Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Neuroplastic changes in hearing loss and deafness are evident across the human lifespan.
There are brief sensitive periods in early childhood during which the central auditory pathways are maximally plastic. Audiological intervention within these critical windows may allow for optimal development of the central auditory pathways, overcoming many deleterious effects of auditory deprivation.
Cross-modal reorganization is a form of compensatory neuroplasticity, which occurs when deprivation in one modality (e.g., auditory modality, as in hearing loss) results in the repurposing of its cortical resources by other modalities (e.g., vision, somatosensation). Cross-modal reorganization is evident in children and adults with congenital or acquired hearing loss ranging from mild to profound in severity. Cross-modal reorganization may explain some of the individual variability affecting behavioral outcomes after intervention.
The noninvasive P1 cortical auditory evoked potential (CAEP) biomarker can be used to objectively assess the development and maturation of the central auditory pathways in infants and children, helping to guide clinical decision-making.
Perhaps the most remarkable ability of the human brain is its capacity for change. Neuroplasticity broadly refers to structural and functional neuronal changes that take place across the lifespan. Early in development, neuroplasticity serves as the catalyst for the formation and refinement of the auditory pathways and the establishment of neural networks. During childhood and into adulthood, experience-related inputs (e.g., sensory inputs) drive neuroplasticity, helping to facilitate language acquisition, learning, and memory. Many pediatric otologic conditions are manifested through cascading aberrations in neuroplasticity during development. Many adult-onset otologic conditions occur as a result of insult, injury, or age-related degradation. In either case, compensatory changes in neuroplasticity may occur in an effort to adapt in the face of auditory deprivation. In this chapter, we will review the forces of neuroplasticity within the context of hearing loss across the human lifespan, focusing on how neuroplasticity can be used to guide clinical intervention and rehabilitation.
Typical development of the central auditory pathways is dependent upon whether a child is receiving sufficient auditory input. In the first part of this chapter, we review evidence of a sensitive period for central auditory development, focusing primarily on electroencephalography (EEG) evidence using the P1 cortical auditory evoked potential (CAEP) biomarker in congenitally deaf children receiving cochlear implants (CIs). We demonstrate how audiological intervention may promote optimal brain and behavioral outcomes if delivered within this sensitive period. In contrast, we will describe how prolonged auditory deprivation beyond the sensitive period may contribute to altered development of the central auditory pathways. Using group and case-study evidence, we demonstrate the clinical utility of the P1 CAEP biomarker in making objective decisions about the clinical course of intervention for special pediatric clinical populations including single-sided deafness, auditory neuropathy spectrum disorder, cochlear nerve deficiency, and multiple disabilities.
In the second part of this chapter, we describe new evidence of cortical neuroplasticity in adult-onset, age-related hearing loss (ARHL), including evidence of cross-modal neuroplasticity by the visual and somatosensory systems and the recruitment of additional brain regions (e.g., frontal cortex) for auditory processing using high-density EEG and other neuroimaging methods. We describe the growing body of research linking untreated ARHL to long-term changes in neurocognitive function, and how compensatory changes in neuroplasticity may contribute to individual variability in behavioral outcomes.
Arguably one of the most successful neuroprosthetic devices is the CI. In the coming decades, our knowledge of neuroplasticity may lead to further advancement in auditory pharmacological, technological, and therapeutic innovations for clinical populations with hearing loss. Our goal should be to harness neuroplasticity to direct more timely, targeted, and individualized intervention and rehabilitation to clinical populations in otology.
Pediatric hearing loss is a very prevalent chronic condition in children, affecting 1 to 3 per 1000 children in the United States. Pediatric hearing loss may be congenital or acquired. Among children, genetic forms of hearing loss account for more than 50% of cases, whereas in utero infections, meningitis, hyperbilirubinemia, ototoxic drug exposure, trauma, and other conditions contribute to the remainder of nongenetic cases. Untreated hearing loss during development may have long-lasting effects. For example, pediatric hearing loss has been linked to receptive and expressive delays in oral language acquisition, delays in educational achievement, behavioral and psychosocial problems, negative impacts on quality of life, and a weighty economic burden from a societal and socio-economic standpoint.
Many forms of audiological intervention exist for the treatment of pediatric hearing loss. Hearing aids and CIs are two of the most common forms of intervention. CIs are used to treat deafness while hearing aids are small electronic devices considered a treatment option for lesser degrees of hearing loss. Hearing aids consist of a microphone, amplifier, and receiver. Hearing aids amplify and convert acoustic sound from the environment into digital signals sent to the ear via the air conduction pathway, providing enhanced audibility. A CI is a biomedical device consisting of an internally implanted receiver/stimulator coupled to an external processor. The CI bypasses the deficient or damaged portions of the inner ear and provides direct electrical stimulation of the auditory nerve, thus restoring hearing in more severe cases of more sensorineural hearing loss. These clinical interventions have provided researchers with a natural framework in which they can study the effects of auditory deprivation on the brain, and the ability for these interventions to restore or reverse deprivation-induced changes in neuroplasticity.
