Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Critical care medicine is a requisite aspect of the perioperative course for a significant portion of children presenting for surgery and other procedures necessitating anesthesia. Accordingly, the pediatric anesthesiologist’s understanding of the tenets of critical care medicine is exceedingly important. Critical care facilitates the optimization of critically ill patients before presentation for some patients, and postoperative care is routinely completed in the intensive care unit (ICU) for many patients. In addition, critical care expertise can be a valuable resource during anesthetic management in select cases. This chapter will review of some of the common physiologic principles of management, focusing on potential interplays between the ICU and intraoperative management. In general, communication with the ICU team is of the utmost importance to ensure that intraoperative goals are supporting fastidious recovery in the ICU.
Actual or impending respiratory failure is a common reason for admission to the pediatric intensive care unit (PICU), and one in six patients admitted requires mechanical ventilation ( ). Although some patients with healthy lungs require mechanical ventilation for nonrespiratory reasons, the majority have acute respiratory failure due to lung parenchymal disease. The treatment of acute respiratory failure remains primarily supportive. The goal of treatment is to avoid further damage to the lungs and other organ systems during the period required for the lungs to heal.
Ventilators commonly used in the pediatric intensive care unit (PICU) are capable of sophisticated measurements and analysis of airway pressure, gas flow, and carbon dioxide. All ventilator modes may be understood by identifying the variables that are controlled (see Fig. 58.1 ). In almost all cases, a preset rate is provided. The size of the breath delivered may be controlled either by setting a tidal volume to be delivered or by setting an airway pressure to be achieved.
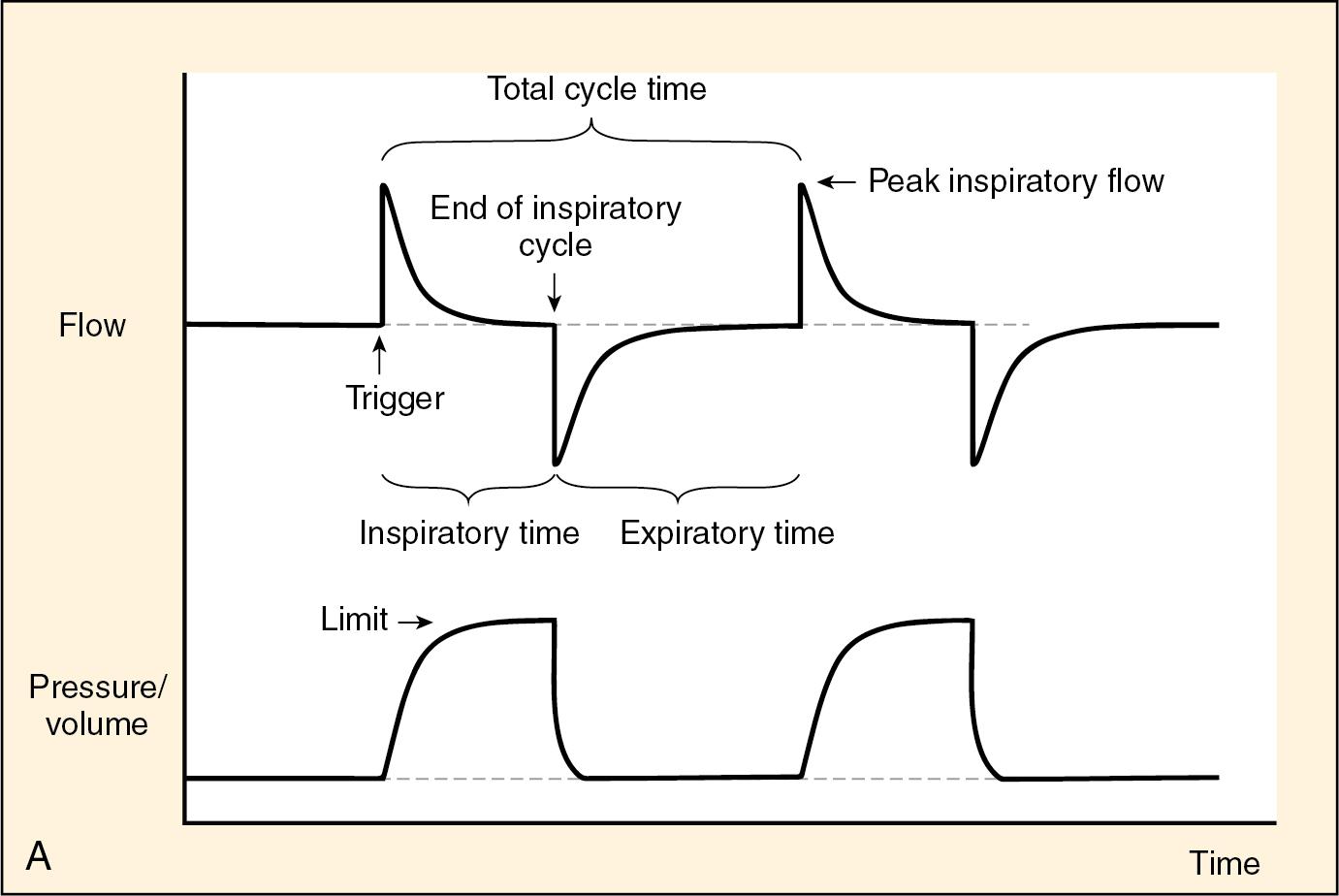
In volume-control modes, the size of the breath is controlled by targeting a volume to be delivered. The ventilator senses air flow and calculates the volume as a product of flow and time. Most sophisticated volume ventilators measure and adjust the gas delivery on the basis of exhaled tidal volumes. When air leaks from the chest by routes other than the gas delivery circuit (e.g., a bronchopleural fistula or a leak around the endotracheal tube), volume delivery may be inaccurate. When compliance of the respiratory system is poor, a percentage of the tidal volume is lost in the circuit because of expansion of the ventilator tubing. The loss of tidal volume may be estimated using a compliance factor that is specific to the diameter of the ventilator tubing. The most sophisticated ventilators (Servo 300i, Siemens Medical