Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
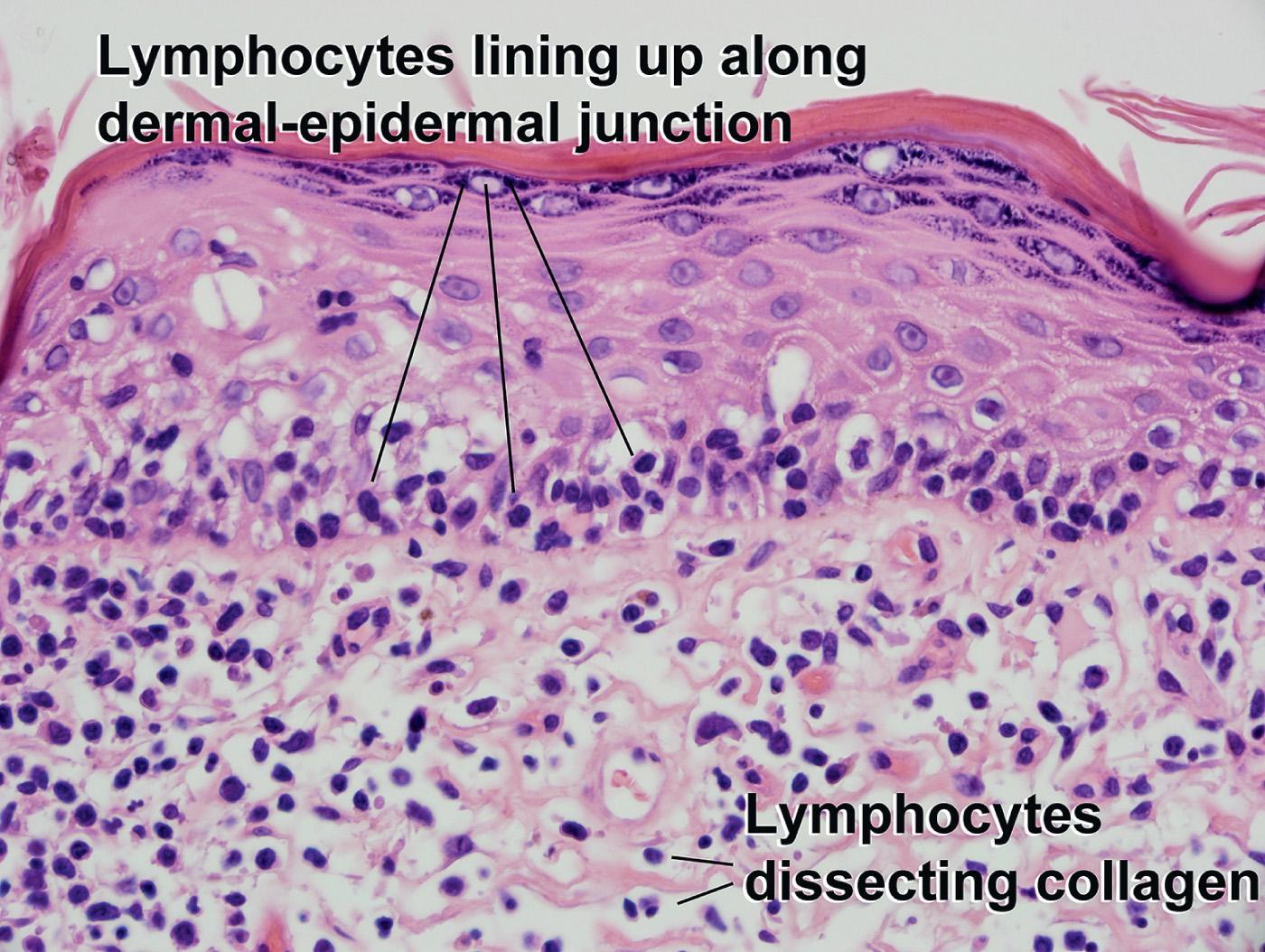
Lymphocytes “line up” along the dermal–epidermal junction (simulates vacuolar interface dermatitis, with a “lymphocyte in every hole”)
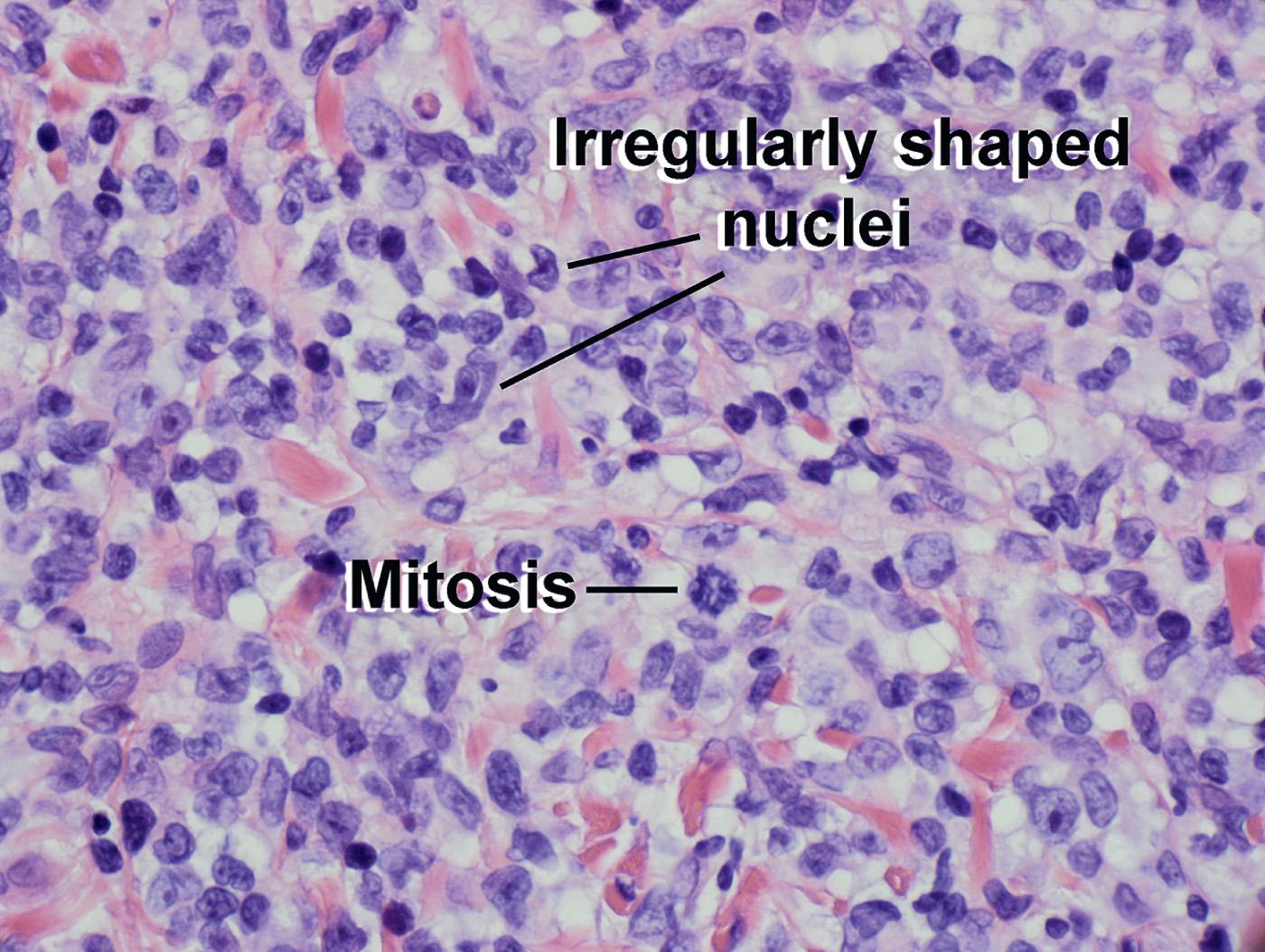
Large dark lymphocytes with irregular nuclear contours and perinuclear haloes (“lump of coal on a pillow”)
Pautrier microabscesses (intraepidermal clusters of atypical lymphocytes, larger than the benign recruited dermal lymphocytes)
Mild bandlike infiltrate in superficial dermis
Sclerosis of the papillary dermis
Eosinophils and necrotic keratinocytes are rarely present
Mycosis fungoides (MF) is the most common type of cutaneous lymphoma. In most cases, the disease is indolent and slowly progressive over a period of years or decades. Three main stages of the lymphoma are recognized: patch, plaque, and tumor. In the patch stage of MF, patients typically present with broad pink or tan, oval-shaped patches with a predilection for the bathing trunk area. The patches may be asymptomatic or pruritic. Both clinically and histopathologically, distinction from eczematous dermatitis is sometimes difficult in the earliest stages of the lymphoma. In the evaluation of patch-stage MF, multiple shave biopsies are often helpful, because the shave technique provides a broad area of epidermis for examination. The typical immunophenotype is CD3+, CD4+, CD8−, CD30−. Aberrant immunophenotypes (with loss of normal T-cell markers, such as CD7) can frequently be demonstrated. Clonal rearrangement of the T-cell receptor gene is helpful in supporting the diagnosis, although the earliest cases may sometimes not have a detectable clone.