Many neuroimaging methods are used to study neuroplasticity in hearing loss in humans: functional magnetic resonance imaging (fMRI), functional near-infrared spectroscopy (fNIRs), positron emission tomography (PET), magnetoencephalography (MEG), and EEG, and each method has significant value. In this chapter, we focus primarily on the utilization of EEG for several reasons. First, EEG is not susceptible to the same types of acoustic artifact during auditory experiments as other neuroimaging techniques, and is very compatible with clinical populations with CI. Second, EEG provides essential millisecond-level temporal resolution superior to other neuroimaging methods, providing detailed timing information about neural processing along the auditory pathway. And finally, EEG is noninvasive, inexpensive, and is relatively easy to apply, making it feasible on both pediatric and adult populations in a clinical setting. In addition to EEG recordings in humans, we will discuss evidence from other neuroimaging methods (e.g., fMRI, PET) and near-field EEG recordings in animal models, which provide us with more detailed spatial resolution about sensory processing in specific brain regions.
While the field of otology is primarily concerned with diagnosis and treatment of disorders of the auditory system, it is useful to first understand neuroplasticity of the central auditory system under the conditions of typical development. During normal development, the central auditory pathways are formed via a series of intricate, sequenced, and time-dependent processes. Both intrinsic neuroplasticity and extrinsic neuroplasticity play a role in typical development of the central auditory pathways. Intrinsic neuroplasticity refers to strictly regulated genetic, molecular, and cellular processes that occur early in development. These intrinsic processes help regulate neurogenesis (the creation of new neurons), migration, differentiation, and synaptogenesis (the creation of new synapses) in the developing auditory cortex. For instance, synaptogenesis within auditory cortex begins in the fetal brain by the 27th week of gestation. Rapid myelination of axons in the auditory cortex peaks within the first 3 months of life, then continues at a slower rate into childhood. By 1 year after birth, all 6 layers of the auditory cortex can be reliably differentiated.
Extrinsic neuroplasticity refers to experience-driven forces (e.g., auditory input), which take place during development and across the human lifespan. Over the first 4 to 12 years of life, auditory stimulation guides the process of synaptic pruning, or refinement, of the central auditory pathways. During this developmental period, the age-old maxim in neuroscience, “neurons that fire together wire together,” holds true. That is, synapses or neuronal connections receiving repetitious and consistent auditory input are strengthened, while unstimulated or underutilized connections are eliminated. Repetitious and consistent auditory stimulation also leads to the long-term potentiation (LTP) and long-term depression (LDP) (strengthening or weakening, respectively) of existing synapses, processes which are known to play a vital role in learning and memory. In the developing auditory cortex, feed-forward connections (e.g., thalamo-cortical connections)—which are thought to be largely intrinsically regulated—develop first. Later on, feed-back cortical connections (e.g., cortico-thalamic connections)—which are thought to be more extrinsically regulated—start developing. In this sense, while a structural and rudimentary neuronal framework is already in place by birth, the central auditory pathways are continually refined through repetitious and consistent auditory experience during infancy and childhood.
The term biomarker is defined by the National Institutes of Health as a “characteristic that is objectively measured and evaluated as an indicator of normal biological processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention.” The P1 CAEP biomarker is an EEG event-related potential (ERP) recorded in response to an auditory stimulus. The P1 CAEP biomarker reflects maturation in synaptic efficiency along the central auditory pathways at the level of the primary auditory cortex (A1) and the thalamus ( Fig. 132.1A4 ). The P1 CAEP response is the dominant response in infants and young children (see Fig. 132.1A1 ), occurring at a latency of approximately 300 ms. As children age, the P1 latency decreases dramatically at first and then gradually (coinciding with the aforementioned intrinsically-regulated and extrinsically-regulated developmental changes over the first few years of life), until eventually reaching adult-like latency between 12 and 16 years of age.
