Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Polycystic kidney disease (PKD) is clinically and genetically heterogeneous and is the most common heritable kidney disease. PKD can be inherited either in a dominant or recessive pattern. Cystic kidney disease is a common feature of ciliopathies and is one of the most common causes of end-stage renal disease in both children and adults.
Although single simple cysts of the kidney are relatively common and have little clinical implication, there is a group of hereditary cystic diseases that are of importance because of their contribution to the development of end-stage renal disease. Each of those diseases has its own genetic abnormality, and each has its own characteristic clinical presentation. The exact mechanisms by which the genetic abnormalities result in the physiologic and pathologic abnormalities are still incompletely identified. Cystic diseases are classified according to their genetic presentation and according to the regions of the kidney that are involved. These classifications are listed in Table 9.1 .
|
Due largely to mutations in: UMOD, MUC1, REN, HNF1B
|
Autosomal dominant polycystic kidney disease (ADPKD) affects between 1 in 500 and 1 in 1000 individuals, and it affects both adults and children. It is found in all racial and ethnic groups and is seen throughout the world. It is the most common of the genetic PKDs. As its name implies, ADPKD is inherited in an autosomal dominant pattern with complete penetrance. Thus the child of an affected parent has a 50% chance of inheriting the abnormal gene. About half the patients affected are unable to give a family history consistent with ADPKD. ADPKD was previously known as the adult form of PKD because it may not become clinically apparent until the third or fourth decade of life. Although 100% of gene carriers will show evidence of the disease, only about 50% progress to renal failure.
ADPKD is a multisystem disorder presenting with many kidney and extra-kidney manifestations (see Table 9.1 ). A careful medical history for kidney diseases, hypertension, stroke (or intracranial hemorrhage), or familial premature death may point out the type of gene mutation or the need for intracranial aneurysm screenings. Less often, patients may present with various manifestations (flank pain, dysuria, hematuria, or hypertension), which may lead to a new diagnosis