Dermatitis generally has more spongiosis and fewer intraepidermal lymphocytes.
Lichenoid drug eruption typically has more apoptotic keratinocytes and eosinophils.
Lymphomatoid drug eruption may look nearly identical. Pautrier microabscesses favor MF.

|
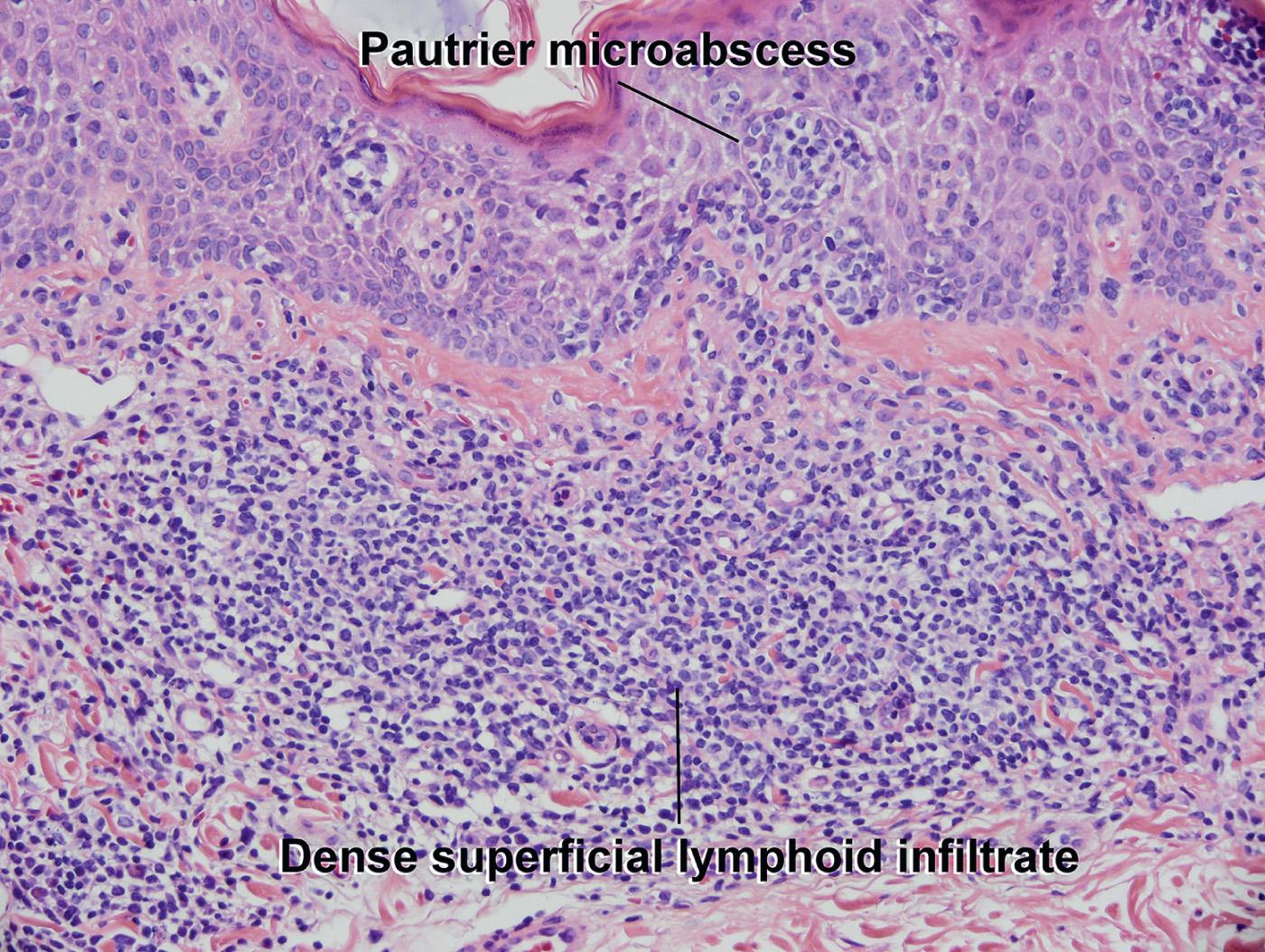
Like patch stage, but with a denser, bandlike infiltrate in the upper dermis
Atypical lymphocytes present in the dermal band
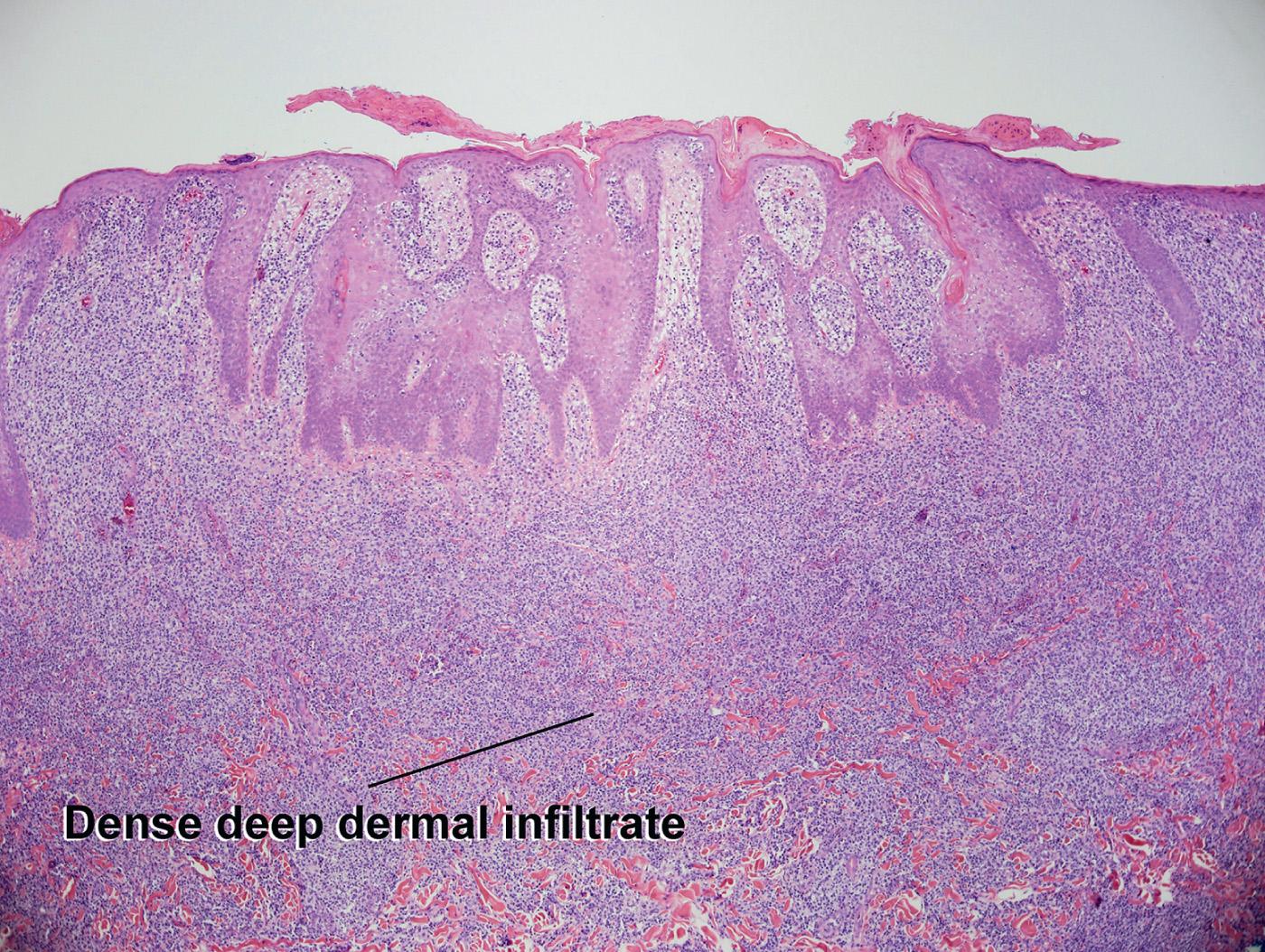
Dense, nodular lymphocytic infiltrate in the superficial and deep dermis
Many atypical lymphocytes present in the dermal infiltrate
Transformation to large-sized lymphocytes in some cases
Acquisition of CD30 expression in some cases
Loss of epidermotropism with progression
Over time, patients with MF may progress to develop thicker plaques and tumors. Whereas patch-stage MF is usually associated with long survival, the prognosis is poorer for those patients who progress to the tumor stage.


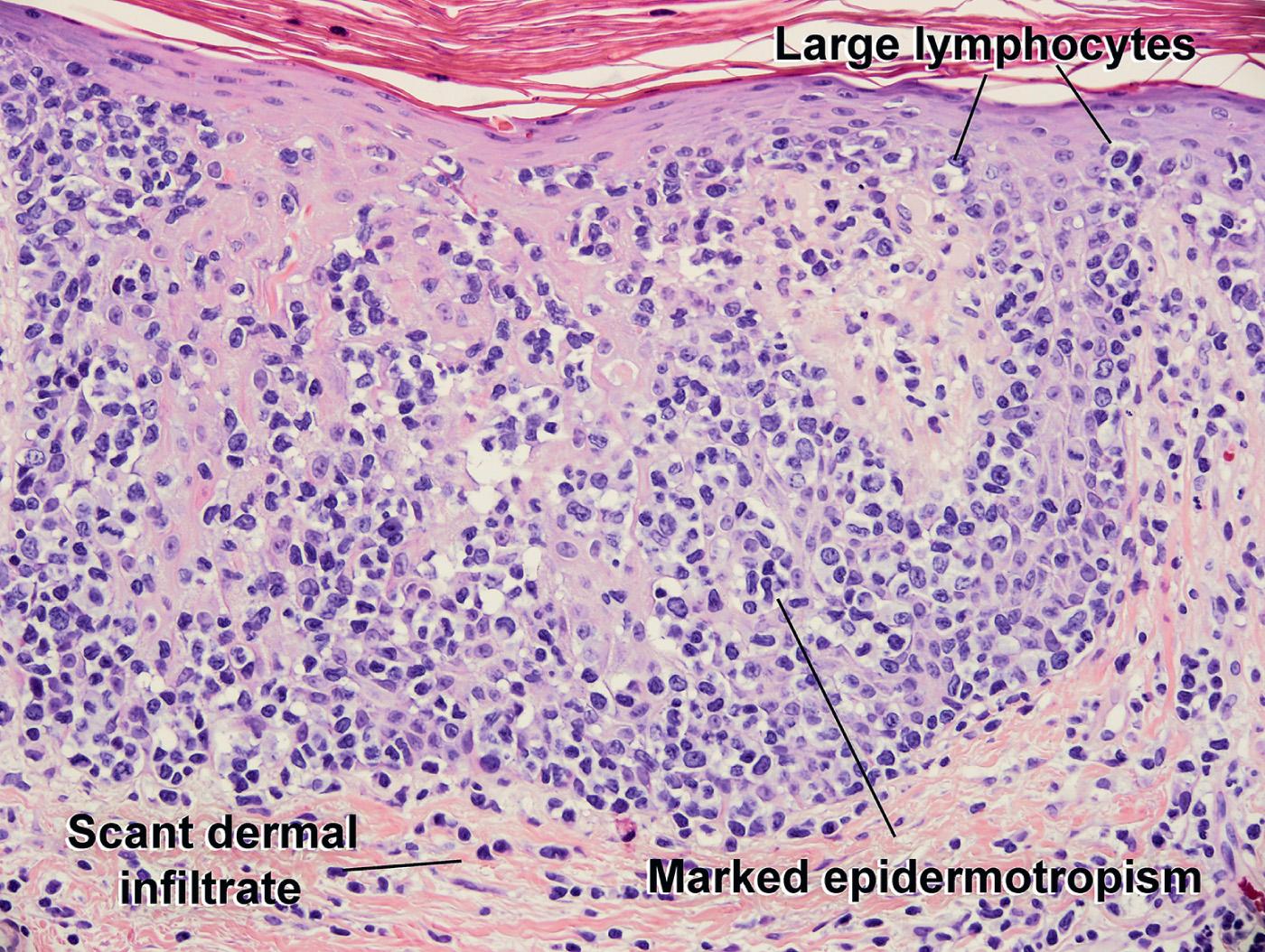
