Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Craniofacial anomalies occur in as many as 1 in 500 to 1000 live births for cleft lip and palate, 1 in 2000 for isolated craniosynostosis, and 1 in 20,000 to 50,000 live births for some syndromic types of craniosynostosis. Many of these deformities may be treated by the otolaryngologist (head and neck surgeon) with pediatric and/or facial plastic training.
Craniofacial defects are often part of a broader syndrome affecting many functional systems.
Appropriate care is best rendered by a multidisciplinary team with diverse expertise.
Craniofacial disorders are etiologically diverse and genetically heterogeneous.
Craniofacial skeletal anomalies are treated surgically with techniques that are reliable and reproducible.
The application of craniofacial surgical principles, developed to treat congenital problems, also serves well for tumor extirpation and trauma repair.
Several materials are currently available to reconstruct the craniofacial skeleton, but there is wide effort to develop more biomimetic bone substitutes.
Craniomaxillofacial plastic and reconstructive surgery is an ever-evolving medical and surgical subspecialty dedicated to restoring form and function to the soft tissues and craniofacial skeleton. Much of the field addresses congenital deformities in the pediatric population, although craniofacial techniques and principles cross over into the management of traumatic and neoplastic deformities in both adult and pediatric patients. Although attempts to alter craniofacial anatomy by surgery or intentional deformation date back thousands of years, much of the growth in this field has occurred in the last few decades. The approaches and techniques allow for safe, reliable manipulation of the craniofacial skeleton, stable fixation, and decreased likelihood of relapse. Craniofacial anomaly refers to any malformation that involves the face, cranium, or cranial base.
Congenital craniofacial deformities commonly occur as isolated defects and less often as part of a syndrome. The Committee on Nomenclature and Classification of Craniofacial Anomalies of the American Cleft Palate–Craniofacial Association classifies craniofacial malformations according to five categories: (1) facial clefts/encephaloceles and dysostoses, (2) atrophy/hypoplasia, (3) neoplasia/hyperplasia, (4) craniosynostosis, and (5) unclassified. Clinical entities such as orbital hypertelorism often exist within a syndrome that clearly fits into one of the aforementioned classifications. Hypoplasia of the midface and micrognathia is one example of atrophy/hypoplasia. Hematologic disorders, neural growth disorders, and overly aggressive cerebrospinal fluid (CSF) shunting may result in secondary craniofacial disorders in children, which may be classified as hyperplasia. Acquired deformities of the craniofacial complex also include those secondary to trauma. Neoplasm (third category) and its treatment are classified as acquired deformities.
The field of craniofacial surgery is large, with many and varied subfields including neurosurgery, plastic or facial plastic surgery, oral-maxillofacial surgery, otolaryngology, head and neck surgery, and pediatric subspecialties of all these. Because the care of such patients is complex, a multidisciplinary team including not only surgeons but also geneticists, pediatricians, anesthesiologists, ophthalmologists, speech and swallowing experts, audiologists, dentists, and orthodontists is often involved. The objective of this chapter is to provide an overview of craniofacial surgery, providing a framework based on the pathophysiology of major craniofacial anomalies and focusing on cranial vault and craniofacial bony defects. Disorders of first and second branchial arch derivatives that give rise to known craniofacial anomalies are briefly described. Trauma and neoplasm are important contributors to craniofacial disease and are examined briefly in this chapter but discussed in greater detail elsewhere in the text. Facial and palatal clefting are very important subjects in craniofacial surgery.
Craniofacial anomalies have been reported to constitute approximately one-third of all congenital defects. Although the incidence of assorted individual deformities and syndromes varies, the overall incidence is considered to be 0.2 to 0.5 per 1000 births. Interestingly, some craniofacial deformities occur at a uniform rate across racial and ethnic populations, whereas others vary in frequency by race and ethnicity.
Craniofacial anomalies have been reported to occur as part of a genetic condition in about 20% of cases. However, with the discovery of new syndromes and the recognition that “isolated defects” often have a genetic etiology, the proportion of genetic cases is likely to be higher. Particularly for syndromic forms of craniosynostosis, transmission often occurs via autosomal dominant or recessive inheritance, and most anomalies have similar incidences in males and females, although metopic and sagittal synostosis are more common in boys and unilateral coronal and lambdoid more common in girls. In general, the pathogenesis of craniofacial anomalies is complex, and their etiology is best described as multifactorial.
According to the functional matrix theory, the growth of the craniofacial skeleton occurs in response to growth of soft tissue structures nearby, that is, neural tissue growth and expansion mediates calvarial growth. The developing brain highly influences the morphology of the basicranium, which in turn functions as a template for subsequent facial growth. This hypothesis reasonably accounts for “what” takes place, but the study of growth factors, growth factor receptors and their underlying genetic transcription factors, and gene regulation have yielded new information on “how” such growth takes place. Currently, craniofacial growth is understood to be a highly complex process, not solely regulated by the central nervous system. During development, the pattern of growth is influenced both by functional needs and by physical and biochemical relationships with nearby structures.
Osteogenesis takes place through one of two pathways. In intramembranous ossification, mesenchyme becomes osteoblasts, which produce randomly oriented collagen fibers within the homogenous matrix collectively known as primary bone. This immature bone is replaced by secondary bone as the osteoid becomes ossified. Endochondral bone formation, which is typical of long bone ossification, is the process in which cartilaginous areas are replaced by the calcification of perichondral matrix. The brain and calvarium typically are 25% of eventual adult size at birth; they double in size by age 6 months and triple in size by age 2 years, reaching 95% of adult size by age 10 years. The cephalic index (CI) is defined as the maximal transverse width (euryon)/maximal anteroposterior (AP) length ×100; normative cephalic indices are usually between 75% and 85%.
Craniofacial physiology is affected by both displacement and remodeling. Displacement occurs when the growth of the surrounding soft tissue together with growth center/sutural activity results in pushing the bone away from its articulation with other bones. Remodeling occurs by resorption and deposition of new bone, resulting in a net vector of growth. The importance of soft tissues in determining growth is exemplified by “adenoid facies.” The entire craniofacial complex system is plastic and in flux throughout the growing period, and it retains some plasticity even after growth is complete, albeit to a far lesser extent. Thus rigid nonbiologic implants such as titanium have the potential to interfere with normal growth if used during active development because they lack the plasticity that is essential for dynamic growth.
Throughout the period of craniofacial growth, an overriding homeostasis exists among the different fields of growth. Small aberrations in one field may be counterbalanced by compensatory growth in another field. This constant equilibrium is very important to bear in mind when planning surgical or orthodontic intervention, as treatment failure or relapse is likely if that equilibrium is significantly disrupted. Generally, interventions are more likely to succeed if the cause of the abnormality is addressed during periods of rapid growth. For example, when treating alveolar clefts, bone grafting is more effective during phases of mixed dentition than after eruption of the permanent cuspid.
