Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
![]() For video s accompanying this chapter see ExpertConsult.com. See inside cover for access details.
For video s accompanying this chapter see ExpertConsult.com. See inside cover for access details.
Corneal surgery in children is challenging and there is reluctance to perform these procedures due to poor outcomes. However, modern imaging modalities and surgical techniques allow for better patient selection and outcomes. Previously, almost all transplants were full-thickness penetrating keratoplasty (PKP). Now lamellar surgery allows for selective replacement of only the diseased part of the cornea ( Table 33.1 ) resulting in reduced rejection rate, need for steroid use, and susceptibility to trauma.
| Anatomical involvement | Procedure of choice | Examples of pathology |
|---|---|---|
| Partial thickness | ||
| Subepithelial | Superficial keratectomy | Basement membrane dystrophies |
| Anterior stromal (<200 µm) | Anterior lamellar keratectomy |
|
| Deep stromal | Deep anterior lamellar keratoplasty (DALK) | Deep scars and limbal dermoid |
| Endothelial dysfunction |
|
|
| Full thickness | ||
| With endothelial dysfunction | Penetrating keratoplasty (PKP) |
|
| Without endothelial dysfunction |
|
|
| With tectonic weakness | Epikeratoplasty (overlay graft), PKP, DALK, patch graft |
|
The choice of the most appropriate procedure depends on detailed ocular examination including anterior segment imaging (ultrasound biomicroscopy (UBM), B-scan ultrasound, and/or optical coherence tomography (OCT)). This may require examination under anesthesia (EUA) or sedation. Relevant factors include partial- or full-thickness involvement of the cornea (see Table 33.1 ), presence of glaucoma, and status of the crystalline lens and iris. The visual potential of the eye may also be limited by retinal and optic nerve disease.
Systemic abnormalities, including cardiopulmonary, central nervous, and genitourinary system abnormalities, are also common and impact safety of anesthesia. Finally, the tremendous importance of the family on the outcome of surgery must be emphasized. Parental commitment to long-term care beyond surgery, which includes compliance with eye drops, spectacle correction, amblyopia treatment, and frequent postoperative visits heavily influences the success of any intervention.
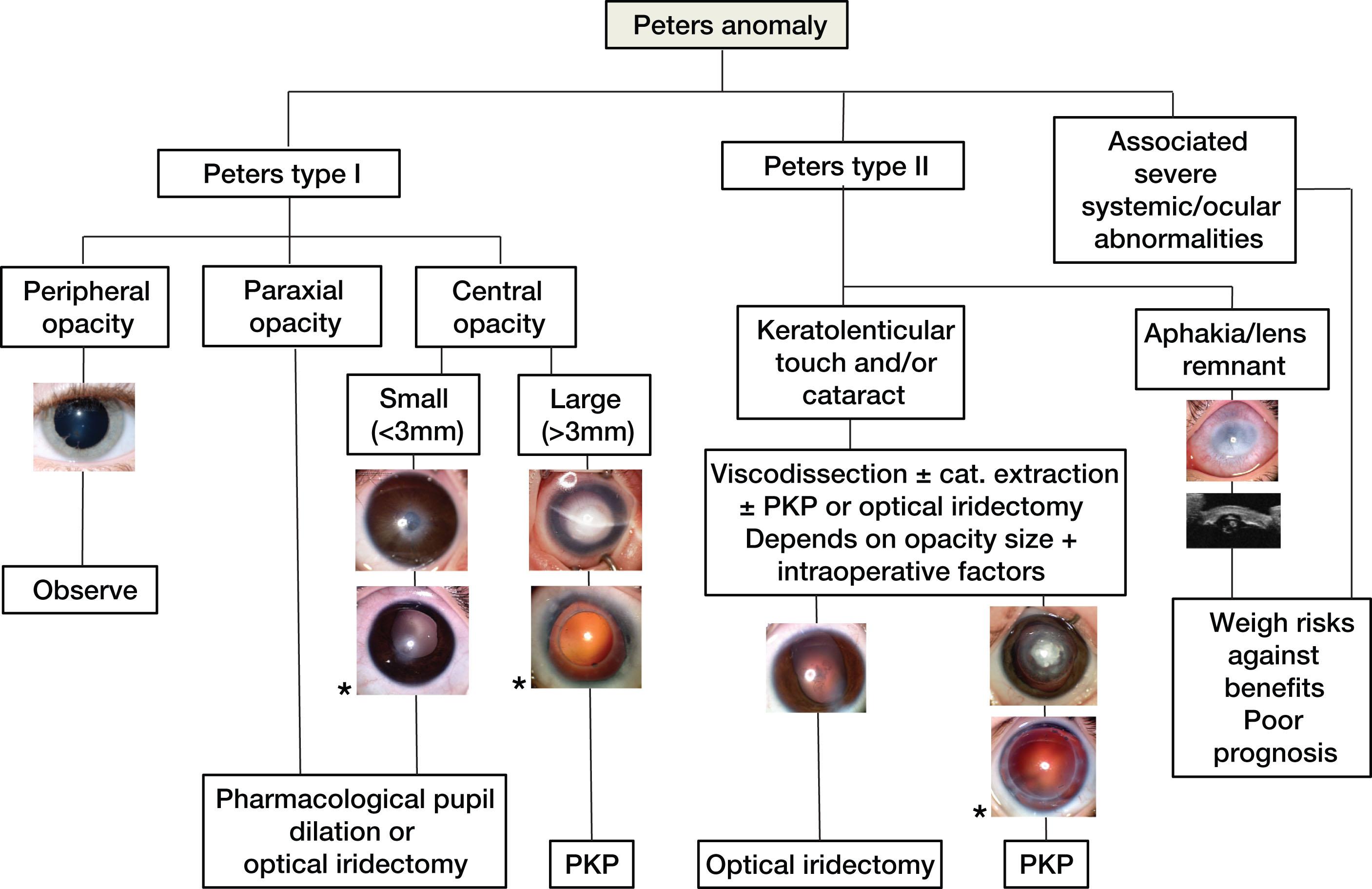
The main indications for surgery in infants are congenital corneal opacities (CCOs), the commonest of which is Peters anomaly (PA). Figure 33.1 shows a treatment algorithm for Peters anomaly that takes into account the phenotypic spectrum of the disease. If the opacity does not involve the central 3 mm of visual axis, management can be limited to the treatment of amblyopia and refractive correction. Opacities partially involving the visual axis with clear corneal periphery may be treated with optical iridectomy, a less invasive and viable long-term alternative to keratoplasty (see Fig. 33.1 ).
