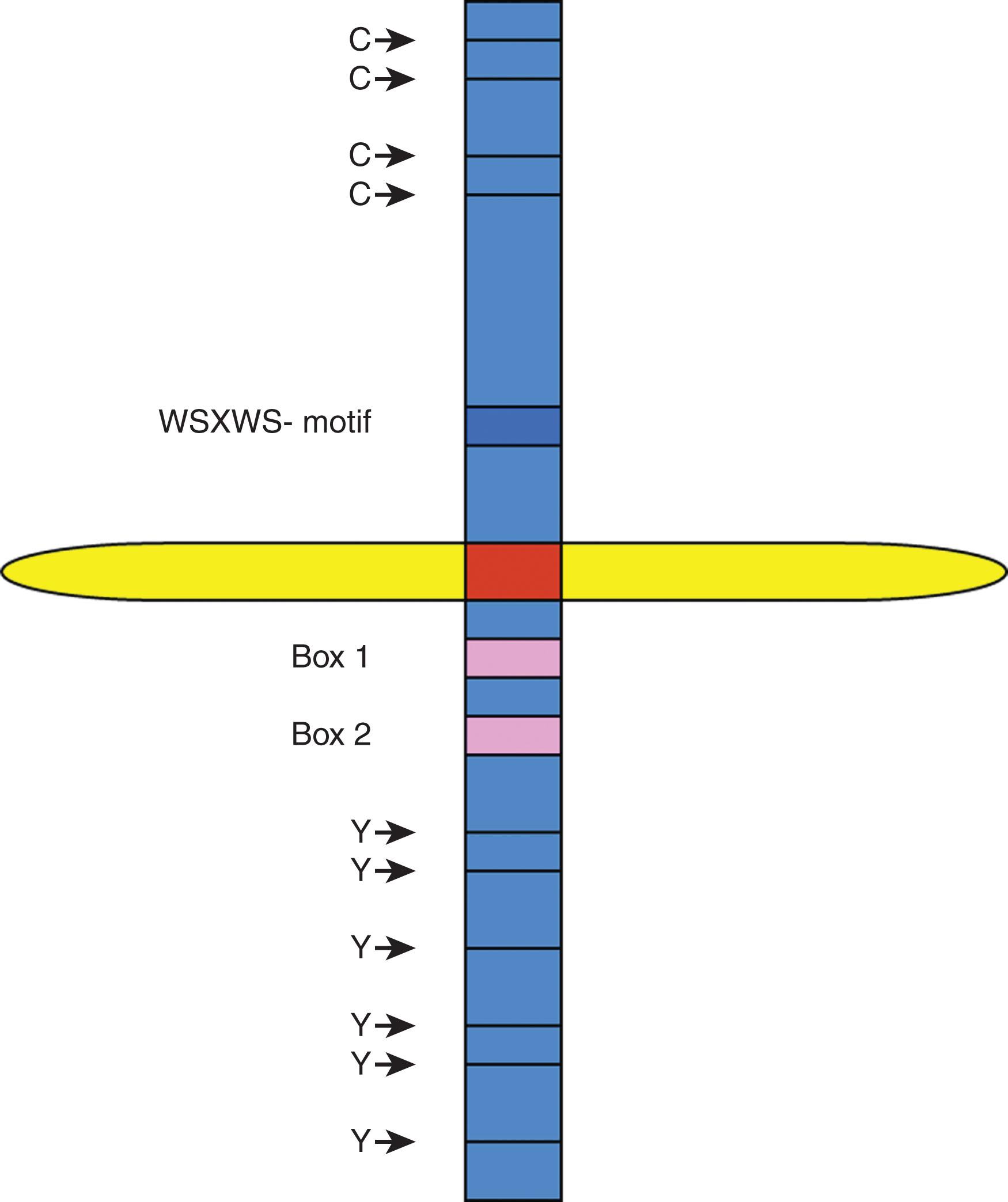
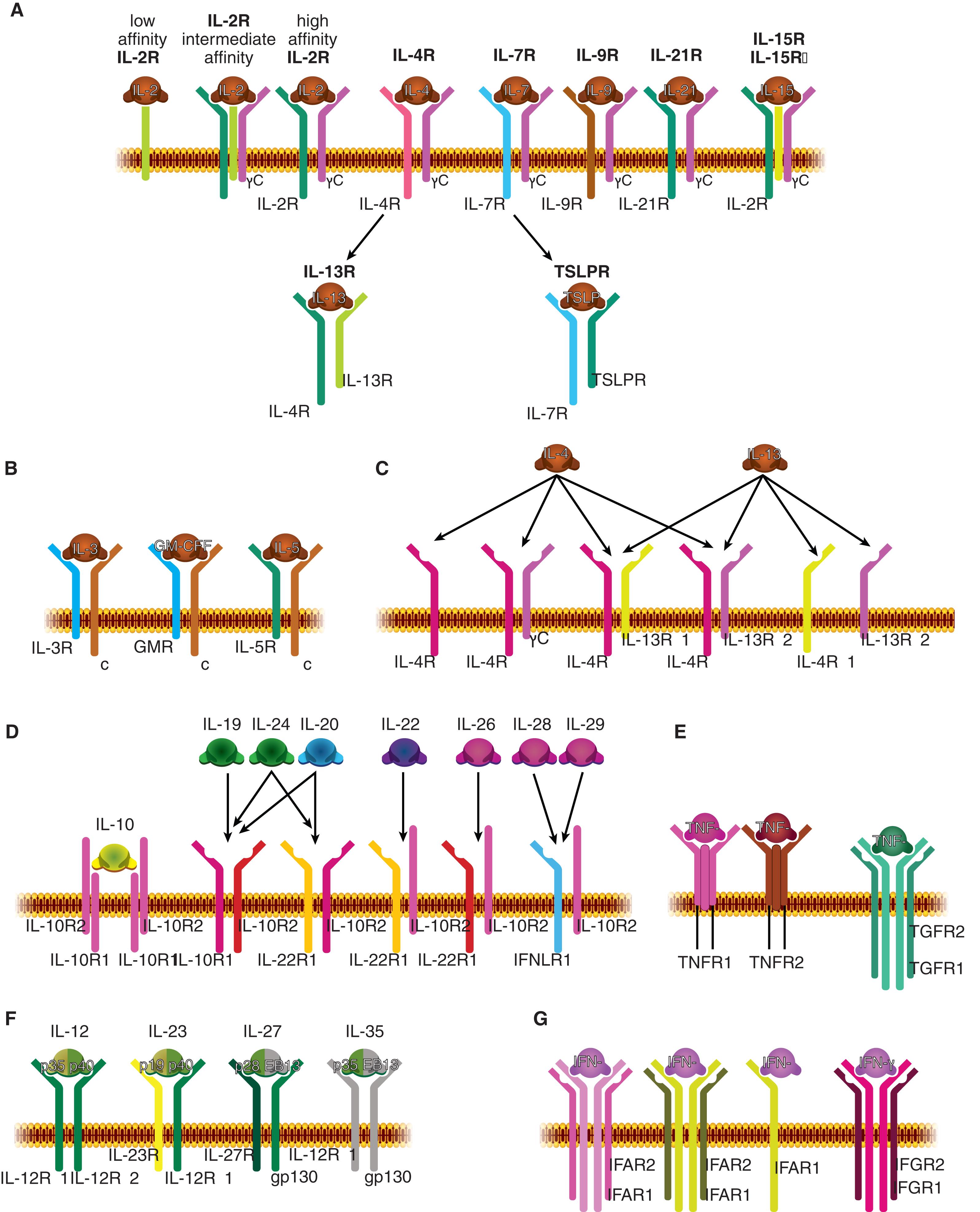
Structural similarities between the various type I cytokines were not immediately recognized. However, cloning of their receptors revealed significant homology in that the extracellular regions contain a common domain with four conserved cysteines (C4) in the N-terminal segment and a tryptophan-serine doublet near the C-terminal end. Mutagenesis studies revealed a structural role for these amino acids in maintaining tertiary receptor structures without being involved in cytokine interactions. A 200-amino-acid region evolutionarily derived from a tandem of two ancestral fibronectin-like domains were named hematopoietin receptor domain or cytokine-binding homology region (CHR) because they mediate interactions with cytokines. α-Receptors of IL-2 and IL-15 of the γc family are atypical cytokine receptors; they do not contain a CHR, but rather they contain sushi domains. Two conserved Box 1/ Box 2 regions are located in the proximal intracytoplasmic segment ( Fig. 11.3 ). By contrast, type II cytokine receptors contain two cysteine doublets (C2-C2) located in the C-terminal end of both fibronectin-derived domains. They retain Box 1/2 regions but lack a tryptophan-serine-x-serine-tryptophan