of ADPKD. Clinical features of ADPKD are numerous with variable presentations for each patient. Flank or loin pain can arise from large cysts by compression or stretching of the surrounding structures.
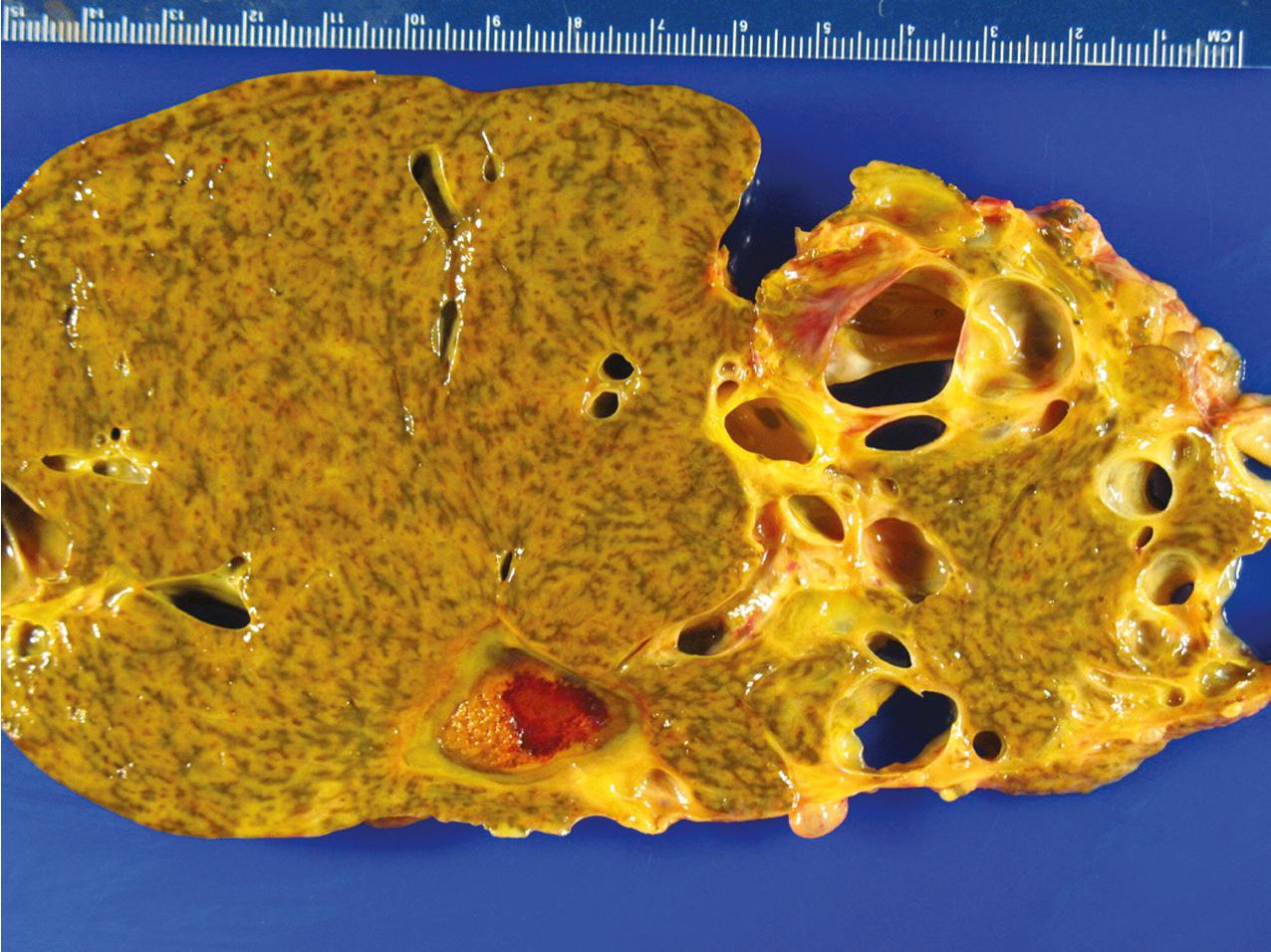
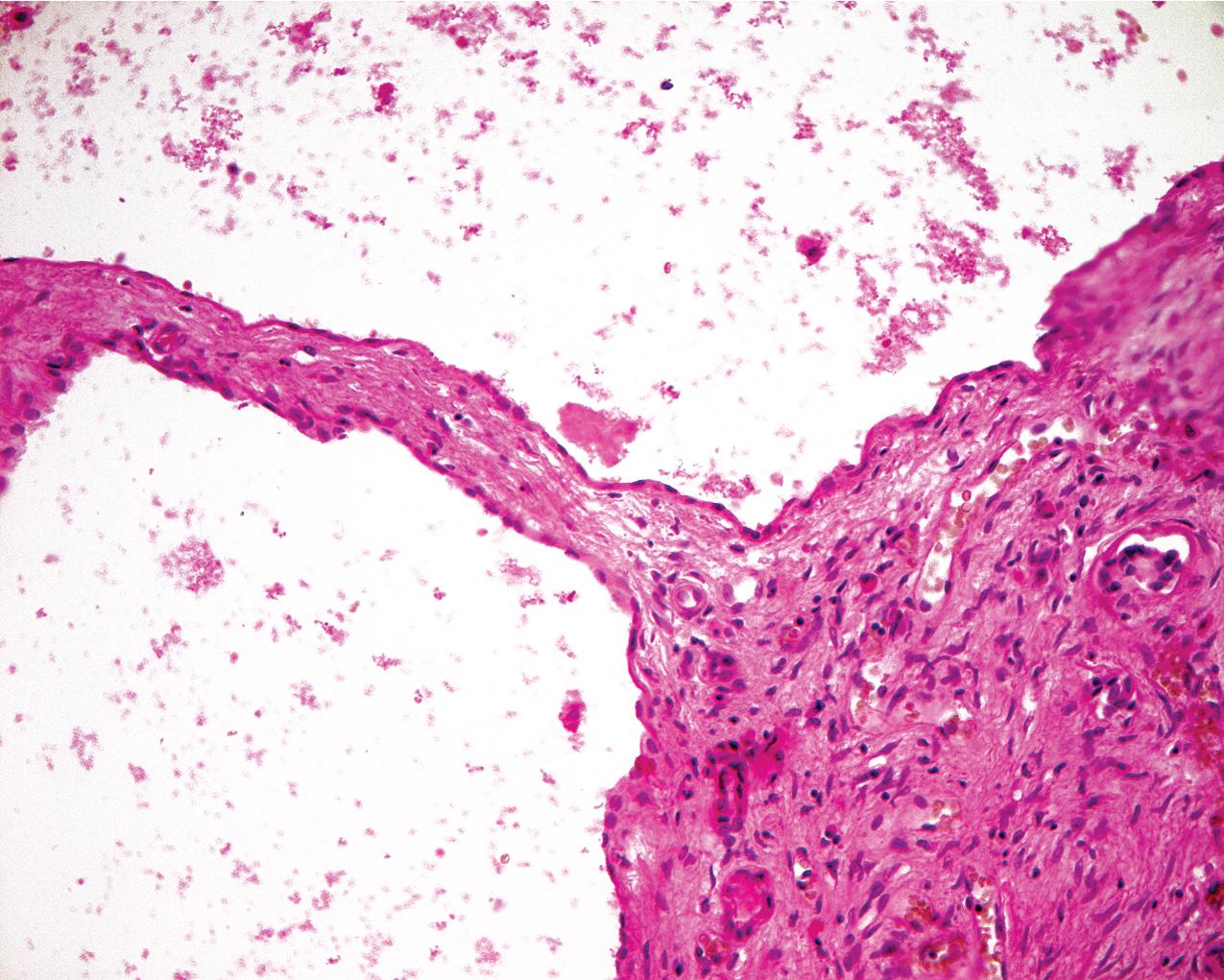
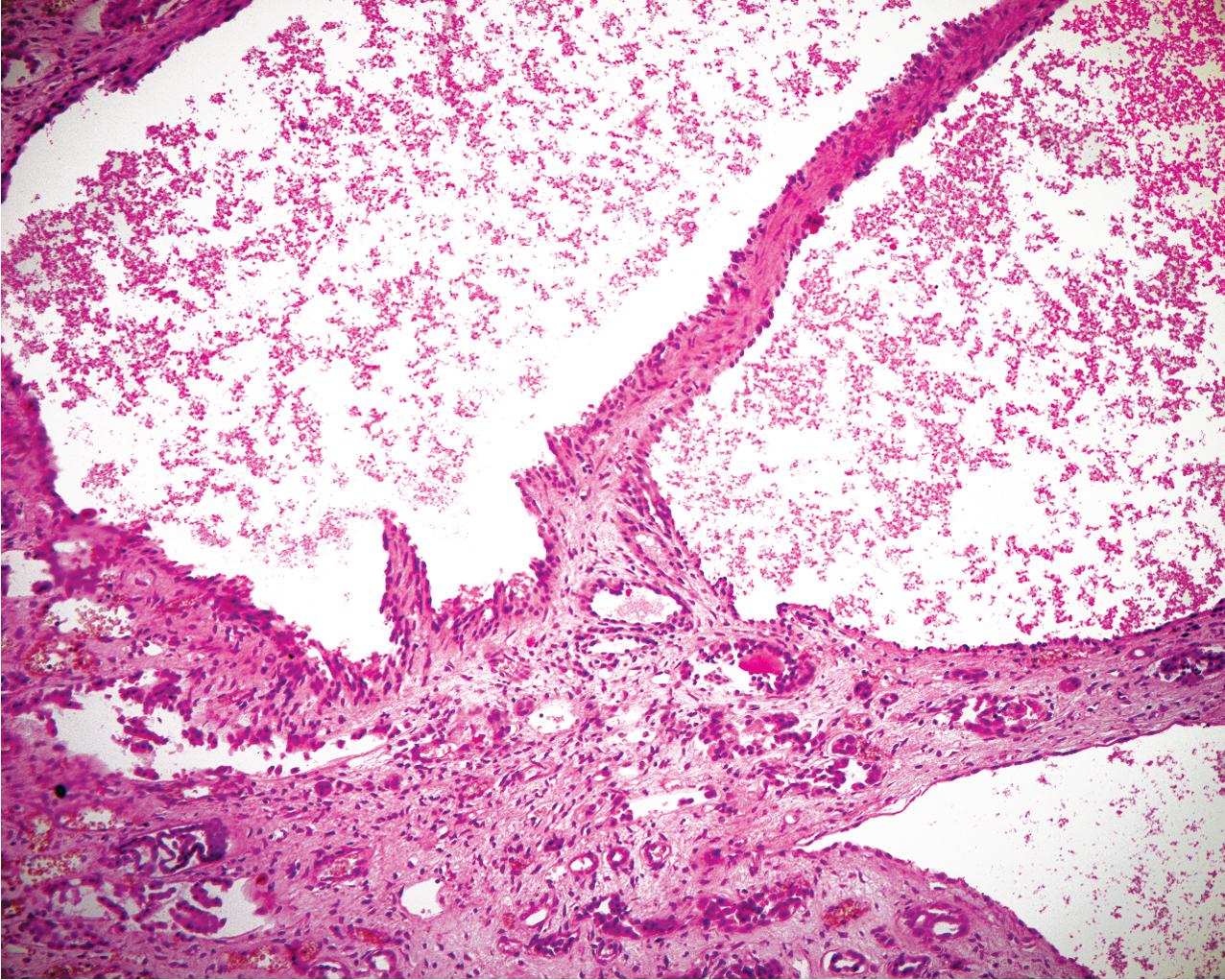
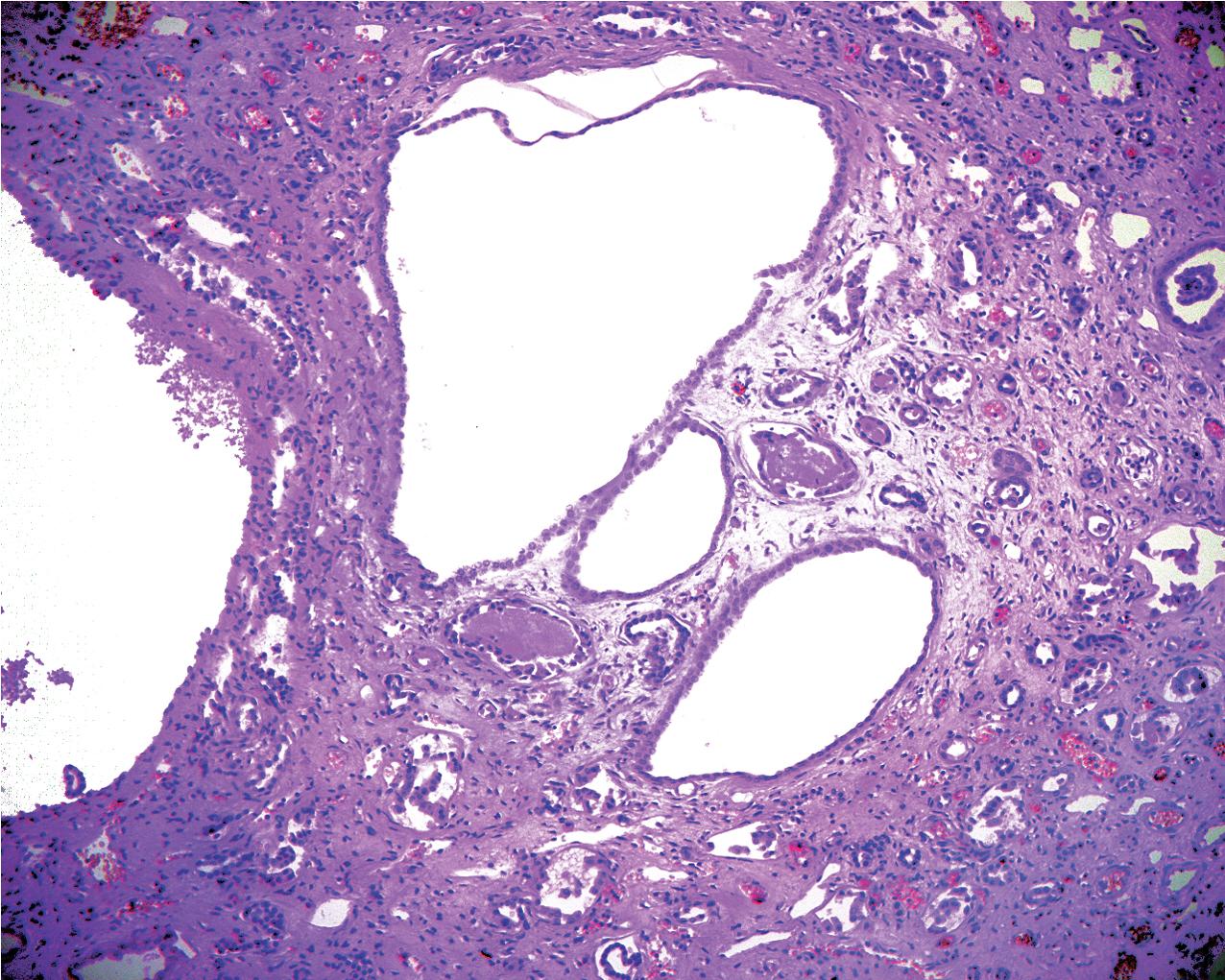
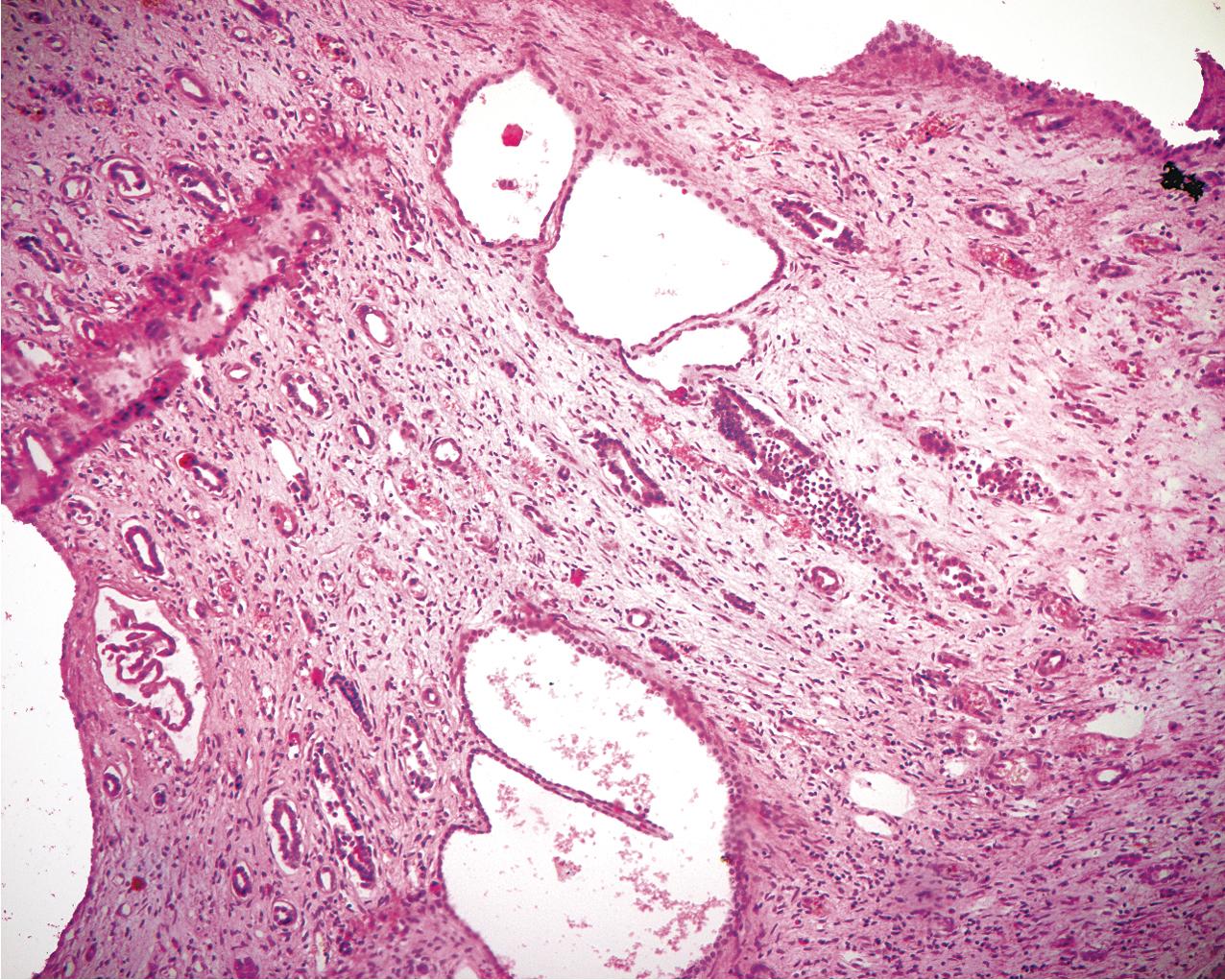
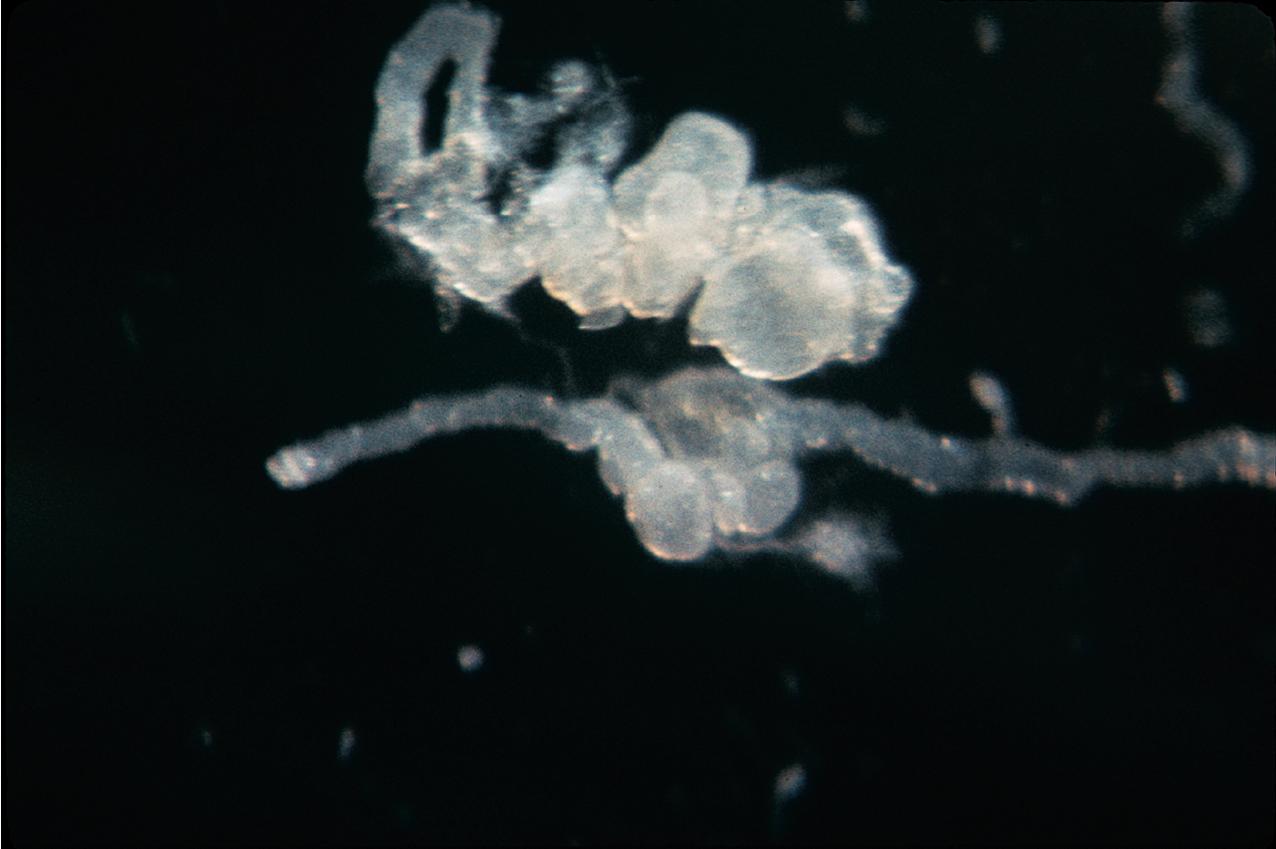
ADPKD results in kidneys that are enlarged and diffusely cystic ( Figs. 9.1–9.4 ). It is important to note that although the cysts appear to involve the entire kidney, only a portion of the total number of nephrons in the kidney are cystic ( Figs. 9.5–9.8 ). The cysts range in size from barely visible to several centimeters in diameter. Microdissection studies have demonstrated the cysts to be spherical dilations or outpouchings from existing renal tubules ( Fig. 9.9 ). As the cysts enlarge, they appear to become detached from their tubule of origin. In the early stages of the disease, the noncystic parenchymal elements remain normal; as the cysts increase in number and grow in size, however, the residual normal tissue becomes atrophic and nonfunctional, which results in the development of end-stage renal disease. ADPKD is a systemic disorder that can have extrarenal manifestations that include cysts in other epithelial organs, such as the liver and pancreas ( Fig. 9.10 ). Patients with ADPKD also show connective tissue defects, such as intracranial aneurysms, cardiac valve abnormalities, aortic dissections, and abdominal wall hernias.