Solutions, Stockholm, Sweden) can adjust for this inaccuracy if that setting is selected. The most accurate measures of tidal volumes are obtained at the endotracheal tube using an inline respiratory profile monitor (CO2SMO Mainstream, Respironics, Murrysville, PA, USA). When the size of the breath is controlled by volume delivered, decreasing compliance in the lung will result in higher pressures achieved. An inspiratory pause gives the pressure in the circuit time to equilibrate between the proximal airway and the alveoli and will help determine whether the alveoli are being distended by high pressures. In the setting of a noncompliant, diseased lung, the duration of inhalation or inspiratory time will affect the pressures achieved in volume-control modes.
In pressure-control modes, the size of the breath delivered is determined by the target pressure and the inspiratory time. Pressure-control modes were developed for use in small infants before sophisticated methods existed to control delivery of very small tidal volumes. The volume delivered at a given airway pressure will depend on the compliance of the lungs. As the patient’s disease process resolves and compliance improves, tidal volumes may become large, placing the patient at risk for ventilator-induced lung injury (VILI). The very high initial flow rates necessary to achieve a given pressure for the duration of the inspiratory time can result in turbulence in a constricted airway, which results in decreased dynamic compliance and smaller tidal volumes delivered.
Pressure-regulated volume control (PRVC) is a volume-controlled mode with a flow pattern similar to a pressure-controlled breath. A target tidal volume is set, and the ventilator applies constant pressure throughout inspiration to achieve that target volume. Volume delivered is measured on exhalation, and the pressure is adjusted in subsequent breaths to deliver the target tidal volume. Because exhalation volume is used to calculate the pressure needed for the next breath, PRVC may not be used when there is significant air leak outside the ventilatory circuit.
PRVC shares with pressure control the advantages of a larger area under the pressure-time curve, leading to higher mean airway pressure (mPaw) and improvement in oxygenation. PRVC shares with volume control the safety of a controlled tidal volume that cannot increase as compliance improves or decrease as compliance worsens. This mode is particularly useful when dramatic improvement in compliance is expected, such as after the administration of surfactant. When PRVC is not available as a synchronized mode, significant inspiratory effort may result in breath stacking or extreme variability in tidal volumes delivered. For this reason, PRVC is usually used with very heavy sedation or neuromuscular blockade (or both), except with the Servo 300i, which can provide synchronized intermittent mandatory ventilation with PRVC.