motif. Both types of receptors bind ligands that display common spatial four α-helix bundle organizations; they use intracellular signaling mediators of Janus kinase (JAK) and signal transducer and activator of transcription (STAT) families. Type I and II cytokine receptors represent homogeneous structural groups of proteins. Sequence homology is noted in limited numbers of cases, including for GH/prolactin (PRL) and for the IL-6 families. Evidence of the common derivation of cytokines is observed in a common four-helix bundle structures, in addition to similar intron-exon relationships and clustering observed for certain cytokine genes, such as genes of the IL-4 family. Receptors can be composed of dimers of a single chain G-CSFR, EPO receptor (EPOR), or TPOR (c-MPL) or are heterodimeric with a common signaling subunit and a unique ligand-binding chain. Heterodimeric receptors are grouped into families based on sharing a common β-chain GM-CSFRα, IL-3Rα, IL-5Rα, or sharing a gp130 receptor (IL-6Rα, leukemia inhibitory factor [LIF] receptor β, ciliary neurotrophic factor receptor α [NTFRα], IL-11Rα IL-12R, IL-23R, oncostatin M receptor α) or sharing a common γ-chain (IL-2Rα, IL-2Rα, IL-4Rα, IL-7Rα, IL-9Rα, IL-13Rα, IL-15Rα, and IL-21Rα) (see Fig. 11.1 ).
