Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The principal physiologic role of the cornea is to allow external light to enter the eye and to contribute to its focusing on the retina. Thus transparency and refractive power are essential for this function.
The cornea consists of the epithelium, Bowman layer, stroma, Descemet membrane, and endothelium.
The functions of the corneal epithelium are regulated by various biologically active agents such as growth factors, cytokines, and chemokines in tear fluid, whereas those of the endothelium are regulated by factors in aqueous humor.
Dynamic homeostasis of the corneal epithelium is maintained by the generation of new epithelial cells from limbal stem cells, centripetal cell movement, differentiation of basal cells into wing cells and then superficial cells, and cell desquamation via apoptosis.
The corneal stroma is composed primarily of extracellular matrix, predominantly type I collagen and proteoglycans. Collagen fibrils are homogeneous in diameter and are aligned at a constant distance from each other to maintain tissue transparency. Keratocytes are the resident cells of the stroma, and although relatively few in number, they play important roles in the maintenance of stromal structure through synthesis and secretion, as well as degradation, of collagen and proteoglycans.
The cornea is one of the most sensitive tissues in the body as a result of its abundant sensory nerve endings. Thus neural regulation is another important factor in the maintenance of corneal structure and function.
Morphogenesis of the eye is achieved by cell lineages of various origins, including the surface and neural ectoderm during embryonic development. Characterization of the development of ocular tissues during embryogenesis is important for understanding the pathogenesis of congenital anomalies of the cornea and anterior eye segment.
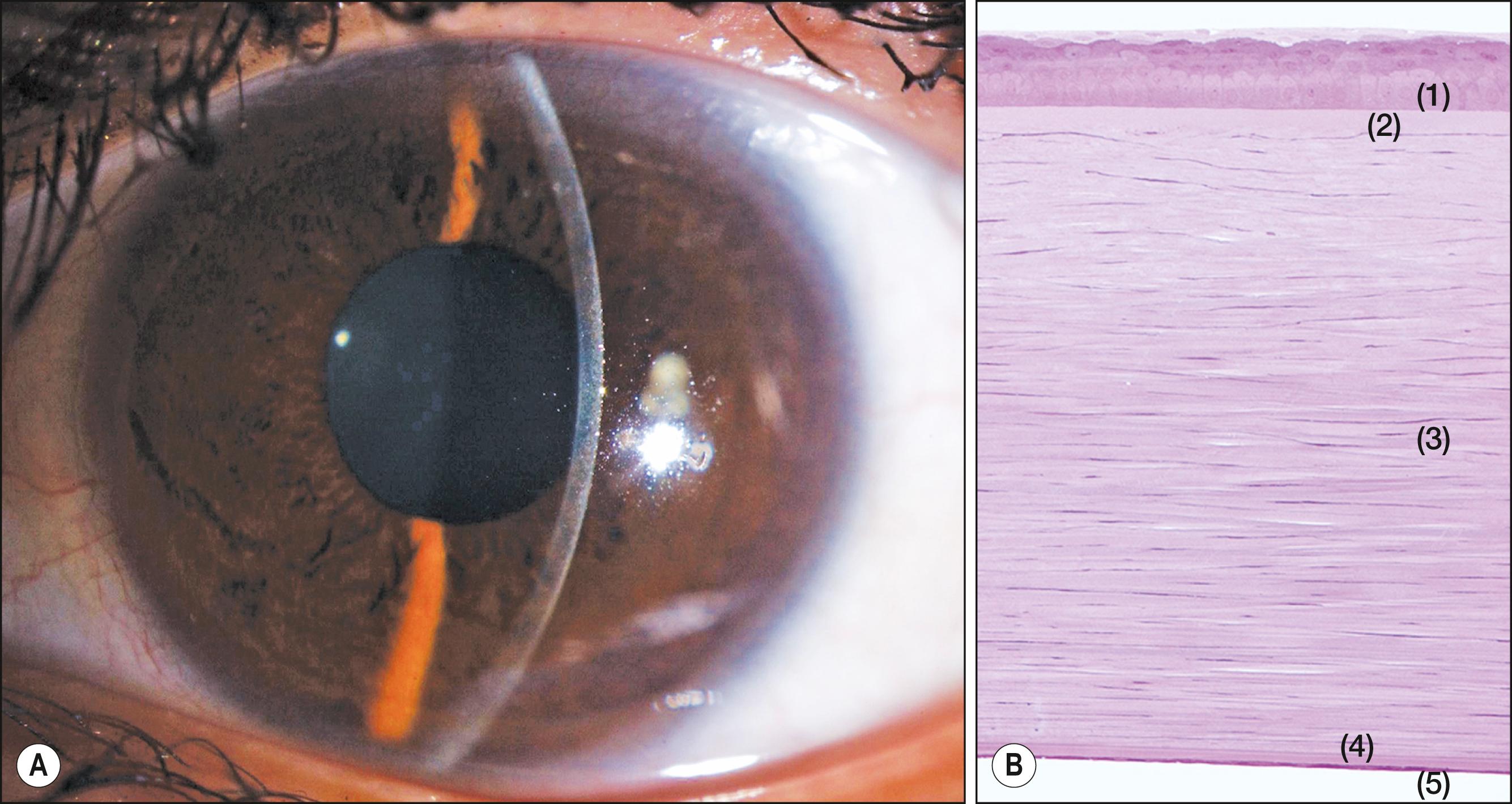
The avascular cornea is not an isolated tissue. It forms, together with the sclera, the outer shell of the eye, occupying one-third of the ocular tunic. Although most of both the cornea and sclera consist of dense connective tissue, the physiologic roles of these two components of the eye shell differ. The cornea serves as the transparent “window” of the eye that allows the entry of light, whereas the sclera provides a “dark box” that allows the formation of an image on the retina. The cornea is exposed to the outer environment, whereas the anterior portion of the opaque sclera is covered with the semitransparent conjunctiva and has no direct exposure to the outside. The differences in the functions of the cornea and sclera reflect those in their microscopic structures and biochemical components ( Fig. 1.1 ).