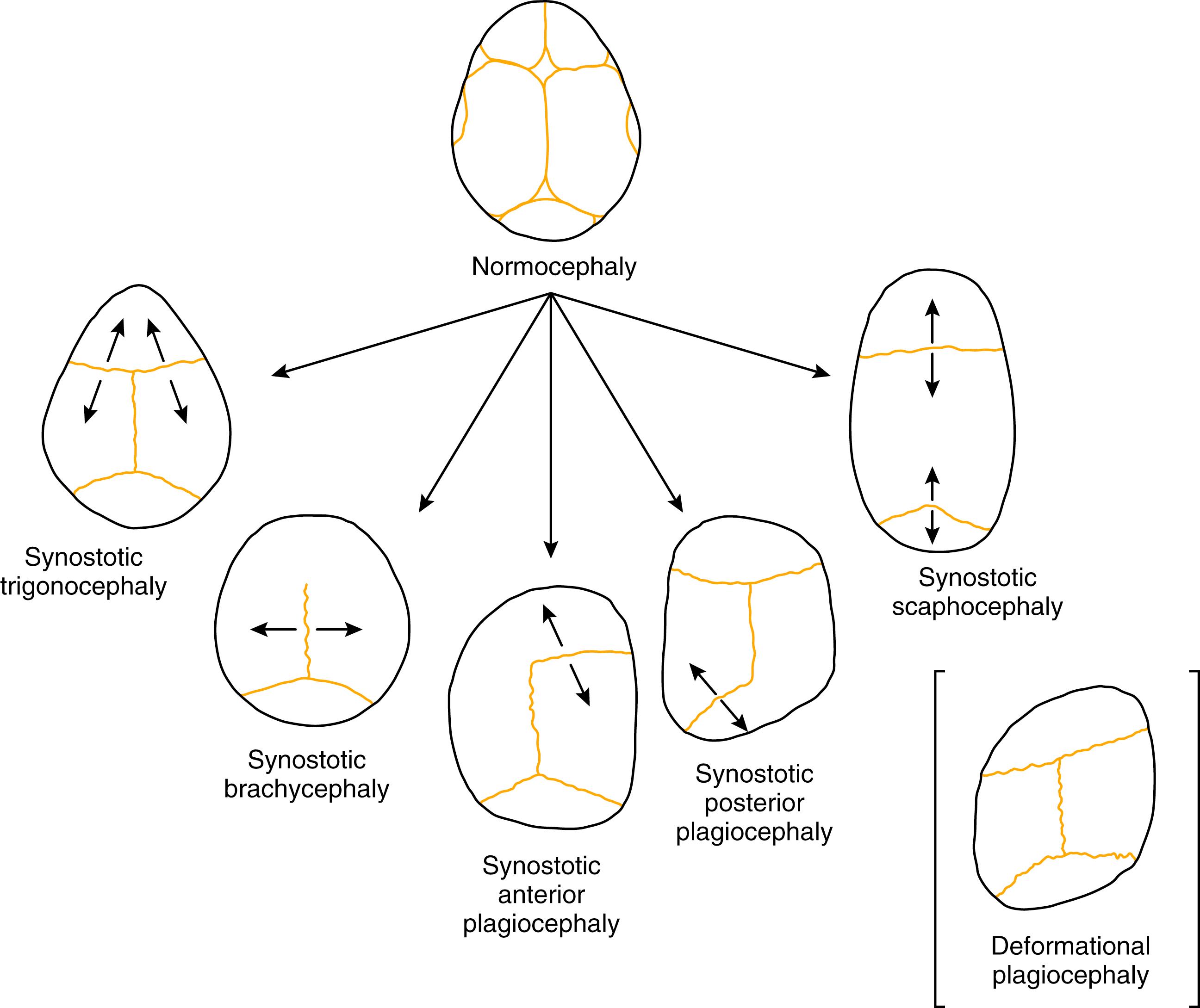
Craniofacial synostosis, or craniosynostosis, occurs when one or more than one suture closes prematurely. The pathophysiology of primary craniosynostosis demonstrates the effects of defective skeletal growth and the importance of homeostasis in craniofacial development ( Fig. 6.1 ). The causes of craniosynostosis are likely multifactorial and the source of some controversy. , Craniosynostosis has been associated with abnormalities in sutural biology, various biomechanical forces, and defects in the primary growth centers of the involved bones. Sutural growth is also affected by surrounding tissue interactions. The contacting dura, for instance, influences the continued patency of sutures and helps regulate their closure. Regardless of the underlying etiology of premature closure of cranial sutures, it is clear that a disruption in normal growth near the affected cranial suture(s) leads to compensatory growth in the cranium, at times the basicranium and even the facial skeleton, depending on both type of synostosis and duration of disease. If the synostotic suture is treated early in life while craniofacial growth is still quite active, normal homeostasis may be restored by releasing the synostotic suture, although sometimes with a tendency to relapse to the presurgical shape, and sometimes with inhibition of growth. Once active growth is largely complete, treatment will have to address the morphologic sequelae of the synostotic suture, including structures affected by compensatory growth. Late repairs are more likely to be stable and more resistant to relapse or negative growth effects, but this stability often occurs at the expense of more difficult and bloody surgeries to achieve desired changes in shape, and increased likelihood of bony lacunae/deficiency compared with early surgery.
Fusion of the facial processes during embryogenesis is another fundamental aspect of facial growth that is critical to normal development. Orofacial clefting results from a defect in this process. For opposing facial processes to fuse in the midline, the intervening epithelia must be eliminated so that the underlying mesenchymal tissue may coalesce. In the palate, epithelial elimination has been shown to occur through apoptosis, transdifferentiation of epithelial to mesenchymal cells, or cell migration. Lack of fusion may occur as a result of excessive apoptosis, underdevelopment of the mesenchyme of the involved subunits, or inadequate migration or reduced proliferation of neural crest ectomesenchyme. Facial clefting may also result from constricting anatomic defects. For example, in the Pierre Robin sequence, mandibular hypoplasia contributes to glossoptosis (inferior or posterior displacement of the tongue), which prevents the palatal shelves from fusing in the midline.
A diverse and heterogeneous group of factors may contribute to abnormal craniofacial development, but etiology remains incompletely understood. Great advances have been made in the last few decades toward an understanding of various genetic defects implicated in the development of craniofacial anomalies. Mutations in a variety of genes have been identified that are directly associated with syndromic or nonsyndromic (isolated) craniofacial defects ( Table 6.1 ). Major categories of proteins encoded by these genes include:
Growth factor receptors: fibroblast growth factor receptors (FGFRs) 1, 2, and 3; tumor growth factor-β receptors (TGFβRs) 1 and 2; PTC and FGD1 gene products
Ephrins: membrane-anchored ligands for Eph receptor tyrosine kinases
Transcription factors: MSX2, ALX4, GLI3, MITF, PAX3, RUNX2, TWIST
Connective tissue structural proteins: type II and XI collagen, fibrillin-1 (FBN1)
| Condition | Craniofacial Phenotype | Gene † |
|---|---|---|
| Loeys-Dietz syndrome (aortic aneurysm, arterial tortuosity, hypertelorism, cleft palate/bifid uvula, craniosynostosis) | Craniosynostosis, cleft palate, hypertelorism | TGFβR1 TGFβR2 |
| Apert syndrome | Craniosynostosis (brachycephaly), wide midline defect closes by coalescence of bony islands, midface malformations; dental crowding, cleft or narrow palate with swellings | FGFR2 |
| Beare-Stevenson cutis gyrata syndrome | Craniosynostosis (kleeblattschädel) or cloverleaf skull | FGFR2 |
| Boston-type craniosynostosis | Craniosynostosis (kleeblattschädel), forehead retrusion, frontal bossing | MSX2 |
| Cleidocranial dysplasia | Delayed suture closure, frontal parietal bossing, wormian bone(s), hyperdontia, tooth eruption defects | RUNX2 |
| Craniofrontonasal syndrome | Craniosynostosis (brachycephaly), central defect between frontal bones, hypertelorism, divergent orbits | EFNB1 |
| Crouzon syndrome | Craniosynostosis (brachycephaly), pronounced digital impressions of skull, midface hypoplasia, shallow orbits | FGFR2 |
| Crouzon syndrome with acanthosis nigricans | Craniosynostosis | FGFR3 |
| Greig cephalopolysyndactyly | Craniosynostosis in small percentage of cases, frontal bossing, sagittal ridging, hypertelorism | GLI3 |
| Muenke-type craniosynostosis (nonsyndromic) | Craniosynostosis (brachycephaly) | FGFR3 |
| Parietal foramina | Symmetric parietal bone defects, cleft lip/palate | MSX2, ALX4 |
| Parietal foramina with cleidocranial dysplasia | Symmetric parietal bone defects | MSX2 |
| Pfeiffer syndrome | Craniosynostosis (brachycephaly) | FGFR1, FGFR2 |
| Saethre-Chotzen syndrome | Craniosynostosis (especially brachycephaly), flat forehead, low hairline | FGFR2, TWIST1 |
| Shprintzen-Goldberg (marfanoid) syndrome | Craniosynostosis (especially lambdoid and sagittal sutures), maxillary and mandibular hypoplasia, palatal abnormalities | SKI |
| Thanatophoric dysplasia II | Craniosynostosis (cloverleaf skull/kleeblattschädel deformity) | FGFR3 |
† Gene abbreviations that have not been defined/spelled out are not acronyms or abbreviations.