Many CSFs have been implicated in the pathobiology of both hematopoietic and nonhematopoietic disorders or have been used after production by recombinant DNA technology to treat patients. Cytokines are involved in regulation of hematopoiesis at the level of HSC, HPC, and more mature blood or other accessory cells. Accessory cells include stromal cells (which are themselves very heterogenous), endothelial cells, cells belonging to lymphoid lineages (T cells, B cells, natural killer [NK] cells, NKT cells), and innate immune cells (monocytes/macrophages, neutrophilic myeloid cells, dendritic cells [DCs]). Cell regulation is not necessarily easily dissectible, but strides have been made in elucidating these intricate cytokine-cell interactions. Cytokine receptor signaling has been reviewed. Cytokines are involved in developmental processes during embryogenesis, through neonatal, and into adult life. They can fight infections and elicit immune responses. Redundancy in cytokine(s), their receptors, and actions are probably nature’s way to protect the body against unwanted insults that may interfere with specific cytokines and their protective actions. ILs and chemokines are part of the cytokine superfamily of molecules. ILs, chemokines, and their receptors are covered in this chapter, as will other biologically active proteins that influence hematopoiesis. Cytokines are secreted by leukocytes, as well as other cells. They act on, but are not limited to, hematopoietic cells to regulate blood cell production; this includes effects on immune cells and during inflammation. Biologically active proteins influence in vitro and in vivo proliferation, differentiation, and survival/apoptosis (programmed cell death) at the level of HSC, HPC, and more mature cells of the hematopoietic system. It is clear that activities of these proteins can be modified by enzymatic cleavage, such as that mediated by dipeptidylpeptidase (DPP) 4 (a.k.a. CD26), and also in context of oxygen tensions that are vastly different in vivo (~1% to 5% O 2 in BM, and also low in blood and other tissues, from that of the ambient air conditions of ~21% O 2 in which most investigators have studied the in vitro and ex vivo activities of these growth regulators). These potent cytokines/chemokines/other growth regulators and their receptors are discussed throughout the chapter. It is not possible to truly understand the action(s) of cytokines and other growth-modulating factors in absence of these modulating/modifying influences on cytokine activities.
Importantly, cytokine-cell interactions can become dysregulated and induce or be involved in pathologic situations. Examples of some adverse pathologic effects of cytokines include the “cytokine storm” elicited following chimeric antigen receptor (CAR) T-cell therapy, during inflammation, and after infection with viruses, such as SARS-CoV-2 (see Chapter 26, Chapter 67, Chapter 68, Chapter 79, Chapter 90, Chapter 152 ). Thus cytokine-cell interactions present a “double-edged sword” for both “good” (physiologic) and “bad” (pathologic) effects. Specific information on cytokines, chemokines, iron-binding proteins, other biologically active proteins, and growth regulation and their receptors follows.
There are many cytokines, including CSFs, ILs, and chemokines, which are enumerated as follows.
Much of the original background on CSFs through the mid to late 1980s has been reviewed, but it is important to understand that the purification and respective cloning of the ligands and receptors of GM-CSF, G-CSF, IL-3, M-CSF, EPO, and TPO have led to the preclinical analyses of their biologic activities in vitro and in vivo, and the eventual evaluation of their effects in humans. For example, EPO has been used to enhance erythropoiesis and production of erythrocytes in humans under a variety of clinical scenarios, GM-CSF, G-CSF, and TPO have been used to accelerate production of myeloid cells, and G-CSF has also been used to mobilize HSC and HPC for eventual collection as autologous and allogeneic stem cell grafts.
The IL family of cytokines now includes IL-1 through IL-38/40, with the first IL, IL-1, purified to homogeneity in 1977. Examples of some of the actions of the ILs and the cell(s) that produce them are summarized in Table 11.1 . ILs are placed in different families based on sequence homology, receptor similarities, and their functional activities. Some of the IL receptors are shown in Fig. 11.4 . Many ILs, as do most members of the cytokine family, have redundant activities and have multiple effects on immune cells and hematopoiesis. Reviews on the IL-1, IL-2, IL-6, IL-10, IL-12, and IL-17 families present in-depth information on many of the known IL molecules. Examples of the hematopoietic actions of some of the ILs from our lab and others, such as IL-1, IL-3, IL-4, IL-5, IL-6, IL-7, IL-8 (also known as the chemokine CXCL8), IL-9, IL-10, IL-11, IL-12, IL-17, IL-18, IL-20, IL-21, IL-26, IL-31, IL-32, and IL-33, have been reported. The receptor for IL-33 is known as suppressor of tumorigenesis (ST2) which is primarily elevated during graft-versus-host disease (GVHD) and is a biomarker associated with a poor prognosis. IL-33 signaling is also associated with hematologic malignancies, autoimmune disorders, allergic inflammation, and gastrointestinal cancers. The ILs work in combination with other ILs, as well as other cytokines, to regulate hematopoiesis in an additive to synergistic fashion.
