Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Coronaviruses (CoVs) are a family of single-stranded positive (plus, +) RNA viruses with the largest RNA genomes of any human pathogenic viruses, ranging from 26 to 32 kb in length. Pathogenic CoVs of humans and domestic and companion animals were identified over many years. With the enhanced surveillance and identification of CoVs that has occurred since the SARS epidemic 2003, it has become increasingly apparent that the CoV family has vast diversity in mammalian and avian species with hundreds of known CoVs, causing both mild species-specific infections and devastating epidemics. While it has long been known that CoVs are able to cross species and adapt to new environments, the emergence of severe acute respiratory syndrome CoV (SARS-CoV) in 2003–2004, Middle East respiratory syndrome CoV (MERS-CoV) in 2012, and SARS-CoV-2 (COVID-19) in 2019 has dramatically confirmed the capacity for CoVs to cause zoonotic (animal to human) infections with pandemic consequences.
The CoV (+)RNA genomes have a 5′ to 3′ orientation, are 5′ capped and 3′ polyadenylated and encode at least five open reading frames (ORFs) ( Fig. 17.1A ). The 5′ ∼20 kb (two-thirds of the genome) comprises two large overlapping open reading frames (ORFs 1a and 1b) that together encode the proteins required for host cell membrane modifications, RNA genome replication, and subgenomic mRNA transcription. In addition, it is has become well appreciated that several of the ORF1a/b encoded proteins serve other functions in virus biology including recombination, and virus evasion of the host immune response. Following entry of the CoV RNA genome into the host cell, ORFs 1a and 1ab are translated by host polyribosomes into large co-amino-terminal polyproteins 1a and 1a/b composed of 16 nonstructural proteins (nsp1–16). Fourteen well-characterized cleavage sites in the polyproteins are co- and post-translationally processed by two or three viral proteases in nsp3 and nsp5 to yield intermediate and mature proteins. For SARS-CoV-2, there are two proteases, nsp3-PLpro and nsp5-3CLpro/Mpro, that mediate the cleavage events and have been shown to be essential for virus RNA synthesis, replication, and virulence. Compared with other (+)RNA viruses, CoVs encode proteins with a much larger number of functions, including an RNA-dependent RNA polymerase, capping enzymes (guanylyl, N7-methyl and 2’O methyl transferases), an RNA-binding protein, an RNA helicase, an endonuclease, an exonuclease, a de-ADP ribosylating enzyme, and processivity factors, as well as proteins of unknown function. , Importantly for understanding SARS-CoV-2 evolution, CoVs express the only known viral RNA-dependent RNA proofreading capability, which mediates significantly higher-fidelity replication than other known RNA viruses and may have supported the evolution of their large, complex, and genetically robust genomes and protein functions. Thus, they may have the advantages of both significantly higher constitutive mutation frequency than DNA viruses or other DNA-based organisms, along with an increased capacity to prevent a large number of highly deleterious mutations. The demonstration of frequent host species movement and adaptation, as well as the dramatic mutational repertoire across the SARS-CoV-2 genome in the many million fully sequenced genomes worldwide (e.g., 4.2 million Omicron sequences as of June 21, 2022), support that CoVs may generate and tolerate vast diversity of genome polymorphisms not only in the spike but across the entire genome.