The timing of surgery for CCOs is a balance between obtaining best visual outcome whilst minimizing risk of complications. In general, a delayed surgery is technically easier, has less risk of glaucoma and graft rejection leading to better graft survival. However, the visual potential may be reduced if surgery is performed after the critical period of visual development. In dense opacities, keratoplasty by 3 months of age allows for preoperative assessments, counseling for parents, and systemic work up while offering adequate visual rehabilitation. Regular preoperative follow-up (every 2–3 weeks) is required to screen for glaucoma, which requires prompt diagnosis and intervention before keratoplasty. The second eye in bilateral cases can be performed a week after the first to simplify the drop regimen, follow-up, and suture removal planning for the family whilst reducing the risk of amblyopia.
Corneoscleral tissue in infants has low rigidity and high elasticity, and easily collapses during surgery. This in concert with elevated posterior vitreous pressure increases the risk of intraocular content expulsion. An experienced pediatric anesthetist delivering deep anesthesia with muscle relaxant, hyperventilation, and intravenous 20% mannitol (0.5 g/kg body weight) can help reduce posterior pressure. Mannitol is best given when the Flieringa ring is secured to the eye (see below). CCOs can be associated with an abnormally anterior conjunctival insertion onto the cornea, hence conjunctival recession helps preserve limbal stem cells. A suitably-sized Flieringa ring is secured to the globe for support. Iridocorneal or keratolenticular adhesions are divided with gentle injection of viscoelastic and blunt division of adhesions. Care should be taken to avoid damage to the lens. Donor and trephination sizing should account for the reduced size of the host cornea whilst ensuring that adequate numbers of corneal endothelial cells are transplanted. Oversizing the donor by 0.5 mm facilitates wound closure and vaulting of the cornea to increase anterior chamber (AC) depth. However, oversizing >0.5 mm may cause excessive corneal steepening, high myopia, and corneal exposure postoperatively. Furthermore, large donor diameter (>8.0 mm) is associated with reduced graft survival in children with PA.
A “sandwich technique” involves suturing the donor to the host tissue prior to complete removal of the host button to minimize open globe time during the surgery ( ![]() ). Non-absorbable interrupted sutures are used in pediatric keratoplasty due to the risks of early suture loosening and infection.
). Non-absorbable interrupted sutures are used in pediatric keratoplasty due to the risks of early suture loosening and infection.
Postoperatively, follow-up is frequent initially and aimed at identifying any signs of glaucoma, suture loosening, infection or rejection, any of which should trigger appropriate intervention. Antibiotic drops are required until sutures are removed. Topical steroids are frequently used initially and tapered over the ensuing months to once daily or alternate daily dose, then maintained in the long term to prevent rejection.
The Multicenter Pediatric Keratoplasty Study (MPKS) reported complications in 75% of cases. The major complications, which can occur early or late, are infection (17%–29%), graft rejection (22%–43%), secondary glaucoma (5%–33%), and graft failure. Other less common complications are wound leak, wound dehiscence, cataract formation, endophthalmitis, retinal detachment, and phthisis bulbi. Studies on clinical outcomes of pediatric PKPs are difficult to compare due to heterogeneous etiology, varying age at surgery, and follow-up period. One-year graft survival as high as 92%–100% has been reported in congenital hereditary endothelial dystrophy (CHED) and posterior polymorphous corneal dystrophy (PPCD). Survival for Peters anomaly and other anterior segment dysgenesis is less favorable and dependent on severity of disease. Reported graft survival includes 62% at 3 years for all types of PA. Survival for PA type I ranges from 64% at 2 years to 74% at 10 years and for type II from 8% at 2 years to 39% at 10 years. Interestingly, successful PKPs in infants have better endothelial cell counts than adults.
Visual outcome is often limited by the degree of amblyopia as well. The spectrum of visual outcomes for PA can range from 20% of eyes undergoing PKP achieving 20/200 or better, and 38% having LP (light perception) or NLP (no light perception) vision. However, encouraging outcomes achieving a visual acuity equal or better than 20/200 are also reported for 48% of patient with type I and 36% for type II PA. Acquired causes of corneal opacity generally have better survival and visual prognosis than congenital abnormalities as patients are often older and have had normal visual development up until the point of surgery. If corneal neovascularization poses a risk for graft rejection, treatment with fine needle diathermy and adjuvant intrastromal bevacizumab can be successful. Finally, to avoid the risk of rejection, a rotational autograft can be considered if enough peripheral clear cornea exists. This technique is ideal for moving linear traumatic corneal scars out of visual axis.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here