| Interleukin (IL) | Produced By | Actions |
|---|---|---|
| IL-1 | Macrophages, B cells, endothelium, fibroblasts, astrocytes | Activates lymphocytes, stimulated macrophages; increases leukocyte/endothelial adhesion |
| IL-2 | T cells | T and B cells proliferation and differentiation; activates NK cells antibody production |
| IL-3 | T cells | Acts as a multi-CSF |
| IL-4 | CD4 + T cells (Th2) | Acts on T and B cells |
| IL-5 | CD4 + T cells (Th2) | Activates eosinophils and production of innate immune cells |
| IL-6 | T and B cells, fibroblasts, macrophages | B-cell differentiation |
| IL-7 | BM stromal cells | Acts on pre-B cells and T cells; stimulates B- and T-cell proliferation |
| IL-8 | Monocytes and fibroblasts | Targets include mature myeloid cells |
| IL-9 | Th9, Th2, Th17, Mast, NKT and TREG cells | Enhances survival of T cells and activates mast cells |
| IL-10 | Th2 cells | Primary targets are Th1 cells; down regulates Th17 cells, and inhibits macrophage IL-12 production |
| IL-11 | BM stromal cells and fibroblasts | Actions on hematopoietic progenitors and osteoclasts |
| IL-12 | Monocytes | Induces Th1 cells, and T cells, and NK cell IFN-γ production |
| IL-13 | Th2, NKT, and mast cells | Acts on monocytes, epithelial cells, and B cells |
| IL-14 | T cells | Activates B cell proliferation and inhibits secretion of immunoglobulins |
| IL-15 | Monocytes, epithelium | Acts on T cells and activated B cells |
| IL-16 | CD8 + T cells and eosinophils | CD4 + T-cell chemoattractant |
| IL-17 | Th17 cells | Acts on epithelial and endothelial cells; causes release of cytokines |
| IL-18 | Macrophages | Cofactor in Th1 cell induction; induces IFN-γ and enhances NK cell activation |
| IL-19 | Th2 cells, antiinflammatory molecules | Antiinflammatory molecule involvement |
| IL-20 | Immune cells and activated epithelial cells | Plays a role in cellular communication |
| IL-21 | NK and CD4 + T cells | Acts on innate and adaptive immune cells, and erythroid progenitor cells |
| IL-22 | T cells and other cells in the innate and acquired immunities | Inhibits IL-4 production |
| IL-23 | Macrophages and dendritic cells | Maintenance of IL-17–producing T cells |
| IL-24 | Monocytes and T and B cells | Involved in wound healing and protects against bacterial infections |
| IL-25 | Dendritic cells | Th2 cells and their production of IL-4 and IL-13 |
| IL-26 | Th17 cells | Causes IL-10, IL-1β, IL-6, and IL-8 production |
| IL-27 | T cells | Proinflammatory molecule |
| IL-28 | TREGs | Acts on keratinocytes and melanocytes |