Interwoven fibrous collagen is responsible for the mechanical strength of both the cornea and sclera, protecting the inner components of the eye from physical injury and maintaining the ocular contour. The corneal epithelium forms an effective mechanical barrier as a result of the interdigitation of cell membranes and the formation of junctional complexes such as tight junctions and desmosomes between adjacent cells. Together with the cellular and chemical components of the conjunctiva and tear film, the corneal surface protects against potential pathologic agents and microorganisms.
The smooth surface of the cornea contributes to visual clarity. The regular arrangement of collagen fibrils in the corneal stroma accounts for the transparency of this tissue. Maintenance of corneal shape and transparency is critical for light refraction, with the cornea accounting for more than two-thirds of the total refractive power of the eye. A functionally intact corneal endothelium is important for maintenance of stromal transparency as a result of regulation by the endothelium of corneal hydration. The cornea thus plays a central role in vision as a result of its transparency and refractive power, and it maintains the eye shell. Each part of the cornea contributes to its transparency and shape, and its anatomy is closely related to its physiology and function. Any change in corneal shape or function may thus impair the formation of a clear image on the retina.
The maintenance of corneal function is dependent on precise and delicate regulatory mechanisms, with humoral and neural regulation playing key roles.
Various hormones, cytokines, and growth factors regulate the various cellular components of the cornea, and such humoral regulatory factors are present in tear fluid and aqueous humor. , Although the cornea itself lacks a vasculature, rich vascular systems in the limbus and conjunctiva are a source of humoral regulatory factors that reach the corneal surface via tear fluid or the corneal stroma by diffusion. Furthermore, the cellular components of the cornea and conjunctiva—including epithelial cells, stromal keratocytes and conjunctival fibroblasts, and endothelial cells—influence each other through the production and secretion of cytokines and growth factors, although the precise mechanisms of these reciprocal interactions remain to be fully clarified. On the other hand, the cornea and adnexa are innervated by the ophthalmic division of the trigeminal nerve, with such neural regulation also being important for maintenance of the physiologic functions of the cornea. ,
Any disturbance in these humoral or neural regulatory mechanisms, such as might arise as a result of infection, dry eye syndrome, or trigeminal nerve dysfunction, can lead to defects in corneal structure and function that manifest clinically.