Solitary or multiple patches or plaques on distal extremities
Atypical large lymphocytes extensively infiltrating the epidermis
CD3+
CD4+ CD8− or CD4− CD8+
Small reactive lymphocytes in papillary dermis
The term pagetoid reticulosis is now limited to the Woringer–Kolopp type, which presents as one or several patches or plaques on the distal extremities. This type of lymphoma is associated with an excellent prognosis. The disseminated Ketron–Goodman type of pagetoid reticulosis has been reclassified into several other types of cutaneous T-cell lymphoma.
Atypical lymphocytes infiltrate the follicular epithelium
Basaloid induction and hyperplasia of follicular epithelium
Eosinophils common
Follicular mucinosis (pools of mucin in the follicular epithelium)
Epidermis usually spared
CD3+, CD4+, and CD8− in most cases
The folliculotropic variant of MF presents with follicular papules and boggy plaques, most frequently involving the head and neck. Follicular mucinosis is often present. Folliculotropic MF is generally associated with earlier progression than conventional MF.

Pendulous folds in intertriginous regions
Preceded by insidious onset of patches, papules, and plaques
Massive dermal and subcutaneous infiltrate ± epidermal involvement
Small T lymphocytes with epidermotropism and mild cytologic atypia
Huge multinucleate giant cells with numerous nuclei, often in wreathlike arrangement
Phagocytosis of lymphocytes by multinucleate cells
Dermal edema or fibrosis
Loss of dermal elastic tissue fibers
CD3+, CD4+, and CD8− immunophenotype
Granulomatous slack skin syndrome is an extremely rare variant of MF with a slowly progressive clinical course. The clinical presentation is striking. In fully developed cases, massive folds of skin extend from flexural areas, such as the axillae or groin. An association with nodal Hodgkin lymphoma has been documented in multiple cases.


Erythroderma with generalized pruritus
Palmoplantar keratoderma
Generalized lymphadenopathy
Peripheral blood Sézary cell count of ≥1000 cells/microliter
Peripheral blood lymphocytes with aberrant phenotype or CD4/CD8 ratio >10
Clonal rearrangement of T-cell receptor genes in blood and/or skin
Histopathology like MF, or may show only nonspecific dermatitis
Although previously believed to be a leukemic variant of MF, Sézary syndrome is now considered to originate from a different subset of T cells (central memory T cell in Sézary syndrome versus skin resident memory T cell in MF). In contrast to MF, Sézary syndrome has a rapidly progressive clinical course and a poor prognosis. The diagnosis is confirmed by evaluation of the peripheral blood. Skin biopsy is sometimes useful in demonstrating a histopathologic pattern similar to MF. It should be recognized, however, that skin biopsy findings may be nondiagnostic in some cases of Sézary syndrome. In an erythrodermic patient, nonspecific biopsy findings (such as spongiotic dermatitis) do not exclude the diagnosis. It is important to evaluate the peripheral blood if there is clinical suspicion for Sézary syndrome.