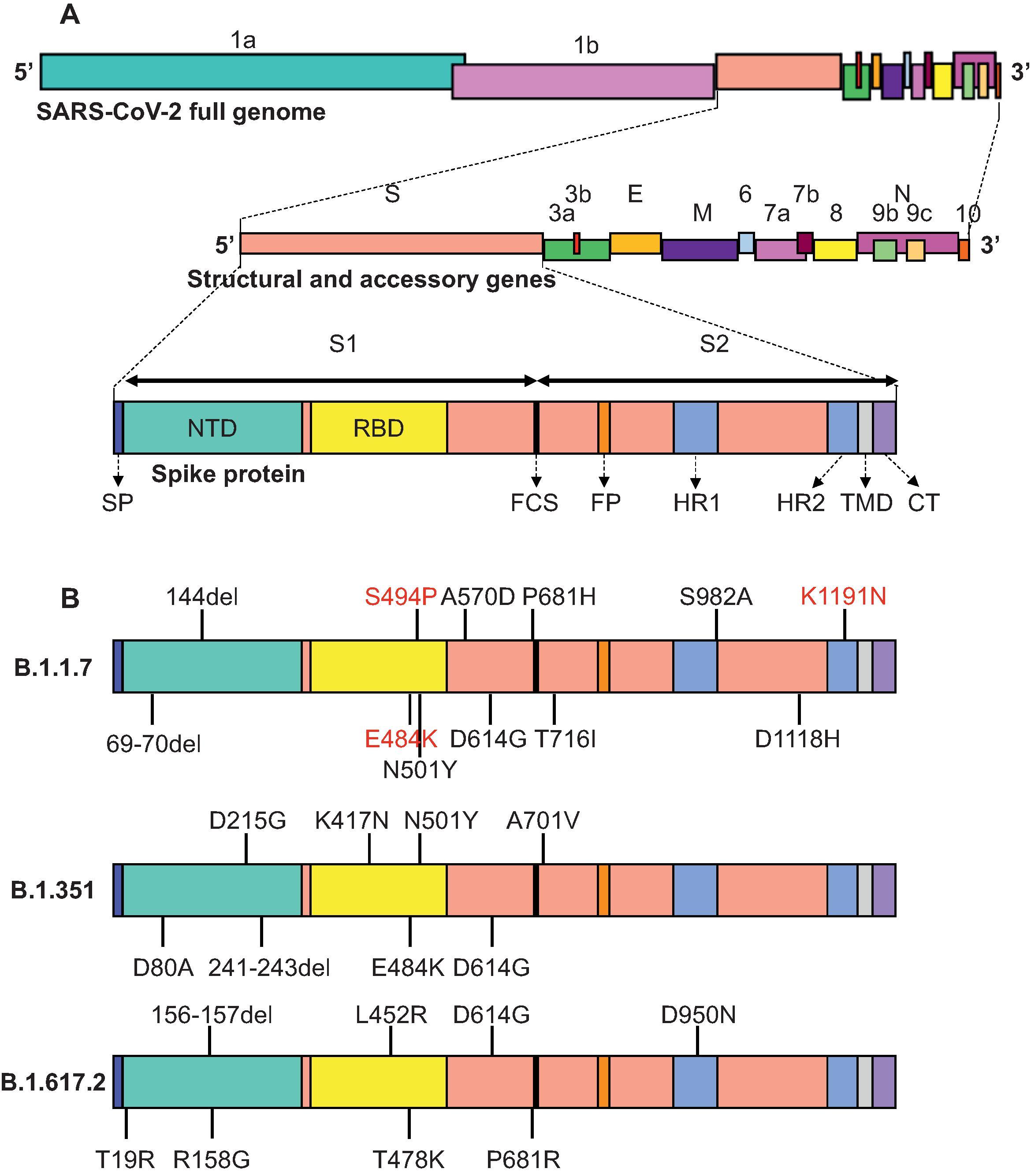
The 3′ third of the genome encodes for the four well-described structural proteins conserved in all known CoV. The membrane (M) and envelope (E) protein are part of the lipid coat of the virus and E is also an ion channel. The nucleocapsid (N) encapsidates the viral RNA. Most important for this chapter, the spike (S) protein is a membrane protein that is responsible for binding to the host cell receptor and effecting virus entry ( Fig. 17.1A ). The host cell receptor for SARS-CoV and SARS-CoV-2 is ACE2 (angiotensin converting enzyme 2), an ectopeptidase with a regulatory role in the renin–angiotensin system (RAS). The S protein is the primary target for the neutralizing antibody response. Most of the neutralizing response is directed to the part of the S protein that actually binds to the receptor (receptor binding domain, RBD) but S protein-specific antibodies directed outside of the RBD also possess neutralizing activity. The S protein is the substrate for the majority of the vaccines described below.