FGFRs are transmembrane receptors for fibroblast growth factors (FGFs). FGFs are involved in regulating cell proliferation, differentiation, and migration through a number of pathways. The receptor is made up of an extracellular domain (receptor-ligand binding), a transmembrane domain, and an intracellular tyrosine kinase enzymatic domain (see Table 6.1 ). There are several variations of the FGFR protein, all of which have important cell growth functions. Signaling via FGFR1, for instance, has been shown to facilitate neural crest migration, and a defective receptor has been linked to midline facial clefting in mice. Defects in FGFR1 have been found to cause Kallmann syndrome type 2, which can include cleft lip and palate. Defects in FGFR1 and FGFR2 have been linked to syndromes such as Pfeiffer syndrome (in which both receptors are defective) and to other forms of craniosynostosis. , Mutations in FGFR2 have been shown to be responsible for Apert syndrome, , Jackson-Weiss syndrome, Crouzon syndrome, and Beare-Stevenson cutis gyrata syndrome.
Skeletal growth in utero and beyond are regulated by FGFR genes. A defective FGFR3 protein is responsible for crouzonodermoskeletal syndrome (Crouzon syndrome with acanthosis nigricans), Muenke-type craniosynostosis, and achondroplasia. In achondroplasia, the epiphyseal growth plates in long bones prematurely fuse, leading to abnormalities in the long-bone growth plates that resemble the gross and histologic pathology seen in synchondroses of the calvaria. In Fgfr3 knockout mice (mice in which the gene that encodes this protein has been eliminated), there is severe retardation of skeletal maturation. ,
The relationship between genetic mutations and the consequent syndromes or anomalies may be complex and difficult to predict. Defects in each of the three domains (in different FGFRs) may be associated with similar craniofacial abnormalities, yet defects in the same domain of one FGFR type may cause different phenotypes. For instance, identical mutations have been discovered in patients with Crouzon, Pfeiffer, and Jackson-Weiss syndromes, suggesting the involvement of other factors in the ultimate phenotypic expression. Conversely, as is evident in Fig. 6.2 , mutations in several distinct domains on one particular FGFR can result in the same phenotype.
As previously mentioned, defects in transcription factors and connective tissue proteins are also implicated in the pathogenesis of craniosynostosis. MSX2 is a homeobox gene that encodes a DNA binding transcription factor. It has been associated with nonsyndromic craniosynostosis (Boston type), , and ALX4, another homeobox gene, has been linked to parietal foramina. Most cases of Saethre-Chotzen syndrome are caused by haploinsufficiency of the TWIST gene, which appears to encode a transcription factor. Mutations in GLI3, a transcription factor gene, are responsible for Grieg cephalopolysyndactyly (a rare syndrome that can include craniosynostosis). , Susceptibility to isolated sagittal synostosis is associated with loci within the BBS9 and near , the s gene, and mutations in FREM1 are associated with an increased risk of metopic synostosis.
Genetic defects encoding several different extracellular matrix constituents may lead to anomalies of the craniofacial complex. Defective collagen, such as that seen in osteogenesis imperfecta, is clearly associated with bony abnormalities, although this disease only occasionally affects the craniofacial skeleton. Defective fibrillin (encoded by FBN1 ) results in Shprintzen-Goldberg syndrome with craniosynostosis and maxillary/mandibular hypoplasia. Genes responsible for Stickler syndrome include COL2A1, COL9A1, COL11A1, and COL11A2, which encode types II, IX, and XI collagen, respectively. , There are additional forms of Stickler syndrome for which the genes are yet unknown.
Several metabolic derangements are known to interfere with craniofacial development, including mucolipidosis, hyperthyroidism, hypophosphatemia, and rickets. , The mucopolysaccharidoses are a group of disorders characterized by a deficiency of lysosomal enzymes that results in accumulation of mucopolysaccharides. Although there are several clinical entities, patients with some of the more severe variations of these diseases have large and dolichocephalic (long and narrow) skulls with premature closure of the sagittal suture and poor pneumatization of the mastoids and paranasal sinuses.
Environmental factors that contribute in utero to craniofacial maldevelopment may be categorized as teratogenic, infectious, nutritional, and mechanical. Fetal alcohol syndrome may manifest with a wide range of defects, including holoprosencephaly. Tobacco use during critical periods of embryogenesis has also been shown to adversely affect craniofacial development. Medications such as hydantoin, phenytoin, valproic acid, and isotretinoin (a vitamin A derivative used in the treatment of acne) can exert deleterious effects on embryogenesis, as can toluene, the environmental contaminant dioxin, and ionizing radiation. , ,
Viral infections during pregnancy may affect expression of cleft lip/palate in patients with mutations of PVRL1 and IRF6. Nutritional intake is also clearly linked to craniofacial development. As cholesterol modulates sonic hedgehog ( SHH ) signaling, which is important in mediating craniofacial development, diets and medications with profound effects on cholesterol levels may cause craniofacial malformations. , The link between a diet deficient in folic acid and neural tube defects has been well documented and has led to the widespread supplemental addition of folic acid to many processed foods throughout the developed world.
Finally, deformation of the craniofacial skeleton may occur because of intrauterine constraint, which may result from persistent or abnormal fetal lies (the relationship between the long axis of the fetus with respect to the long axis of the mother). Other factors associated with intrauterine constraint include breech presentation, early pelvic engagement of the head, oligohydramnios, primigravidity, multiple gestations, uterine malformations, amniotic bands, and defects in fetal neuromuscular development. ,
Several postnatal conditions predispose an infant to disrupted craniofacial growth and secondary craniosynostosis. Hematologic diseases such as thalassemia, sickle cell anemia, congenital hemolytic icterus, and polycythemia vera are associated with hyperplasia of the bone marrow, leading to bony overgrowth in the skull, which may in turn cause the calvarial sutures to fuse prematurely. Iatrogenic craniofacial anomalies may occur in patients who require cranial ventricular shunts. Excessive shunt volumes may result in a lack of tension across the sutures, which produces an environment that mechanistically resembles conditions predisposing to microcephaly. Trauma and neoplasms are rare causes of acquired craniofacial deformities.