| IL-29 | Virus-infected cells, dendritic cells, TREGs | Upregulates viral protective responses |
| IL-30 | Monocytes in response to Toll-like receptor agonists such as bacterial lipopolysaccharides | Acts on many hematopoietic cells |
| IL-31 | Th2 and dendritic cells | Acts as proinflammatory and chemotactic factor |
| IL-32 | NK cells and monocytes | Induces cytokine production (IL-6, IL-1β) and inhibits IL-15 production |
| IL-33 | Mast cells and Th2 cells | Mediates Th2 responses |
| IL-34 | Phagocytes and epithelial cells | Enhances IL-6 production and is involved in differentiation of antigen-presenting cells |
| IL-35 | Regulatory B cells | Immune suppressor |
| IL-36 | Phagocytes | Acts on T and NK cells |
| IL-37 | Phagocytes | Regulates innate immunity and causes immunosuppression |
| IL-38 | Placenta, B cells, etc. | Acts on T cells, inhibits IL-17 and IL-22 production |
| IL-39 | B cells | Acts on differentiation and expansion of neutrophils |
| IL-40 | BM, fetal liver, activated B cells | Involved in development of humoral immune responses |
Chemokines, originally considered chemotactic cytokines, are a large family of cytokines that usually signal through G protein–coupled heptahelical receptors ( Table 11.2 and Fig. 11.5 ). There is redundancy within the chemokine family, with some chemokines binding multiple chemokine receptors and, in more rare occasions, nonchemokine receptors, and some chemokine receptors binding multiple chemokines. Fig. 11.6 diagrammatically denotes the many functions of chemokines and chemokine receptors. Chemokines can act as monomers but can form homodimers, heterodimers, and higher aggregates. Specific chemokines and their receptors have been implicated in various phases of HSC regulation. They can act as positive and negative regulators of HSC and HPC and have been implicated in the survival, proliferation, and mobilization of these cells. For example, IL-8/CXCL8, which acts through the chemokine receptors CXCR1 and CXCR2, has been implicated not only in the negative regulation of hematopoiesis, but CXCL8 and CXCR2 have been implicated in leukemia/MDS. Interestingly, DEK, a heterochromatin remodeling agent that has cytokine-like activity, can bind and signal through the chemokine receptor CXCR2 and can compete with IL-8/CXCL8, as well as macrophage inflammatory protein (MIP)-2, at the level of CXCR2. Extracellular DEK potently enhances cytokine-induced ex vivo expansion of mouse BM and human cord blood (CB) HSCs, plus has other effects. Another well-recognized