The ocular surface is composed of the cornea, conjunctiva, lacrimal glands, and other adnexa. The outermost portion of the cornea and conjunctiva is an epithelium that directly faces the environment. The anterior corneal surface is covered by tear fluid, which protects the cornea from dehydration and helps to maintain a smooth epithelial surface. Tear fluid contains many biologically important ions and molecules, including electrolytes, glucose, immunoglobulins, lactoferrin, lysozyme, albumin, and oxygen. Moreover, it contains a wide range of biologically active substances such as histamine, prostaglandins, growth factors, and cytokines. The tear film thus serves not only as a lubricant and source of oxygen and nutrients for the corneal epithelium but also as a source of regulatory factors required for epithelial maintenance and repair. The posterior surface of the cornea is bathed directly by the aqueous humor. The highly vascularized limbus, which is thought to contain a reservoir of pluripotent stem cells, constitutes the transition zone between the cornea and the sclera or conjunctiva. The structure and function of the corneal epithelium and endothelium are therefore regulated by biologically active factors present in tear fluid and aqueous humor, respectively.
The sclera, a tough and nontransparent tissue, shapes the eye shell, which is approximately 24 mm in diameter in the emmetropic eye. The sclera is the continuation of the corneal stroma and does not directly face the environment. The anterior part of the sclera is covered with the bulbar conjunctiva and Tenon capsule, which consists of loose connective tissue and is located beneath the conjunctiva ( Fig. 1.2 ). The nontransparency of the sclera prevents light from reaching the retina other than through the cornea, and, together with the pigmentation of the choroid and retinal pigment epithelium, the sclera provides a dark box for image formation. The scleral spur is a projection of the anterior scleral stroma toward the angle of the anterior chamber and is the site of insertion for the anterior ciliary muscle. Contraction of this muscle thus opens the trabecular meshwork. At the posterior pole of the eyeball, where the optic nerve fibers enter the eye, the scleral stroma is separated into outer and inner layers. The outer layer fuses with the sheath of the optic nerve, dura, and arachnoid, whereas the inner layer contains the sieve-like structure of the lamina cribrosa. The rigidity of the lamina cribrosa accounts for the susceptibility of retinal nerve fibers to damage during the development of chronic open-angle glaucoma. The sclera contains six insertion sites of the extraocular muscles, as well as the inputs of arteries (anterior and posterior ciliary arteries) and outputs of veins (vortex veins) that circulate blood through the uveal tissues.