Endemic in Japan, Caribbean, southeastern United States, and central Africa
Hypercalcemia
Osteolytic bone lesions
Organomegaly
Lymphadenopathy
Dermal and/or subcutaneous lymphoid infiltrates
T cells with multilobed nuclei
Epidermotropism in some cases
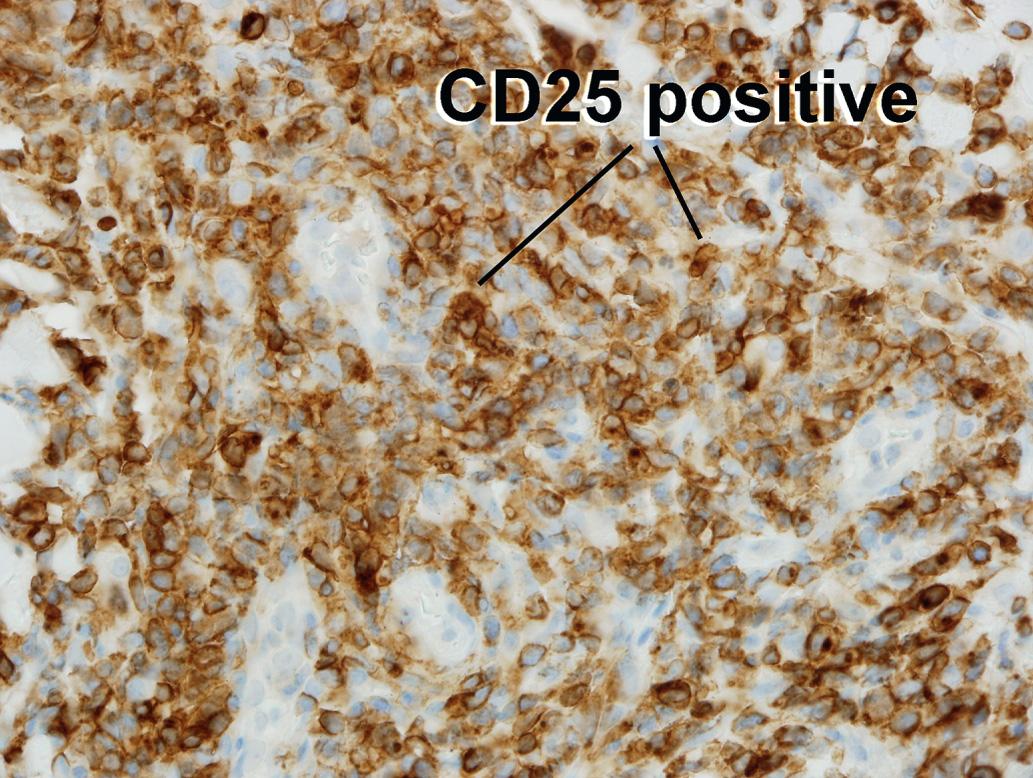
CD3+, CD4+, CD8−, and CD25+ neoplastic cell population in blood, nodes, and skin
Peripheral blood flower cells with multilobed nuclei
Clonal integration of the HTLV-1 genome within neoplastic cells
Clonal rearrangement of T-cell receptor genes
Adult T-cell leukemia/lymphoma is remarkable for its well-established viral etiology, that is, human T-cell leukemia virus type 1 (HTLV-1). HTLV-1 infection is transmitted by sexual contact, blood transfusion, and mother-to-child vertical transmission. In areas with the highest rates of HTLV-1 infection, such as southwestern Japan, only a relatively small percentage of infected individuals eventually develop the lymphoma or leukemia. ATCLL may occur as an acute or smoldering disease. The skin is involved in up to 50% of patients. Nodules, papules, and plaques may occur. The most specific diagnostic finding is the clonal integration of the HTLV-1 genome within lymphoma cells. This feature is helpful in distinguishing the smoldering variant of ATCLL from MF.

Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here