chemokine and its receptor is SDF-1/CXCL12 and CXCR4, respectively. stromal cell-derived factor (SDF)-1/CXCL12 is involved in normal and leukemic HSC/HPC survival, proliferation, chemotaxis, migration, and mobilization. SDF-1/CXCL12 also binds to CXCR7, but CXCR4 appears to be the main hematopoietic signaling receptor for SDF-1/CXCL12. Blocking of CXCR4 by the CXCR4 antagonist, AMD3100/plerixafor, has been used to induce the mobilization of peripheral blood HSC and HPC and to synergize with G-CSF in this mobilization. Chemokines are affected by a number of posttranslational modifications, including by DPP4, which is discussed later in this review. They are inducible by several different stimuli and are involved and are players in response to infection and inflammation. There are also viral genes that encode chemokine receptors. Chemokine receptors can also regulate infectivity to viruses. For example, individuals with specific mutations in CXCR5 are immune or less susceptible to infection with human immunodeficiency virus (HIV), but thus far only very few patients with HIV have been “cured” by allogeneic stem cell transplantation using donor cells from stem cell donors with this specific variant of mutated CXCR5.
| Name(s) | Human Receptor(s) | Mouse Receptor(s) |
|---|---|---|
| CCL Chemokines | ||
| CCL1 (I-309) | CCR8, DARC | CCR8 |
| CCL2 (MCP-1, MCAF) | CCR2, CCR4, CCR11, D6, DARC | CCR2, CCR4, D6, DARC (JE = mouse ligand along with MCP-1) |
| CCL3 (MIP-1α, LD78) | CCR1, CCR4, CCR5, D6 | CCR1, CCR4, CCR5, D6 |
| CCL3L1 (LD78β) | CCR1, CCR3, CCR5, D6 | ___ |
| CCL3L3 (LD78β) | CCR1, CCR3, CCR5 | ___ |
| CCL4 (MIP-1β) | CCR1, CCR5, CCR8, D6 | CCR5, CCR1 |
| CCL4L1 | CCR1, CCR5 | ___ |
| CCL5 (RANTES) | CCR1, CCR3, CCR5, CCR4, D6, DARC | CCR1, CCR3, CCR5, CCR4 |
| CCL6 | ___ | CCR1 |
| CCL7 (MCP-3) | CCR1, CCR2, CCR3, D6, DARC | CCR1, CCR2, CCR3 |
| CCL8 (MCP-2) |
|
___ |
| CCL9/10 (MIP-1γ) | ___ | CCR1, CCR3 |
| CCL11 (Eotaxin) | CCR3, CCR5, D6, DARC | CCR3, DARC |
| CCL12 (MCP-5) | ___ | CCR2 |
| CCL13 (MCP-4, CKβ) |
|
___ |
| CCL14 (HCC-1, MCIF, CKβ1) | CCR1, CCR3, CCR5, D6, DARC | ___ |
| CCL15 (MIP-1δ, MIP-1γ, LKN-1, MIP-5) | CCR1, CCR3 | CCR1, CCR3 |
| CCL16 (HCC-4, LEC, LMC) | CCR1, CCR2, CCR5, CCR8, H4, DARC | ___ |
| CCL17 (TARC) | CCR4, CCR8, D6, DARC | CCR4 |
| CCL18 (PARC, CKβ7, MIP-4) | PITPNM3, CCR8, DARC | ___ |
| CCL19 (MIP-3β, ELC, Exodus-3) | CCR7, CCR11 | CCR7, CCR11 |
| CCL20 (MIP-3α, LARC, Exodus-1) | CCR6 | CCR6 |
| CCL21 (6CKine, Exodus-2) | CCR7, CCR11 | CCR7, CCR11 |