Craniofacial anomalies range from mild functionally asymptomatic defects to severe anomalies that are not compatible with life such as some types of holoprosencephaly. The craniofacial surgeon may be consulted prenatally based on abnormalities identified on ultrasound or other types of antenatal testing, or sometime thereafter when a baby or child is noted to have an abnormal head shape or unusual craniofacial dysmorphism. A comprehensive history and examination may prompt further consultation with other experts if a syndrome is suspected or discovered. Evaluation by a skilled geneticist in conjunction with genetic testing may also yield important information, although genetic testing does not necessarily change the diagnosis or determine management. The majority of cases of craniosynostosis are isolated with no other associated anomalies.
Primary craniosynostosis refers to premature closure of one or more cranial sutures, which leads to characteristic growth inhibition of calvarial bone perpendicular to the affected suture line (see Fig. 6.2 ). Because the brain is initially rapidly growing, compensatory growth occurs in other parts of the skull where skull growth is unimpeded. Both the growth restriction and the compensatory changes of calvarial shape occur in largely uniform and predictable ways that are characteristic of the underlying disease process, although there is variability in terms of severity and presentation of these deformities. Secondary synostosis may occur with any congenital, metabolic, infectious, or other disorder that leads to brain underdevelopment and subsequent microcephaly.
In the past, single-suture synostosis was thought to rarely lead to functional consequences or neurodevelopmental delays. However, there is a growing body of research suggesting that even children with single-suture synostosis have a higher incidence of neurodevelopmental delays compared with children without synostosis. Speltz and colleagues in 2015 conducted a multiinstitutional analysis of unoperated single-suture synostosis patients compared with a control group at age 7 years old. The synostosis children had poorer scores on intelligence testing and increased incidence of learning delays and disabilities compared with controls, with the highest incidence in unilateral coronal and lambdoid synostosis and the lowest incidence in sagittal synostosis.
Other reports have suggested a greater frequency of neurodevelopmental delays in trigonocephaly. It is unanswered whether the bitemporal calvarial constriction and supraorbital retrusion that accompany significant trigonocephaly impair development of the brain, or whether an underlying problem with brain growth in those areas leads to the characteristic restrictions of the skull. In one study, the severity of metopic synostosis was not associated with greater delays, whereas a more recent study did find more severe cases of trigonocephaly had greater language processing delays. The role of surgery in minimizing neurodevelopmental delays and behavioral problems remains unanswered, , although limited data show postsurgical improvements in neurodevelopmental delays.
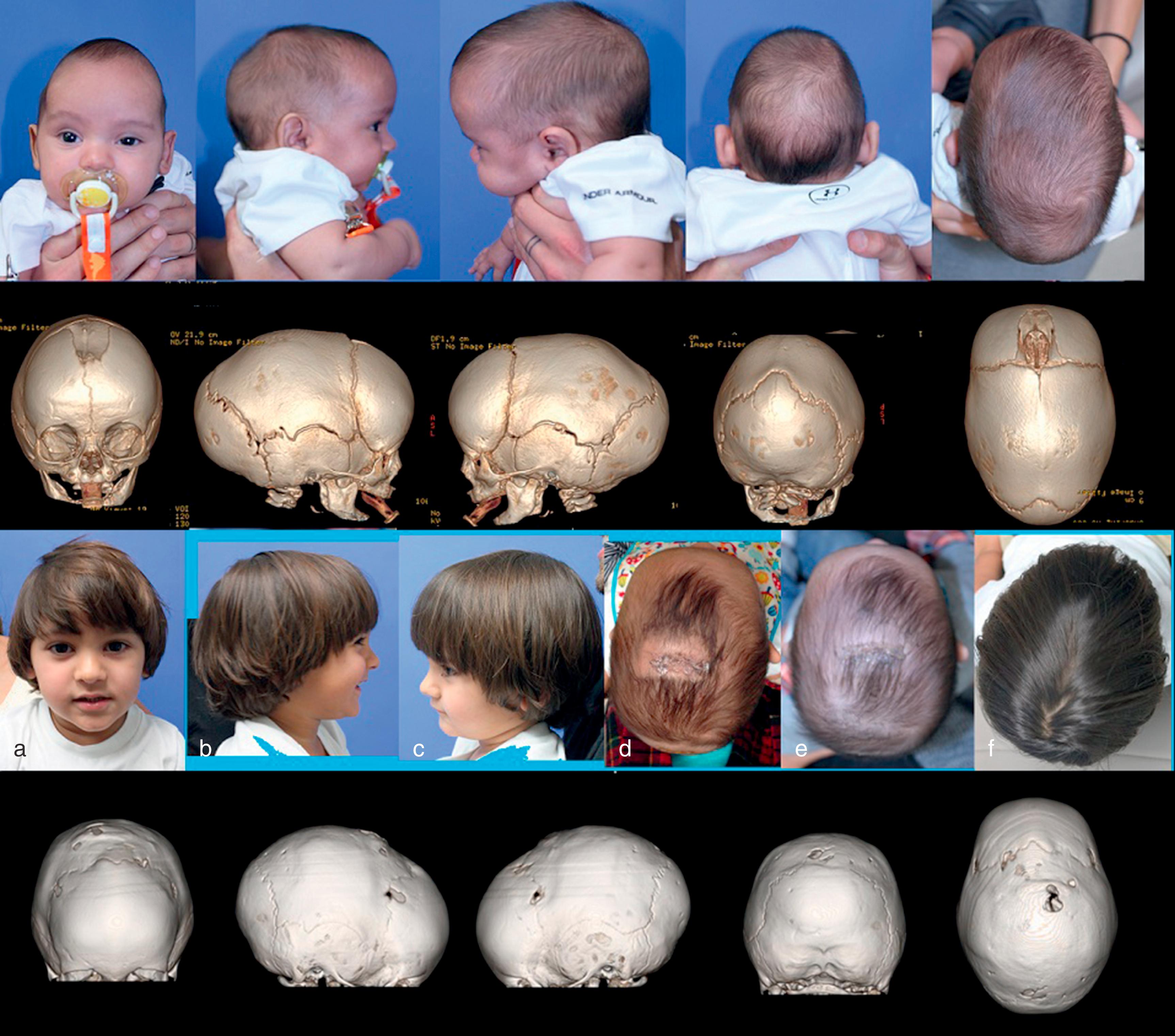
Sagittal synostosis is the most common type of craniosynostosis, typically presenting with scaphocephaly or dolichocephaly (boat-shaped head/long head). In sagittal synostosis, the sagittal suture, or part of it, prematurely fuses (see Fig. 6.2 ). The compensatory growth occurs either posteriorly (leading to occipital cupping, also referred to as “occipital bullet” or bathrocephaly), anteriorly (leading to frontal bossing), or both. The fused sagittal suture restricts growth biparietally, resulting in a head shape that is long and narrow with a CI of less than 75%. A low CI is relatively sensitive, although not specific for sagittal synostosis. Scaphocephaly without synostosis is sometimes found in otherwise normal children with skull measurements that are outside normal range or as a result of prematurity or postnatal positioning. Synostosis may vary from partial to complete synostosis with varying degrees of severity.