This ventilation is a time-triggered, pressure-limited, inverse-ratio mode of ventilation. It has some potential advantages including improved alveolar recruitment and oxygenation, preserved spontaneous ventilation, reduced barotrauma, reduced left ventricular transmural pressure, and improved hemodynamic stability. It has been proposed to be beneficial for patients with acute respiratory distress syndrome (ARDS). Airway pressure release ventilation (APRV) may be contraindicated in patients with severe obstruction to exhalation, such as status asthmaticus.
Although there is evidence to demonstrate improved physiologic parameters in animal models, there is no evidence this mode of ventilation improves mortality in the pediatric patient with ARDS ( ). In fact, some literature indicates there may be increased mortality ( ; ).
Effort above a preset rate may or may not be supported. The most common form of support is pressure support. Pressure support has been shown to facilitate weaning, and it may also improve patient comfort. Pressure support is frequently used to recondition weakened respiratory muscles while still providing mandatory breaths to prevent atelectasis; however, data to support this approach do not exist ( ).
All ventilation modes generate inhalation pressures above a set positive end-expiratory pressure (PEEP). The application of PEEP improves functional residual capacity and minimizes shearing forces that result from repeated opening and closing of lung units. Increasing PEEP also increases MAP, which improves ventilation/perfusion (V/Q) matching and oxygenation. High levels of PEEP may impair venous return to the thorax and thus cardiac preload. When there is significant resistance to exhalation, as in status asthmaticus, the patients may have an increase in intrinsic PEEP, which increases the resting lung volume and decreases inspiratory capacity. Intrinsic PEEP can be measured with an expiratory pause pressure.
Over the past few years, there has been an increase in the use of noninvasive ventilation to prevent or delay endotracheal intubation. Noninvasive positive pressure ventilation (NPPV) includes continuous positive airway pressure (CPAP), which is used to increase functional residual capacity (FRC) and to overcome inspiratory airway collapse, and biphasic positive airway pressure, which also supports respiratory effort with positive inspiratory pressure. Studies in adults have shown clear benefit to NPPV, especially when endotracheal intubation can be avoided, and with no decrement when endotracheal intubation is merely delayed ( ). NPPV has been demonstrated successfully in pediatric patients with neuromuscular disease, upper airway obstruction, status asthmaticus, and cystic fibrosis ( ).
There are many delivery devices for noninvasive ventilation, but most are poorly adapted to infants and toddlers. Skin breakdown under the mask or nasal delivery device is common, so strict attention to skin care is necessary when noninvasive ventilation goes on for more than a few hours. It is ideal to have more than one delivery device (e.g., nasal prongs and a mask) when NPPV is used for more than a few days, as rotation between devices can avoid skin breakdown. These devices are commonly used in the long term at home for children with neuromuscular disease.
Successful use of NPPV requires careful patient selection and close monitoring for signs of response or failure. NPPV should be used in patients with normal mental status and normal airway protective reflexes. Patient cooperation is necessary, and NPPV for a severely anxious patient is unlikely to succeed. It is also not very likely to succeed for patients with poor mask fit, copious secretions, or significant acid-base disturbances. NPPV should not be used in patients with upper gastrointestinal (GI) bleeding, recent upper airway or GI tract surgery, or hemodynamic instability. Patients on NPPV should be carefully monitored for synchrony between patient and ventilator. A decrease in respiratory rate is a fairly reliable sign of effective response and is usually seen within 1 hour of initiation of therapy. In a patient with severe hypoxemic respiratory failure, converting to endotracheal intubation from a high level of support with NPPV can result in severe desaturations. The decision to intubate should be considered when the patient’s fraction of inspired oxygen (Fi o 2 ) requirement rises above 60%.