| CCL22 (MDC) | CCR4, D6 | CCR4 |
| CCL23 (MPIF, CKβ8, MIP3) | CCR1 | CCR1 |
| CCL24 (Eotaxin-2) | CCR3 | CCR3 |
| CCL25 (TECK, CKβ15) | CCR9, CCR11 | CCR9, CCR11 |
| CCL26 (Eotaxin-3, MIP-4α) | CCR3, CX 3 CR1 | ___ |
| CCL27 (CTACK, PESKY, Eskine) | CCR10 | CCR10 |
| CCL28 (MEK) | CCR3, CCR10 | CCR10 |
| CXC Chemokines | ||
| CXCL1 (GRO-α, GRO1, NAP3 DCIP-1) | CXCR2, DARC | CXCR2, DARC |
| CXCL2 (GRO-β, MIP-2α, GRO2, MIP-2) | CXCR2, DARC | CXCR2, DARC |
| CXCL3 (GRO-γ, MIP-2β, KC) | CXCR3B, CXCR3, DARC | ___ |
| CXCL4L1 (PF4V1) | CXCR3B, CXCR3 | CXCR3 |
| CXCL5 (ENA-78) | CXCR2, DARC | ___ |
| CXCL6 (GCP-2) | CXCR1, CXCR2, DARC | CXCR1, CXCR2, DARC |
| CXCL7 (NAP2) | CXCR1, CXCR2 | CXCR1, CXCR2 |
| CXCL8 (IL-8, NAP-1, GCP-1) | CXCR1, CXCR2, DARC | ___ |
| CXCL9 (MIG) | CXCR3, CXCR3B, DARC | CXCR3 |
| CXCL10 (IP-10) | CXCR3, CXCR3B, DARC | CXCR3 |
| CXCL11 (I-TAC) | CXCR3, CXCR7, CXCR3B, DARC | CXCR3, CXCR7 |
| CXCL12 (SDF-1) | CXCR4, CXCR7 | CXCR4, CXCR7 |
| CXCL13 (BCA-1, BLC) | CXCR3, CXCR5, DARC | CXCR5 |
| CXCL14 (BRAK) | Unknown | Unknown |
| CXCL15 (Lungkine) | ___ | Unknown |
| CXCL16 (---) | CXCR6 | CXCR6 |
| CXCL17 (DMC) | Unknown | Unknown |
| LIX (---) | ___ | CXCR1, CXCR2, DARC |
| CX3C Chemokines | ||
| CX3CL1 (Fractakine/Neurotactin) | CX3CR1 | CX3CR1 |
| C Chemokines | ||
| XCL1 Lymphotactin, SCM-1α, ATAC) | XCR1 | XCR1 |
| XCL2 (SCM-1β) | XCR1 | ___ |
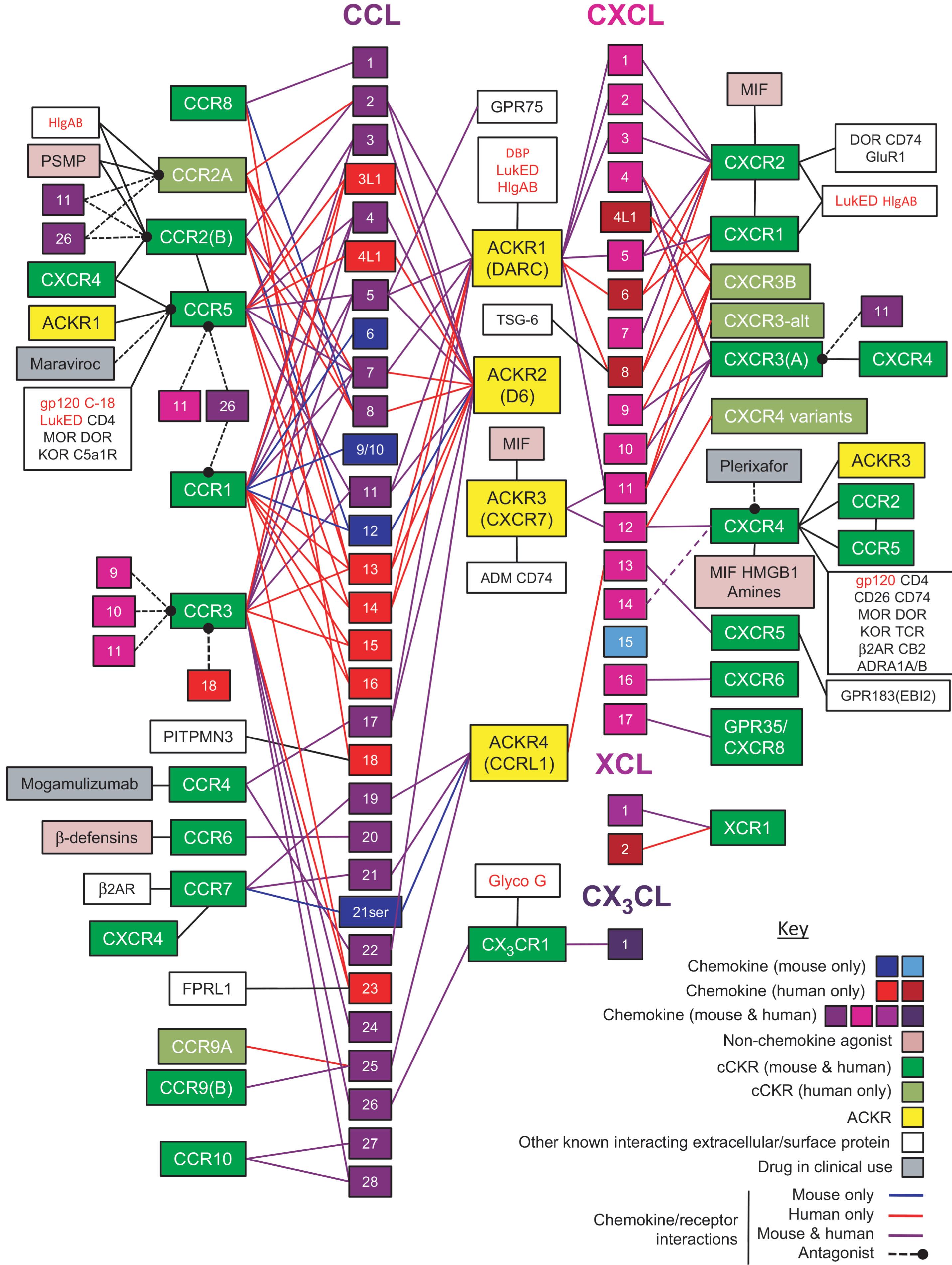
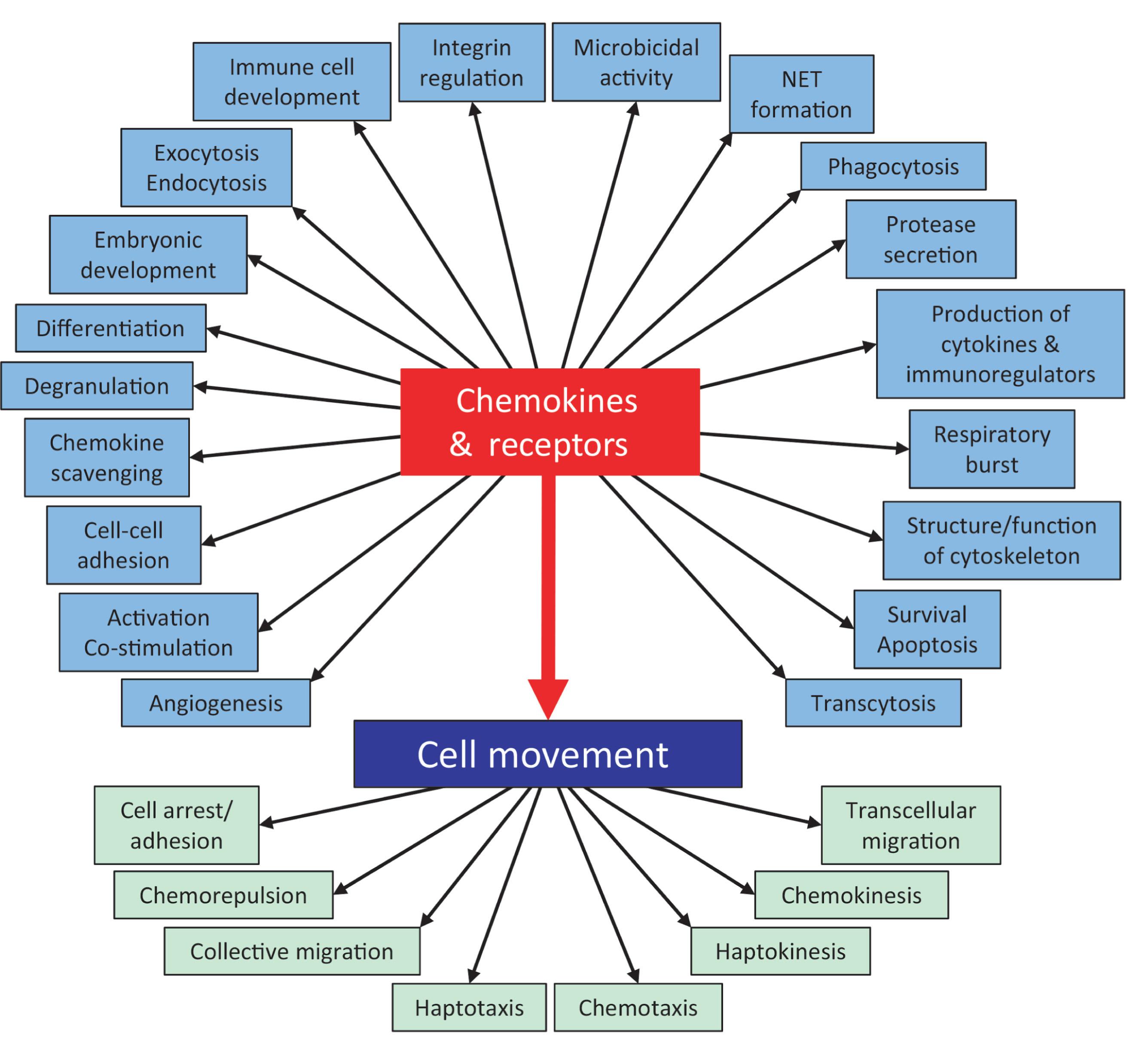
The complex network of cytokines and chemokines and their overlapping and diverse effects on a variety of cellular processes is truly incredible and goes beyond regulation of normal cells. Although redundant chemokine/chemokine receptor interactions are likely the body’s way to protect itself, this “protection” can elicit serious consequences. Many chemokines and their receptors are part of the triggered cytokine release syndrome (CRS), which represents a side effect following CAR-T-cell therapy and viral infections, such as that induced by SARS-Cov-2 during COVID-19 infections. These events lead to excessive elaboration of chemokines and other cytokines and their receptors, representing the “double-edged sword” discussed earlier in terms of health benefits and disease-related problems. Greater knowledge of the production and actions of chemokines, and their intracellular signaling cascades that are induced through chemokine and cytokine receptors, is crucial for future benefits for normal cell regulation and their modification and to dampen their adverse effects during disease.