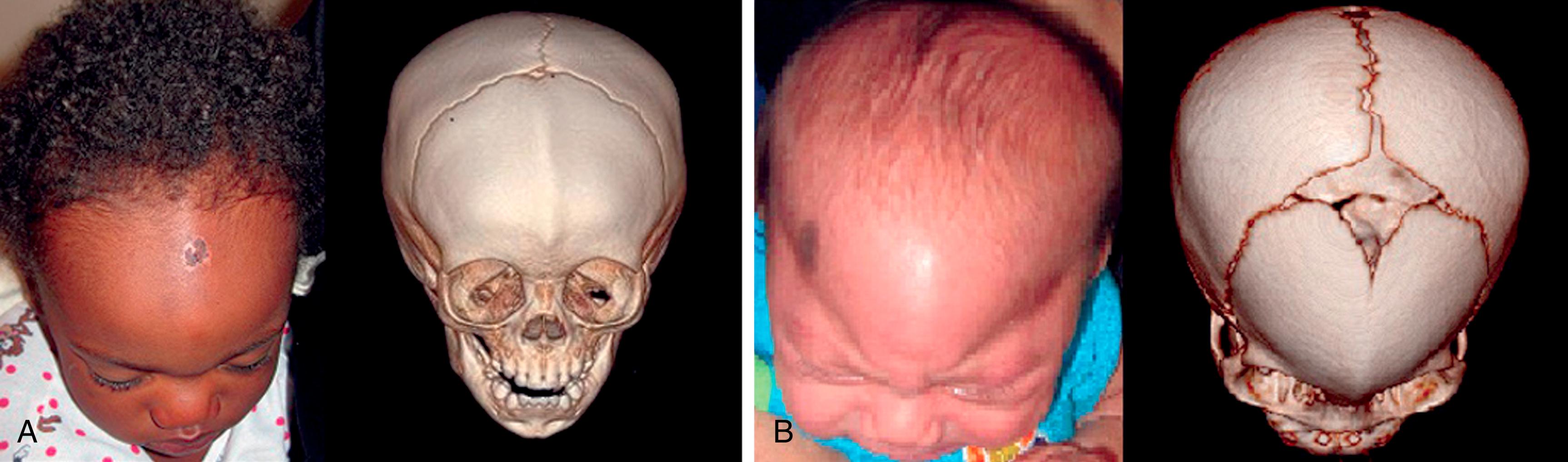
Trigonocephaly is associated with metopic suture synostosis and refers to the characteristic triangular shape of the head when viewed from the vertex or “birds-eye” view. There is a decrease in bifrontal diameter. There can be associated hypotelorism, but functional consequences or neurodevelopmental delays are rare. The suture area is thickened, resulting in an anterior midsagittal ridge, which has been described as resembling the keel of a ship. Metopic craniosynostosis may present along a spectrum from mild, physiologic cases manifested by a vertical ridge in the frontal bones termed a metopic ridge ( Fig. 6.3A ) to more severe cases of trigonocephaly (see Fig. 6.3B ). The metopic suture is unusual in that it fuses much earlier than most of the other sutures, often by 9 months of age. When the metopic suture fuses, it completely disappears both radiographically and clinically, and thus the diagnosis of trigonocephaly is clinical and not radiographic.
Plagiocephaly, meaning twisted or slanted head, is generally used to describe positional or deformational flattening that is nonsynostotic. It typically involves the occiput as the result of repeated postnatal supine sleep positioning. Intrauterine compressive forces likely play some role in deformation plagiocephaly (DP), as evidenced by a higher incidence in males, who are generally larger and more prone to intrauterine constraint. It is estimated that 20% to 50% of all babies develop DP. DP has increased since the early 1990s, when the American Academy of Pediatrics issued recommendations to place sleeping infants in the supine position in an effort to reduce sudden infant death syndrome.
Many babies favor one side of the head when sleeping, particularly premature infants who are slower to develop neck control. This chronic sleep position leads to characteristic unilateral occipital flattening, which often, however, not always, leads to anterior shifting of the same-sided ear and ipsilateral frontal bossing. A “parallelogram” shape of the vertex skull results, although positional brachycephaly in which the entire occiput is flattened is also common. Congenital torticollis of the sternocleidomastoid muscle is present in about 20% of patients with DP, generally occurring contralateral to the side of head tilt. It was long debated whether the torticollis preceded the DP or vice versa, although work by Rogers suggested the former. Congenital torticollis is manifested by ipsilateral head tilt and contralateral head twist and is likely related to fetal positioning and/or intrauterine constraint. It may be treated with neck stretching exercises and physical therapy, and rarely surgery.
Mild positional flattening may self-correct as babies gain increased strength to turn their necks and roll over. When diagnosed in the first months of life, DP can be treated by encouraging “tummy time” or prone positioning while the child is awake. Repositioning the infant to minimize compressive forces on the flattened side and attempt to increase pressure on the more prominent occiput may also be effective. However, once a child reaches 5 to 6 months of age, repositioning maneuvers are less likely to be successful in correcting head shape. For severe deformities, an orthotic molding helmet or band may be useful ( Fig. 6.4 ). Helmets are most effective when worn full time for 3 to 6 months and when started before 6 months of life. There is growing evidence to suggest an association of developmental delays with DP. However, as more severely asymmetric cases of DP are not associated with greater delays, some authors attribute the delays to the underlying factors associated with the pathogenesis of DP and not the DP itself. Treatment of DP is, thus, largely aesthetic; however, it is important to look for and recommend services/treatment for associated delays.