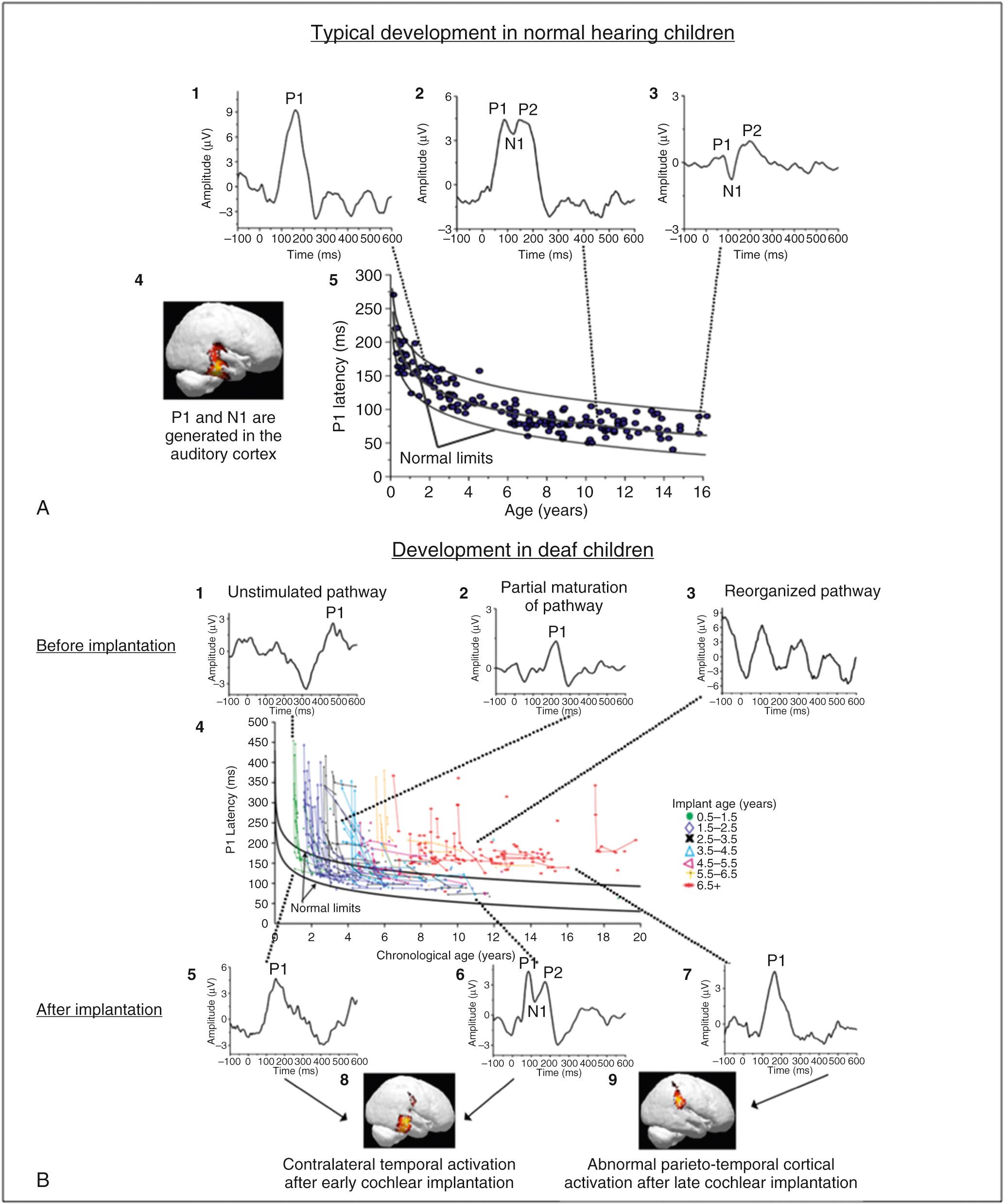
Our laboratory has established normative data for P1 CAEP latency in typically developing, normal hearing children, from infancy through adolescence, providing a basis by which we can examine the effects of auditory disorders such as deafness and hearing loss. Fig. 132.1A5 depicts P1 CAEP latencies in individual normal hearing children as a function of age. The top and bottom black lines in Fig. 132.1A5 represent the 5% and 95% confidence intervals for normal P1 CAEP latency, where P1 latency values falling outside of the top black line indicate significant delays in maturation of the central auditory pathways with 95% confidence. Because P1 CAEP latency varies with age, it can be used as a clinical biomarker of the maturation of the auditory cortex. That is, by comparing P1 latencies in clinical populations with hearing loss to these normative values, we can determine whether the central auditory pathways are maturing at an age-appropriate rate, or whether there are statistically significant delays in typical maturation of these pathways. In many previous studies, we have utilized the P1 CAEP biomarker to evaluate the efficacy of intervention with hearing aids, to establish candidacy for CI, and to monitor development of the central auditory pathways.
The morphology of the CAEP response can also be used as an objective indicator of the maturational status of the central auditory pathways. While the P1 response is the predominant waveform component in the CAEP response in infants (see Fig. 132.1A1 ), as a child ages, extrinsic input to the auditory system drives higher-level development of the central auditory pathways and eventually the emergence of two later waveforms, the N1 and P2 components, can be observed (see Fig. 132.1A2 and 132.1A3 ). The N1 and P2 components are negative-going and positive-going peaks in the CAEP waveform following the P1 response in time. While the P1 response reflects cortical processing at the primary auditory cortex (A1) and thalamus, the N1 and P2 components reflect higher-level auditory cortical processing at the level of secondary auditory cortex (A2), cortico-cortical processing between areas of the auditory cortex, and feedback (cortico-thalamic) processing between A2, A1, and the thalamus (see Fig. 132.1A4 ).
Our laboratory and other laboratories have systematically examined the emergence of the N1 CAEP response under typical development. For example, in a study by Campbell, Cardon, and Sharma (2015), we evaluated the occurrence of the N1 CAEP response in typically developing, normal hearing children at various ages. Results from this study indicate that the N1 CAEP response was present in approximately 57% and 71% in normal hearing children between ages 3 to 6 years and ages 6 to 9 years, respectively. In children 9 to 12 and 12 to 15 years of age, 100% of these children exhibited present N1 CAEP responses. Thus, the morphology of the CAEP response (e.g., presence or absence of the N1 CAEP component) can be used to assess whether higher-level auditory cortical maturation is occurring at a normal rate in clinical populations with hearing loss.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here