CoV vaccine development began many years ago, but the intensity of research devoted to engineering and testing vaccines was accelerated by the COVID-19 pandemic. Prior to the SARS epidemic of 2003–2004, CoVs were most noteworthy as causative agents for severe diarrhea and respiratory disease in domestic and companion animals, including swine, cows, dogs, cats and chickens. These infections, as described below, were the impetus for initial efforts to develop vaccines. In humans, two viruses that caused the common cold in humans, HCoV-229E and HCoV-OC43, were recognized but not studied to a great extent due to their predominantly mild illness, ubiquitous seroconversion by age 5, and probability of frequent reinfection due to limited protective immune responses. The SARS epidemic, which resulted in infection of about 8500 people with an overall 10% mortality, demonstrated that previously unknown zoonotic CoVs could cause severe and lethal respiratory disease in humans. Since virus was only transmitted once infected individuals developed symptomatic lower respiratory disease, public health measures including quarantine of patients and known contacts, were effective in preventing transmission and controlling the epidemic. This “Achilles heel” of SARS-CoV biology resulted in its eradication from human populations, although not presumably from its natural endemic host, which remains to be definitively identified. The Middle East respiratory syndrome (MERS) was first recognized in humans living on the Arabian peninsula in 2012. MERS-CoV is endemic in camels and occasionally crosses species to infect humans. Like SARS-CoV, MERS-CoV is only transmissible from humans once they develop symptomatic pulmonary disease and thus it exhibits poor interhuman transmission except in hospitals. From 2012 and continuing to the present, MERS-CoV has been of significant concern because of its known endemicity in camels and continued cause of an episodic human pneumonia that has a high mortality (>30%). MERS-CoV could pose a threat to human health and give rise to pandemic disease if it better adapted for transmission between humans and caused a broader range of disease.
COVID-19, caused by the beta-CoV SARS-CoV-2, was first recognized as a new disease entity in December 2019. SARS-CoV-2 causes severe pneumonia in 10–15% of infected individuals. It became quickly apparent that the virus readily transmitted between humans, likely because it replicates to high titers in the upper respiratory tract. As of June 26, 2022, there were worldwide more than 543 million COVID cases with ∼6,330,000 deaths (1.2% mortality) https://covid19.who.int/ .
While COVID-19 has been managed to some extent by public health measures in some countries, these measures have been insufficient in many parts of the world. The ongoing pandemic has emphasized in a cataclysmic manner the importance of development of vaccines, antivirals, and other countermeasures against SARS-CoV-2 and other coronaviruses. Here, we discuss only vaccine development.
Over the past 40 years, CoVs caused substantial losses in farmed animals such as swine, cows, and chickens, and also severe disease in companion animals, especially cats. As a consequence, efforts were made to develop vaccines for these animals, which ultimately helped inform SARS-CoV, MERS-CoV, and SARS-CoV-2 vaccine development. Three lessons learned from these efforts were particularly relevant for human CoV vaccine development.
The alphacoronavirus, transmissible gastroenteritis virus (TGEV) is a cause of severe diarrhea in neonatal and juvenile swine. Live-attenuated and killed virus TGEV vaccines were only modestly efficacious because the vaccines did not consistently elicit high lactogenic IgA responses, which were required for protection. TGEV has largely been eliminated from swine populations because a naturally occurring mutant of TGEV, named porcine respiratory coronavirus (PRC) emerged and displaced TGEV. , Remarkably, PRC was a TGEV variant that contained a partial deletion in the S protein and no longer infected the enteric tract. Instead, it caused a mild respiratory disease, which elicited an immune response in dams that protected young swine from TGEV. This naturally occurring TGEV vaccine illustrates the potentially high efficacy of a live-attenuated virus vaccine.
In a second example, a live-attenuated virus vaccine against the avian CoV infectious bronchitis virus (IBV) provided significant protection against the lethal bronchial and renal disease caused by IBV, when assessed using homologous virus. However, several strains of IBV may circulate at any time and there was limited cross protection, which necessitated the administration of multiple combined live-attenuated virus vaccines. These vaccines recombined in nature, resulting in new strains of IBV that could cause serious clinical disease and loss of vaccine protection. These analyses of IBV vaccines illustrate the remarkable capacity for CoV recombination and also a potential limitation of administering multiple live-attenuated CoV vaccines.
In the case of feline coronavirus FCoV-FECV (feline enteric CoV), infection results in acute mild diarrhea in domestic and feral cats. In some cats the virus undergoes mutation to a variant known as feline infectious peritonitis virus (FIPV) that causes a fatal granulomatous peritonitis/serositis (FIP). FIP is proposed to result from a gain in ability of FIPV to infect macrophages. Some FCoV/FIPV vaccines that targeted the S protein elicited high titers of neutralizing antibody. Paradoxically, this anti-S protein antibody response enhanced disease by increasing virus entry into macrophages, resulting in the only experimentally confirmed in vivo example of this type of antibody disease enhancement (ADE) during a coronavirus infection.