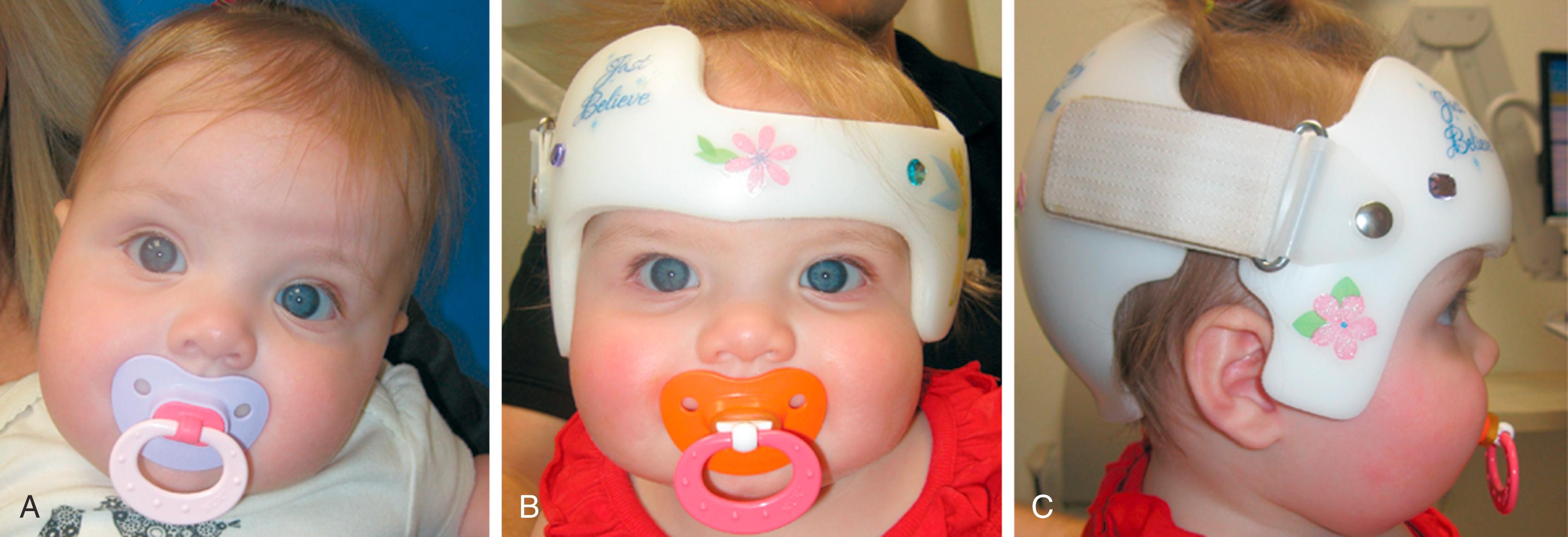
Controversy abounds regarding the treatment of DP. There is a large amount of Level II evidence that helmets are effective and some Level III evidence suggesting helmets are on the one hand better or on the other hand equivalent to repositioning. There is Level I therapeutic evidence that many children will improve without need for a molding helmet. In one randomized controlled study, participants were randomized to either repositioning techniques or a trademarked sleep wrap. There was no difference in groups, with 80% deemed good and 42% having normal head shapes after the study was complete. To date, the only randomized controlled study comparing helmets and repositioning showed equivalent results in the 2 groups of 42 patients treated in the first 6 months of life. The study excluded infants with the most severe asymmetries and torticollis, and their observed normalization rates of 26% in the helmet group and 23% in the repositioning group were lower than other studies have reported.
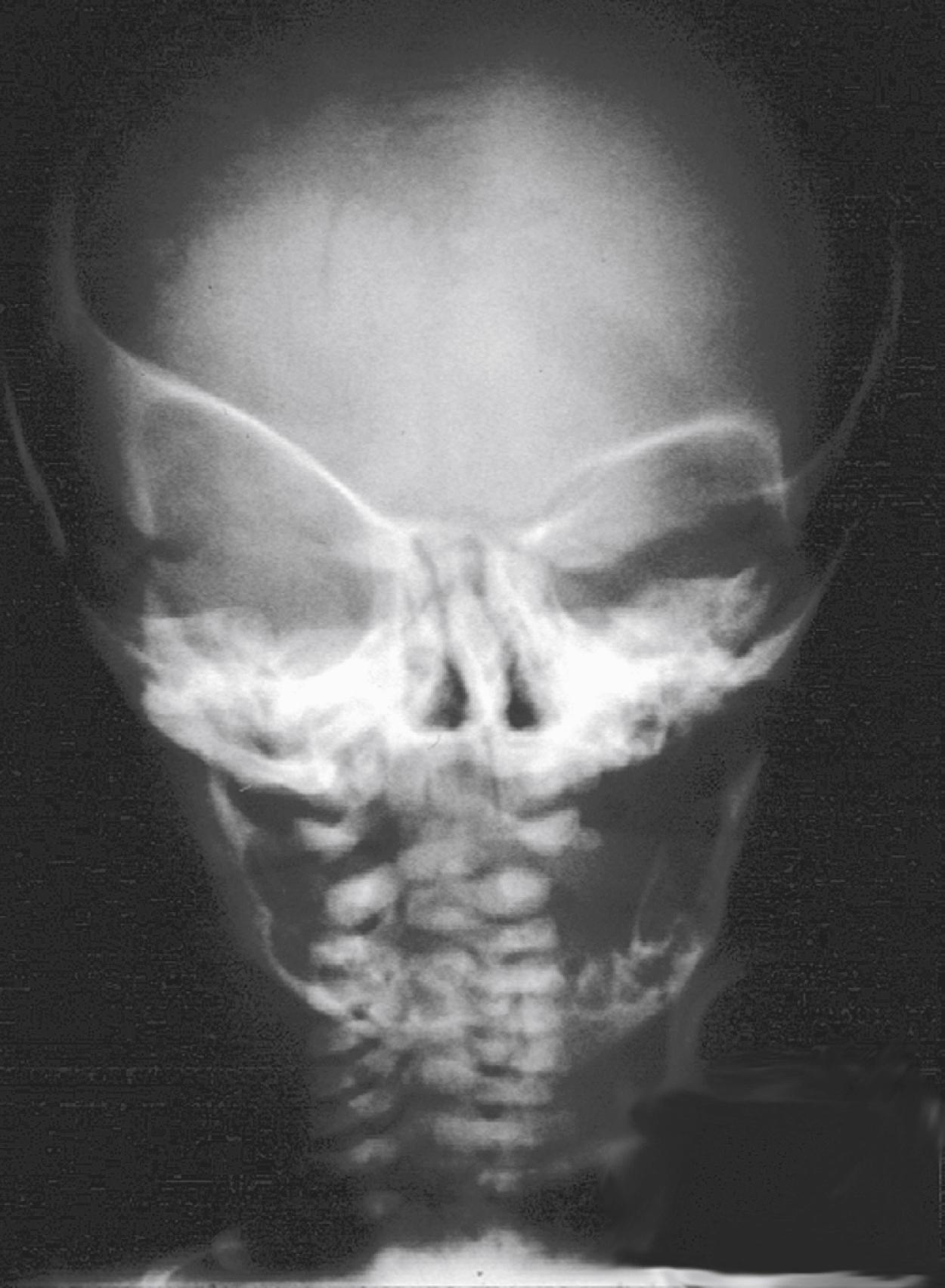
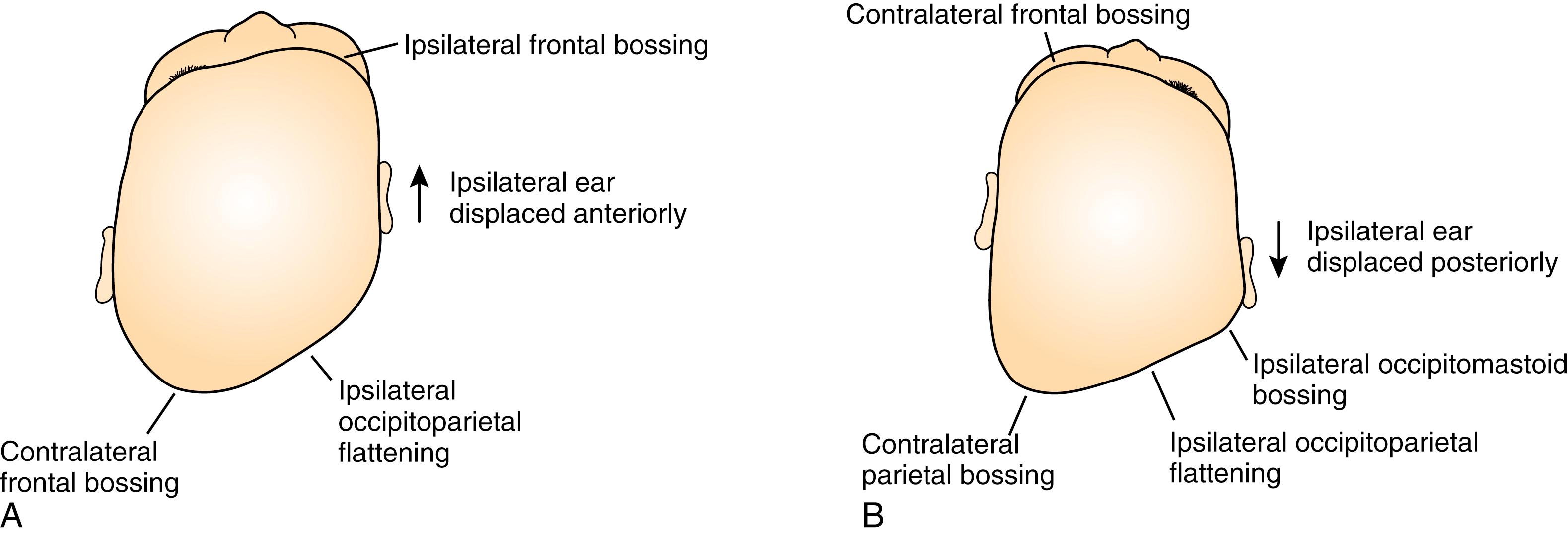
Synostotic plagiocephaly may be either anterior or posterior , referring to unilateral coronal synostosis and lambdoid synostosis, respectively ( Fig. 6.5 ). In coronal synostosis, the forehead of the affected side is flat, whereas the supraorbital rim is elevated and displaced posteriorly, either at or posterior to the plane of the cornea. The supraorbital rim normally lies about 1 cm anterior to the cornea. The nasal root may be deviated to the side of the defect, and the contralateral forehead is bossed. If the supraorbital rim is pushed down, it may lead to vertical dystopia, which lends a quizzical or cockeyed squint to the eyes, manifested as the “Harlequin” appearance on plain x-ray imaging ( Fig. 6.6 ). The ipsilateral auricle is anteriorly displaced, but the occiput is minimally affected.