NF-κB (nuclear factor kappa-light chain enhancer of activated B cells) is a protein complex controlling transcription of DNA, as well as cytokine production and cell survival. NF-κB is involved in cellular responses to stress, cytokines, irradiation, and bacterial or viral antigens. Aberrant NF-κB regulation is associated with cancer, inflammation, autoimmune diseases, septic shock, viral infection, and disordered development of immune cells. In an inactivated state, NF-κB is located in the cytosol with inhibitory protein IκBα. Through a variety of extracellular signals including cytokine and chemokine, the enzyme IκB kinases is activated, which then phosphorylate IκBα, resulting in ubiquitination dissociation of IκBα from NF-κB and degradation of IκBα by the proteasome. Activated NF-κB then translocates to the nucleus and binds to specific sequences of DNA termed response elements. The DNA/NF-κB complex recruits other proteins. There are numerous NF-κB target genes that include cytokines, chemokines, and their modulators (see https://www.bu.edu/nf-kb/gene-resources/target-genes/ ). Upstream signal transduction of NF-κB activation has been described. Reviews and articles are reported on a role for NF-κB in cytokine gene regulation, chemokine gene transcription, and tumor growth, inflammation, cancer, multiple myeloma, and thrombosis. Many inhibitors of NF-κB signaling have been described. Overall, the impact of NF-κB on cytokines and chemokines and resultant physiologic and pathologic effects cannot be underestimated. With the multitude of cytokines and other factors that can influence NF-κB signaling, it is not at all surprising that NF-κB can regulate hematopoiesis. NF-κB has been shown to play an important role in the differentiation of HSCs and HPCs down both the myeloid and lymphoid pathways. The Rel/NF-κB transcription factors are mainly composed of five members (c-Rel, p65/RelA, RelB, NFKB1/p50, NFKB2/p52). P65 and p50 are ubiquitous proteins expressed in all hematopoietic lineages. However, the distinct expression pattern of the other Rel/NF-κB factors in HPCs indicates their importance in HPC differentiation. Lymphocytes, monocytes, granulocytes, and erythrocytes express c-Rel. RelB is mostly expressed in DCs and lymphocytes. DCs, macrophages, and lymphocytes express p52. Using mouse knockout animal models, investigators have shown the importance of these factors in regulating hematopoietic differentiation.
Cytokines and/or chemokines when combined have additive to synergistic effects on proliferation of HPCs. This is especially so for the potent costimulating cytokines SCF (which acts through the c-kit receptor) and FL (which acts through the FL3 receptor). Efforts have been made to specifically engineer cytokines and chemokines for greater efficacy. This includes the GM-CSF/IL-3 fusion protein, PIXY32, which has been used in a clinical trial to promote hematopoietic recovery following chemotherapy-induced multilineage myelosuppression in patients with sarcoma, as well as IL-3/EPO, and IL-8/PF4 fusion. These have had only limited clinical testing. By contrast, molecularly engineered forms of small molecule agonists of the thrombopietin receptor (eltrombopag and romiplostim) are currently used to treat patients with immune thrombocytopenic purpura as well as aplastic anemia (see Chapter 31, Chapter 128 ).
Enhancement of ex vivo expansion of HSCs/HPCs for both preclinical and clinical use is an ongoing area of research. Current clinical trials on ex vivo expansion have been described. There are currently three sources of cells that have been used for clinical HCT. This includes BM, cytokine- and/or other reagent-induced mobilized peripheral blood, and umbilical cord blood. There have been a number of preclinical studies to expand numbers of CB HSCs and HPCs ex vivo. Some such as SR1, UM171, nicotinamide, prostaglandin (PGE) 2 analog, and other reagents are currently in clinical trials. None of the ex vivo expansion procedures work without addition of combinations of cytokines such as SCF, TPO, and FL during the ex vivo culture period. Hence there is a need to add the reagent(s) of choice with SCF, TPO, and FL during the ex vivo culture period. Importantly, use of serum-free cultures and a short time of cell culture has benefits for potential use of the generated cells for clinical application. What is clear is that the most effective ex vivo expansion studies for clinical use is not yet known. Moreover, there are numerous other ex vivo expansion studies not yet tested clinically, and which of these may eventually be the procedure of choice for clinical use is as yet unknown.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here