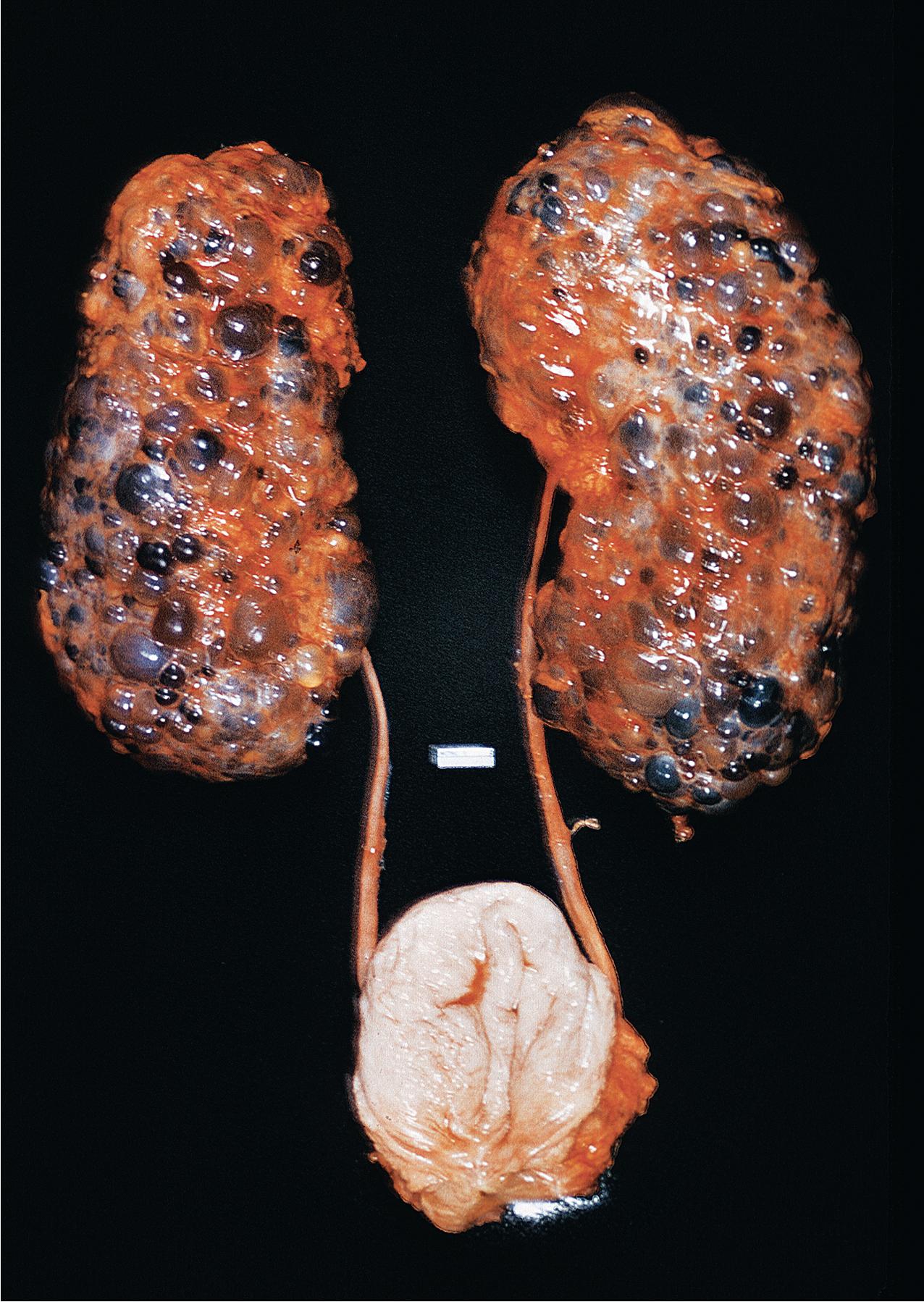
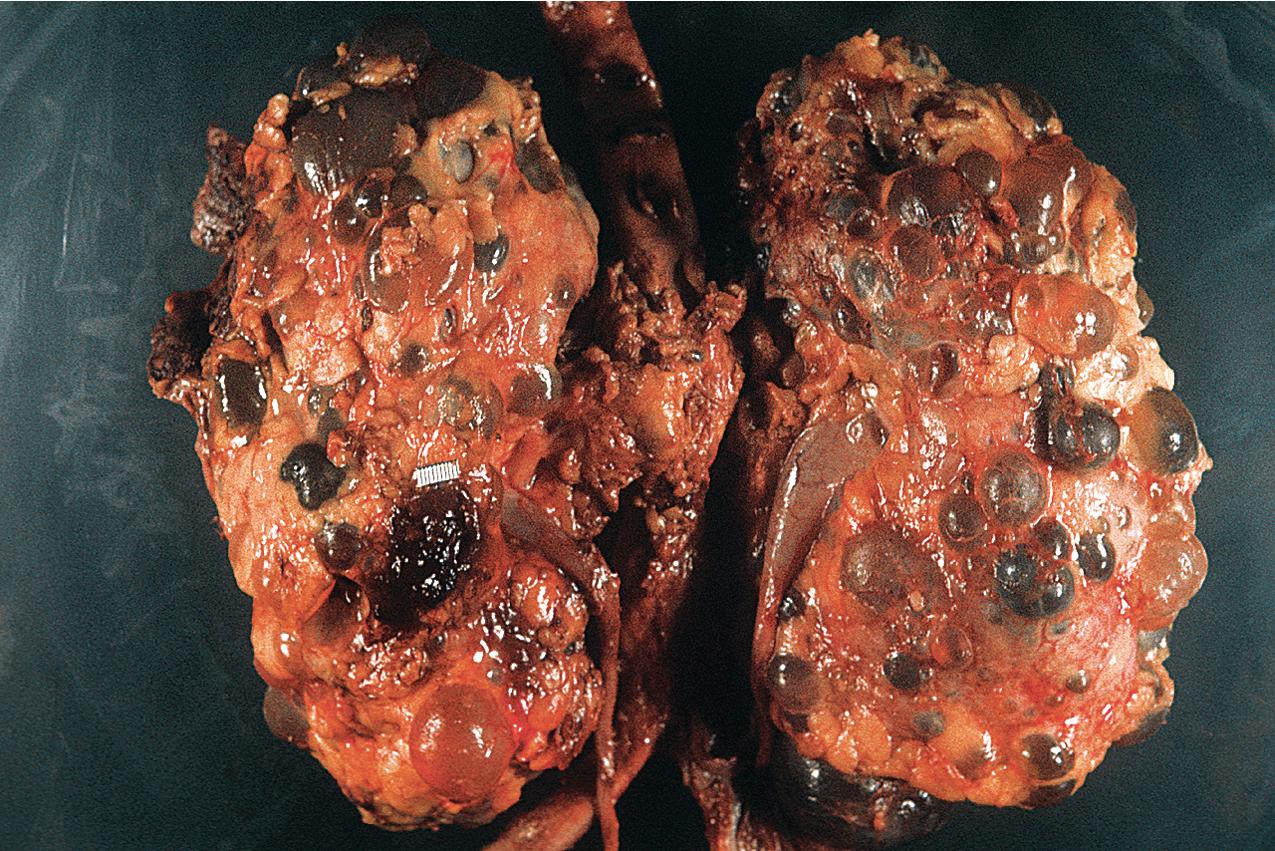
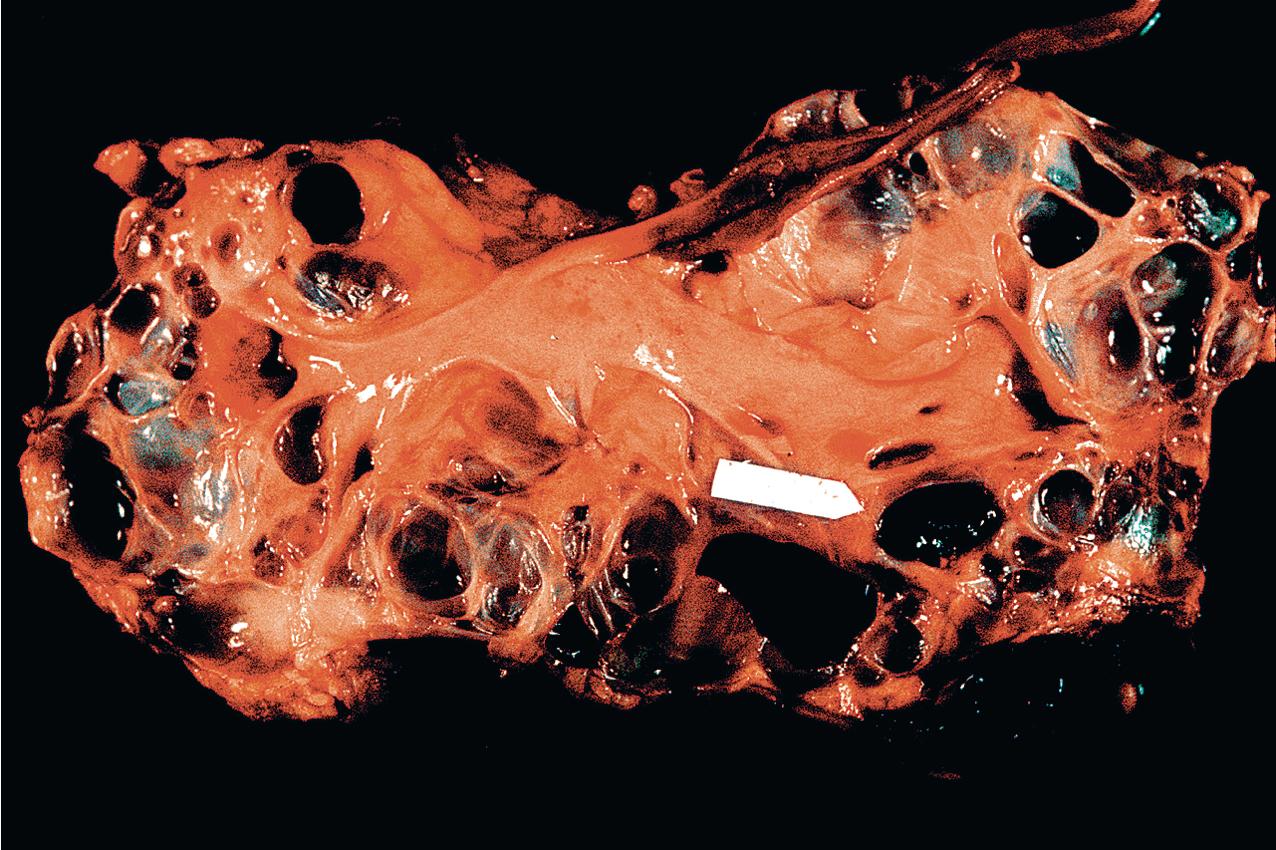
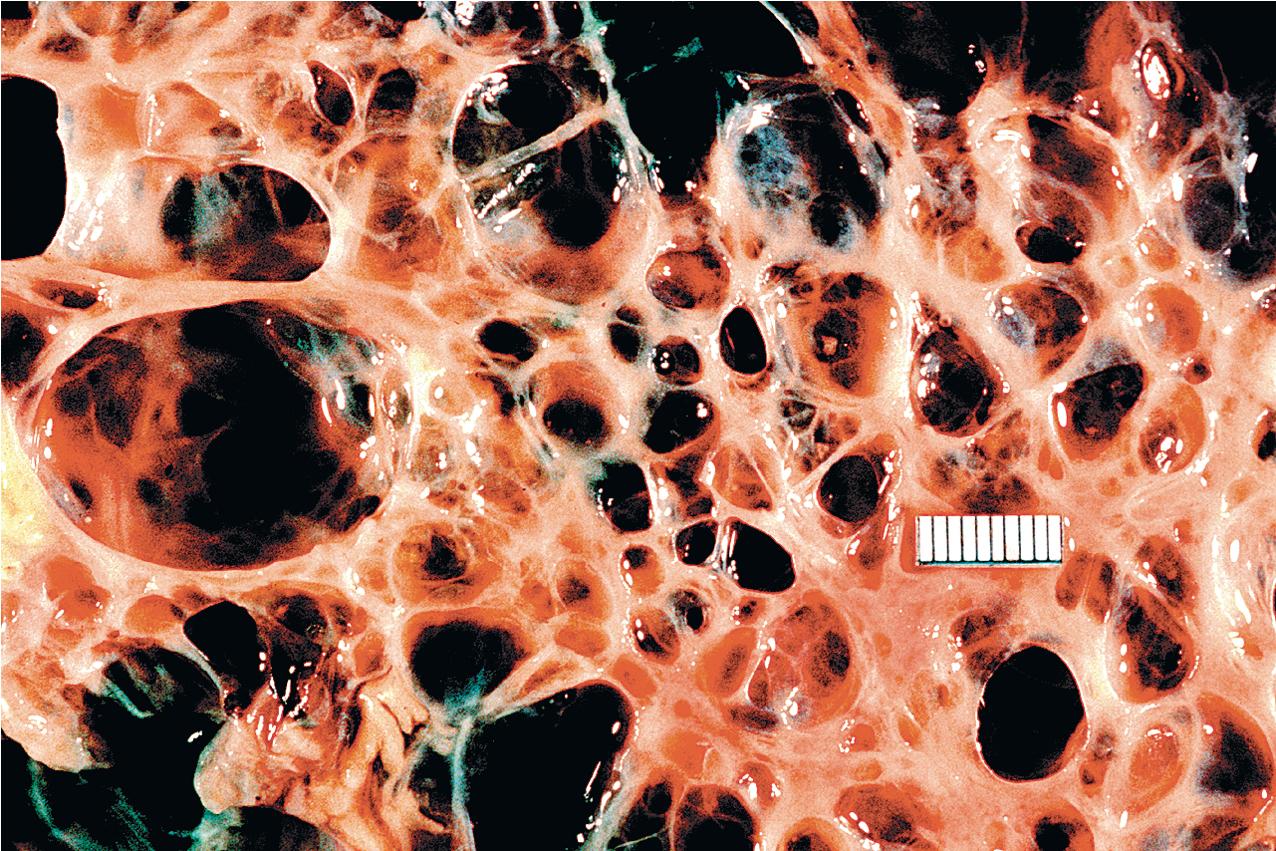
ADPKD is caused by genetic mutations in chromosome 16 for PKD1 or chromosome 4 for PKD2. PKD1 and PKD2 encode proteins called polycystins. Polycystins are a family of eight transmembrane proteins united by sequence homology. Polycystins appear to play key roles during development. In mature organs, their roles in primary cilia, shear stress sensation, alteration of intracellular calcium, and planar cell polarity (which regulates tubular diameter) have been shown. ADPKD is a ciliopathy that arises from abnormalities in the primary cilium. Numerous mutations, including deletions, substitutions, and frame shifts, have been identified in