Respiratory failure is defined as failure to oxygenate or ventilate ( ) and can be broadly separated into four categories: disordered control of breathing, upper airway obstruction, lower airway obstruction, and parenchymal lung disease (see Table 58.1 ). Physiologic support can be as simple as oxygen supplementation for mild parenchymal disease or as significant as invasive mechanical ventilation or extracorporeal membrane oxygenation (ECMO) support.
| Etiology | Examples | Potential Therapies |
|---|---|---|
| Disordered control of breathing | Residual anesthetic Neuromuscular weakness (including residual neuromuscular blockade) Central apnea (e.g., neonates) Encephalopathy Respiratory splinting due to poor pain control |
Invasive mechanical ventilation Rule out/reverse residual medication effects Consider noninvasive positive pressure ventilation (NPPV) +/− backup rate for selected patients |
| Upper airway obstruction | Postextubation stridor Obstructive sleep apnea |
|
| Lower airway obstruction | Mucus plugging Asthma Congenital malacia of large airway Airway foreign body |
|
| Parenchymal disease | Atelectasis Pulmonary edema Lung contusion Pneumonia/pneumonitis Pediatric acute respiratory distress syndrome (PARDS) |
|
Preoperative respiratory failure can be attributed to many etiologies and is beyond this review, but consideration for postoperative respiratory failure is a frequent task for the anesthesiologist.
Several pediatric pulmonary conditions warrant special consideration by the anesthesiologist. First, pediatric acute respiratory distress syndrome (PARDS) is associated with significant morbidity and mortality ( ). The oxygenation index (OI = [F i o 2 × mean airway pressure × 100]/P a o 2 ) defines the severity of disease and also provides some prognostic value, with a predicted 40% mortality when OI exceeds 16 (see Box 58.1 ). Because a relatively large part of long-term morbidity and mortality is related to iatrogenic lung injury during the acute phase of illness, overall strategies for PARDS target safe, but not perfect, oxygenation and ventilation; protecting the long-term health of the lungs is prioritized over “normal” laboratory values.
Although there are few outcome data for optimal management of PARDS, patients with PARDS are generally mechanically ventilated with delivered tidal volumes of 5 to 8 mL/kg ideal body weight to minimize barotrauma and volutrauma. Open lung strategies should be employed with PEEP titration to avoid lung decruitment (atelectotrauma). Permissive hypoxemia (oxygen saturation goal >88%) and hypercarbia (pH target >7.25 without regard to Pa co 2 ) are also acceptable targets for postoperative management and can facilitate less injurious ventilation settings. Similarly, oxygen toxicity is known to occur at high oxygen concentrations, and Fi o 2 should therefore be kept as low as safely possible (ideally <0.4), even if this requires oxygen saturation goals that are not normal. Adjunctive therapies such as inhaled nitric oxide, recombinant surfactant administration, corticosteroids, prone positioning, long-term neuromuscular blockade, and advanced modes of ventilation (such as high-frequency oscillatory ventilation [HFOV], high-frequency percussive ventilation [HFPV], and APRV) may have utility in select patients, but at this time have insufficient evidence to support their routine recommendation for pediatric patients. In severe cases of reversible lung disease, ECMO support (when initiated early and in centers with appropriate expertise) can be a lifesaving measure, allowing time for potential lung recovery to occur while minimizing further lung injury.
A second special consideration is the weak child; weakness may be due to genetic neuromuscular disease such as spinal muscular atrophy, acquired neuromuscular disease such as spastic cerebral palsy, or functional disease such as thoracic insufficiency syndrome. Although each of these conditions, and indeed each individual patient, often requires custom-tailored management, in general these patients present unique challenges to the anesthesiologist due to their inability to recruit and maintain lung units normally and their decreased secretion mobility and clearance. These features put such patients at high risk of perioperative adverse respiratory complications including prolonged intensive care admission and even requirement for tracheostomy following “elective” surgery.
Strategies for management of weak children can include (1) preoperative optimization with both inhalational and mechanical therapies for lung recruitment; (2) intraoperative consideration for whether to control ventilation, whether to place an invasive airway, and how best to maintain lung recruitment; and (3) postoperative planning including extubation strategies, postoperative destination (often an ICU), and pain management. For some procedures, these patients may be managed with noninvasive respiratory support and maintained airway clearance mechanisms. For others, endotracheal tubes (ETTs) are needed and may be required into the postoperative period. Interdisciplinary planning between surgery, anesthesiologist, and intensivist is critical for optimal care of these patients throughout the perioperative period.