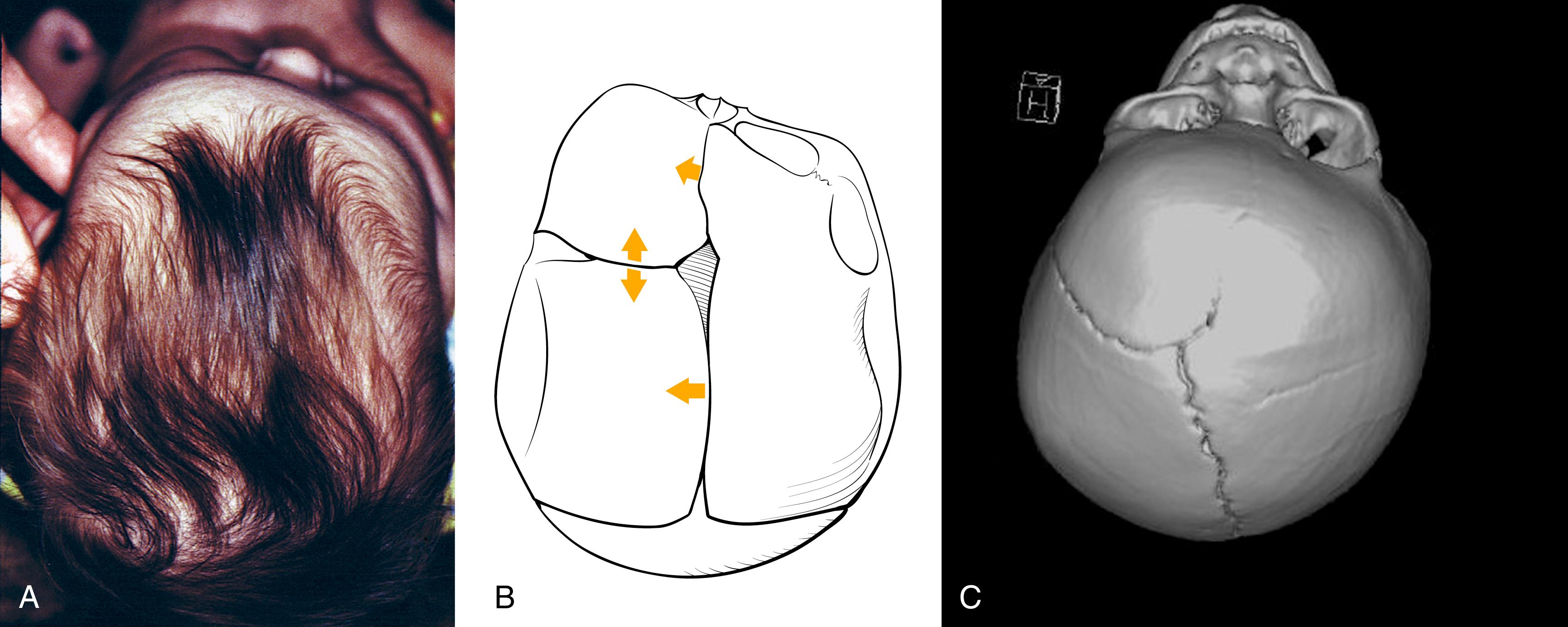
In unilateral lambdoid synostosis, there is an occipital flatness on the affected side, and the ipsilateral auricle is posteriorly displaced. The appearance when viewed from above has been termed a “trapezoid” shape, as the forehead is not typically affected. True lambdoid synostosis is a rare entity estimated to occur in 1 of 100,000 live births. Lambdoid synostosis may be mistaken for the much more common entity of DP, as both involve a flat occiput. However, the diagnosis of DP can usually be established by a thorough history, as it is usually progressive and related to supine positioning on the affected side. Unlike DP, lambdoid synostosis does not characteristically present with ipsilateral frontal bossing, and the auricle is posteriorly rather than anteriorly displaced ( Fig. 6.7 ).
Brachycephaly, or “short head,” occurs when the transverse dimension of the head is as long as the AP dimension. The CI approximates 1. The head has an overall round appearance from the vertex view, as do the head and face from the frontal view. Brachycephaly is most commonly nonsynostotic and related to positioning ( Fig. 6.8 ).