Other forms of vaccine-induced ADE have been described in animals experimentally infected with SARS-CoV. One example in mice is a Th2 response associated with eosinophilia, observed during SARS-CoV infection following immunization with inactivated SARS-CoV. In a second example, vaccination of macaques with a modified vaccinia Ankara virus encoding the SARS-CoV S protein induced an immune response that changed the character of macrophages targeting the infected lungs from a wound healing to a proinflammatory phenotype. This change resulted in greater expression of several proinflammatory molecules, such as CCL2 and IL-8. While in both of these cases the changes in the inflammatory response did not result in more severe clinical disease, they do emphasize the importance of both preclinical studies and postuse analysis of immune responses for candidate and approved vaccines for SARS-CoV-2.
An outstanding question in understanding vaccine efficacy is defining the immune correlates of protection. For SARS-CoV-2, while correlates of protection are partially defined for human populations, some information is available from studies of other coronaviruses. Prior administration of sublethal doses of infectious virus, live-attenuated virus, and inactivated viruses induce protective levels of neutralizing antibody. The protective nature of the antibody to MERS-CoV has been demonstrated using adoptive transfer assays in mice, in which sera or purified antibodies from human MERS-CoV-infected patients have been administered to unimmunized mice prior to or shortly after infection with a lethal dose of virus. , Similarly, convalescent plasma has been used therapeutically in human respiratory virus infection, including SARS-CoV-2. Of note, convalescent plasma is no longer used in COVID-19 because of limited efficacy. Antibodies are often induced against other SARS-CoV viral proteins, especially the nucleocapsid protein, but none of these has been protective and, in some settings, may contribute to worse disease. , In all cases, sufficient levels of neutralizing antibody directed against the CoV in question is protective against rechallenge.
Many of the reports about correlates of protection are supported by studies of COVID-19 convalescent patients and vaccine recipients. In one study, SARS-CoV-2 PCR positivity was detected in 1.09/10,000 versus 0.13/10,000 days at risk in seronegative and seropositive healthcare workers, respectively. Of note, only two seropositive patients became infected, and both remained asymptomatic. Other studies have used modeling to quantify the level of antibody required for protection. These analyses indicate that neutralizing antibody levels are useful biomarkers for vaccine efficacy and protection after natural infection. Based on these findings, immune-bridging using neutralizing antibody titers has been employed to authorize vaccine use in populations in the absence of full Phase III trials efficacy data. One apparent exception to the correlation of neutralizing antibody titers with vaccine protection is the observation that protection was observed by 14 days after primary vaccination in the absence of appreciable antibody titers. Whether this protection is due to the innate immune response, virus-specific T-cell responses or a level of antibodies that are low but still protective requires further study. More precise measurement of correlates of neutralizing antibody-mediated protection will require multicenter studies with standardization of assays and extensive sharing of data.
It is likely that virus-specific T cells are required to develop an optimal SARS-CoV-2-specific immune response. CD4 T cells are required to orchestrate a proinflammatory response involving cytokine and chemokine expression and a regulated ingress of inflammatory cells into the infected lungs. One type of CD4 T cells, T follicular cells (Tfh), is required for antibody isotype switching and selection of memory B and plasma cells that produce high-affinity antibodies. CD8 T cells are required to lyse infected cells. Virus-specific T cells are required for initial virus clearance after challenge in experimentally infected animals. For example, depletion of either CD4 or CD8 T cells prior to infection with a murine CoV results in delayed virus clearance and prolonged clinical disease, with a fatal outcome in most cases. On the other hand, vaccination of mice under conditions that elicit only a CD8 T-cell response results in nearly complete protection against infection with virulent mouse-adapted MERS-CoV. Thus, virus-specific CD8 T cells are sufficient to protect against challenge, if these cells are present in large enough numbers.
Virus-specific T cells have been identified in SARS and MERS survivors. , The clinical severity of MERS ranges from mild (even asymptomatic) to severe, and MERS-CoV-specific T-cell responses were detected in all survivors, while antibody responses had often waned to undetectable in patients with very mild disease. , , In SARS survivors, both virus-specific antibody and T-cell responses were detected at least15 years after the epidemic ended, demonstrating a long-lastingresponse. , , This longevity may have occurred because SARS-CoV always caused clinically evident disease, unlike MERS-CoV or SARS-CoV-2. However, since SARS-CoV was eradicated from human populations in 2004 and there were only about 8500 total cases, it is not known whether antibody and/or T-cell responses correlated with protection and if SARS survivors would be protected if challenged with SARS-CoV.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here