The decision to continue invasive mechanical ventilation postoperatively may also dictate a change in selection of endotracheal tubes. While a Cochrane review did not support the routine use of nasotracheal tubes, found that selected patients at risk for long-term invasive ventilation with a low risk of sinusitis may benefit from improved tube securement with a nasal ETT. Bent ETTs (nasal Ring, Adair & Elwyn [RAE] and oral RAE) make both suctioning and tube positioning more challenging in the postoperative arena and should be changed to straight tubes whenever possible. Long-term airway damage such as subglottic stenosis is a major concern in these patients and should preclude oversizing of ETTs. Cuffed ETT with cuff pressure titrated to minimal occlusive volume is an ideal method of ensuring tracheal tissue perfusion and integrity.
With regard to modes of invasive mechanical ventilation, there is insufficient outcome data to support a recommendation for any particular mode of ventilation for children, with or without respiratory pathology ( ). Common modes of conventional mechanical ventilation employed in pediatrics include pressure controller modes and flow controller modes of ventilation. In a pressure controller mode of ventilation, a constant pressure is delivered for each mechanical breath. Examples are pressure control (PC), synchronized intermittent mandatory ventilation-pressure control (SIMV-PC), and some hybrid modes of ventilation. In pressure controller modes of ventilation, pressure is the controlled variable, and the delivered tidal volume will vary based on the resistance and compliance. Inspiratory flow will decelerate with time in order to maintain constant pressure. With flow controller modes of mechanical ventilation, inspiratory flow is the controlled variable, usually set to target a particular tidal volume, and pulmonary pressure is the dependent variable. Examples are volume control (VC) and synchronized intermittent mandatory ventilation-volume control (SIMV-VC). Pressure will vary based on resistance. For all of the previously mentioned forms of ventilation, triggering variables includes pressure, time, and/or flow.
Intermittent mandatory ventilation (IMV) is a mode of ventilation that allows patients to breathe spontaneously in between ventilator cycled breaths. This is most commonly used in a synchronized mode (SIMV), in which the mandatory ventilator breaths are synchronized with the patient’s own respiratory effort. This mode of ventilation is often employed with the addition of pressure support, in which the patient’s respiratory efforts above the mandatory set ventilator rate are supported with a preset amount of positive pressure. This SIMV can be used with PC, in which pressure limited breaths are given over a set inspiratory time, or with VC, in which a set tidal volume is delivered over a set inspiratory time with a constant flow.
Modern intensive care ventilators, as well as some anesthesia workstations, are capable of delivering hybrid modes of ventilation. These modes (with proprietary names such as Pressure-Regulated Volume Control and Volume Control with AutoFlow) combine the convenience of set tidal volumes with a decelerating inspiratory flow pattern to allow for some of the benefits of both PC and VC modes. For most of these hybrid modes, a microprocessor analyzes the pressure-volume relationship of each individual breath in real time and uses that information to determine an optimal decelerating flow pattern for the subsequent breath.
The most commonly used modes of noninvasive ventilatory support are high flow nasal cannula, CPAP, and bilevel positive airway pressure (BiPAP). A high-flow nasal cannula provides a heated, humidified flow of oxygen and/or air. Flow is delivered through nasal prongs and can vary in amount ranging from 1 to 2 L/kg per min in infants and children and up 40 L/min in adults. With both CPAP and BiPAP, there is a range of nasal and full-face interfaces that can be used. CPAP delivers a continuous level of positive pressure, and the patient’s own respiratory effort is responsible for all inspiratory flow. BiPAP, as the name suggests, has two set pressures (inspiratory positive airway pressure [IPAP] and expiratory positive airway pressure [EPAP]) and can also have a backup rate set, but does not require one.
Pediatric shock states are all characterized by inadequate metabolic substrate delivery to tissues. Broadly broken down into hypovolemic, distributive, cardiogenic, and obstructive categories, shock is a common reason for children to require postoperative critical care services. Because hypovolemia is a problem frequently managed in the operating room and addressed elsewhere in this text, we will not discuss it in this chapter other than to remind readers that many pediatric patients will not become hypotensive until they are profoundly hypovolemic. Similarly, congenital and acquired cardiac disease are not in the scope of this chapter; please see Chapter 57 (Cardiopulmonary Resuscitation) for details about management of cardiac arrest and Chapters 30 (Anesthesia for Congenital Heart Disease) and 48 (Cardiovascular Disorders) for details about management of patients with congenital heart disease. This chapter will focus on distributive, neurogenic, cardiogenic, and obstructive shock categories in patients with structurally normal hearts.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here