the genes encoding the polycystins, all of which, however, appear to diminish cellular function and alter cellular physiology and cell proliferation/cell/matrix interactions, leading to cyst formation. The phenotype produced by these abnormal proteins is essentially identical in morphology, and the clinical presentation is similar, except that PKD2, the less common genotype, progresses to end-stage renal failure at a slower rate. Mutations in the gene GANAB and DNAJB11 missense mutations have been identified in “genetically unresolved” ADPKD patients; 13 GANAB encodes glucosidase II subunit a (GIIa), which is required for ciliary localization of polycystin-1, and DNAJB11 is a molecular chaperone that regulates apoptosis and P53 regulation of cell proliferation. These mutations have also been identified in autosomal dominant polycystic liver disease (ADPLD), and the common mutations explain the phenotypic overlap between the two diseases.
Autosomal recessive polycystic kidney disease (ARPKD) is a rare disorder that occurs in 1 in 6000 to 1 in 55,000 live births. It was previously termed infantile polycystic disease because it manifests at birth and results in significant perinatal mortality. In approximately three-fourths of cases, ARPKD results in death within a few days of birth when associated with pulmonary hypoplasia. Occasional patients have survived through adolescence. A diverse subset of those who survive the neonatal period have clinically significant congenital hepatic fibrosis, which can lead to portal hypertension.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here