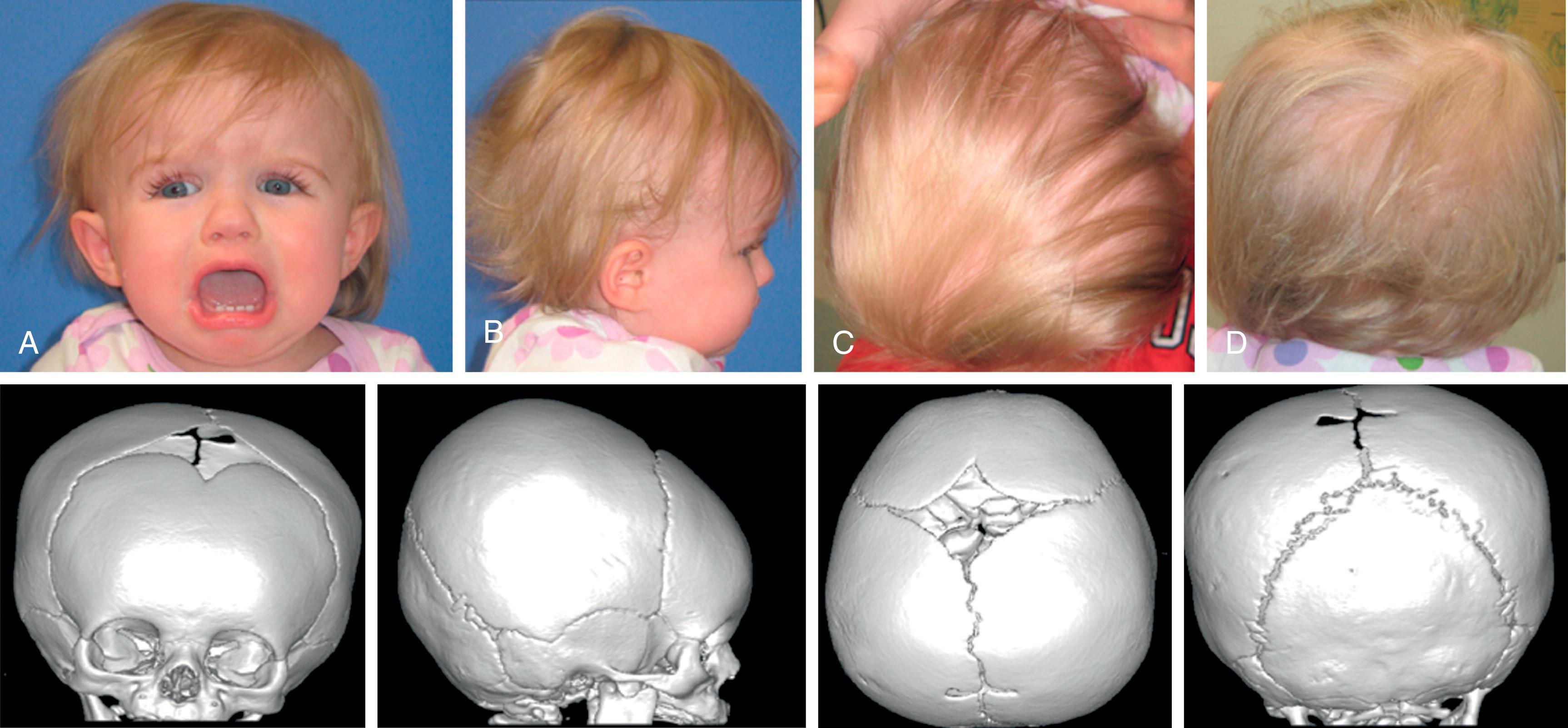
Brachycephaly when present at birth is usually associated with bilateral coronal synostosis. Synostotic brachycephaly is frequently associated with a syndrome (see Table 6.1 ; Fig. 6.9 ). It is often accompanied by turricephaly (tall head) or acrocephaly (pointed head). As a result of this sutural synostosis, the skull is unable to expand in the AP dimension. To accommodate the expanding intracranial content, the skull grows in the lateral and superior dimensions. The neonate’s cranium therefore has a smaller AP distance and greater lateral dimensions. The forehead comes right off the nose with a low hairline, and the glabellar depression is absent. Multisuture synostosis, including synostotic brachycephaly, carries a higher incidence of elevated intracranial pressure (ICP); thus an expert funduscopic exam is important to rule out papilledema.
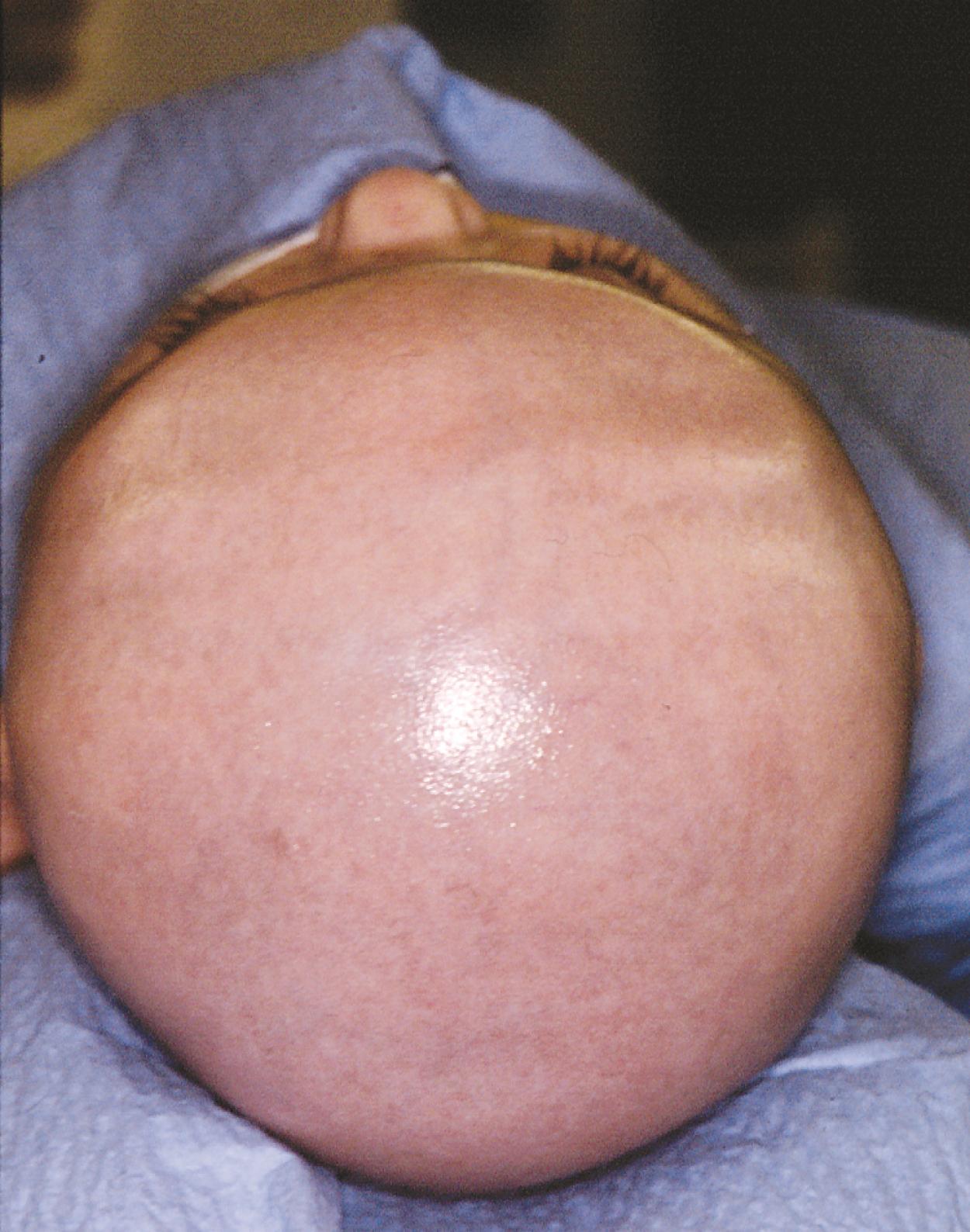
Acrocephaly, also known as oxycephaly, consists of a skull that is high and conical ( Fig. 6.10 ). This abnormality typically develops with multisuture synostosis involving both coronal and sagittal sutures. Compensatory growth occurs through the anterior fontanelle.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here