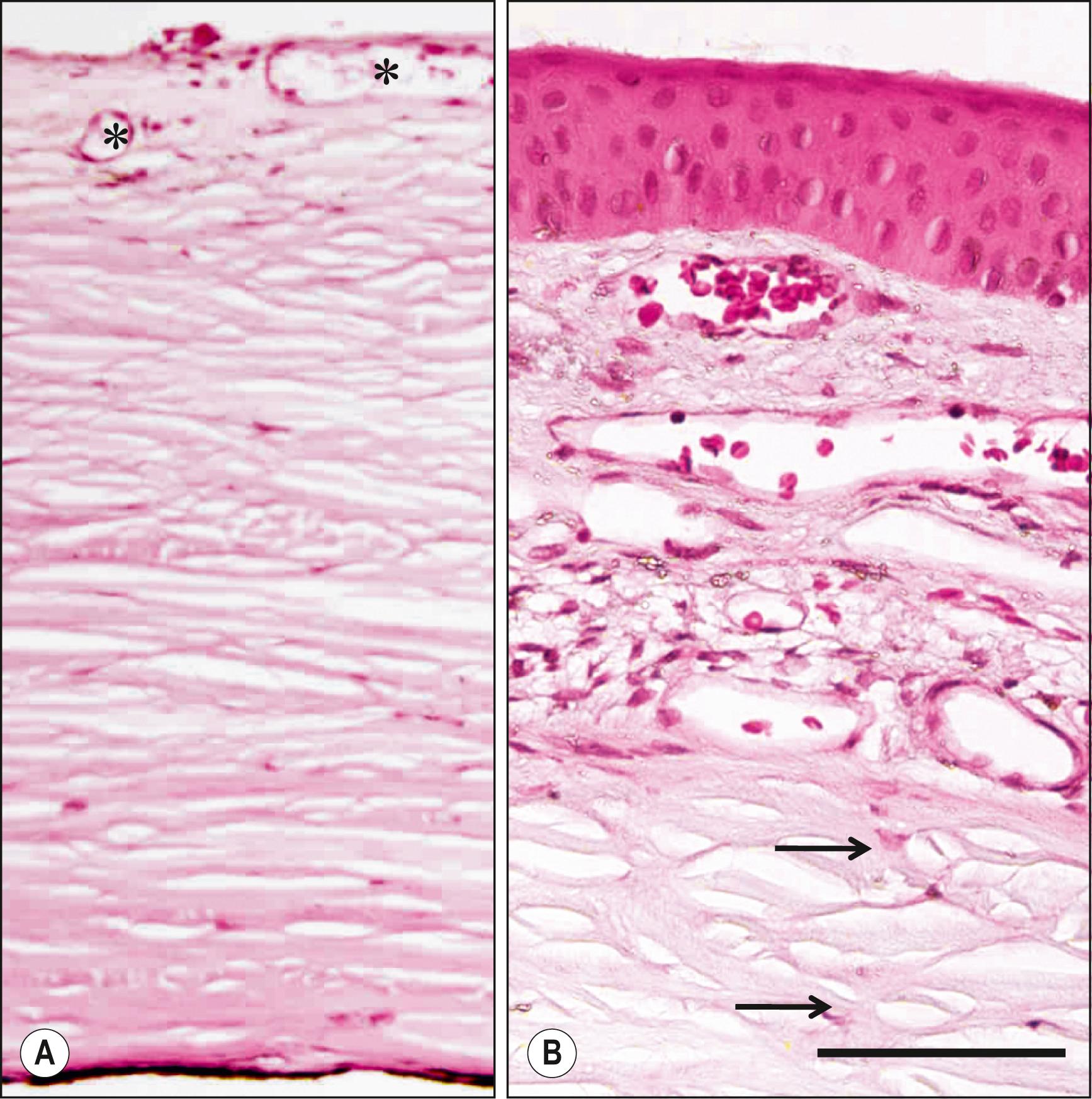
The anterior corneal surface is convex and aspheric (see Fig. 1.1 ), and it is transversely oval as a result of scleralization superiorly and inferiorly. The adult human cornea measures 11–12 mm horizontally and 9–11 mm vertically. It is approximately 0.5 mm thick at the center, with the thickness increasing gradually toward the periphery, where it is approximately 0.7 mm thick. The curvature of the corneal surface is not constant, being greatest at the center and smallest at the periphery. The radius of curvature is between 7.5 and 8.0 mm at the 3 mm central optical zone of the cornea, where the surface is almost spherical. The refractive power of the cornea is 40–44 diopters, constituting approximately two-thirds of the total refractive power of the eye.
The optical properties of the cornea are determined by its transparency, surface smoothness, contour, and refractive index of the tissue. Given that the spherocylindrical surface of the cornea has both minor and major axes, changes in corneal contour caused either by pathologic conditions such as scarring, thinning, or keratoconus or by refractive surgery render the surface regularly or irregularly astigmatic.
The total refractive index of the cornea is determined by the sum of refraction at the anterior and posterior interfaces, as well as by the transmission properties of the tissue. The refractive indices of air, tear fluid, corneal tissue, and aqueous humor are 1.000, 1.336, 1.376, and 1.336, respectively. The refractive power of a curved surface is determined by the refractive index and the radius of curvature. The refractive power at the central cornea is approximately +43 diopters, being the sum of that at the air–tear fluid (+44 diopters), tear fluid–cornea (+5 diopters), and cornea–aqueous humor (−6 diopters) interfaces. Most keratometry and topography measurements assume a standard refractive index of 1.3375.
The corneal and conjunctival epithelia are continuous and together form the ocular surface. They are both composed of nonkeratinized, stratified, squamous epithelial cells. The thickness of the corneal epithelium is approximately 50 μm, which is approximately 10% of the total thickness of the cornea (see Fig. 1.1 ), and it is constant over the entire corneal surface. The corneal epithelium consists of five or six layers of three different types of epithelial cells: superficial cells, wing cells, and columnar basal cells, the latter of which adhere to the basement membrane adjacent to the Bowman layer ( Figs. 1.1, 1.3 and 1.4 ).
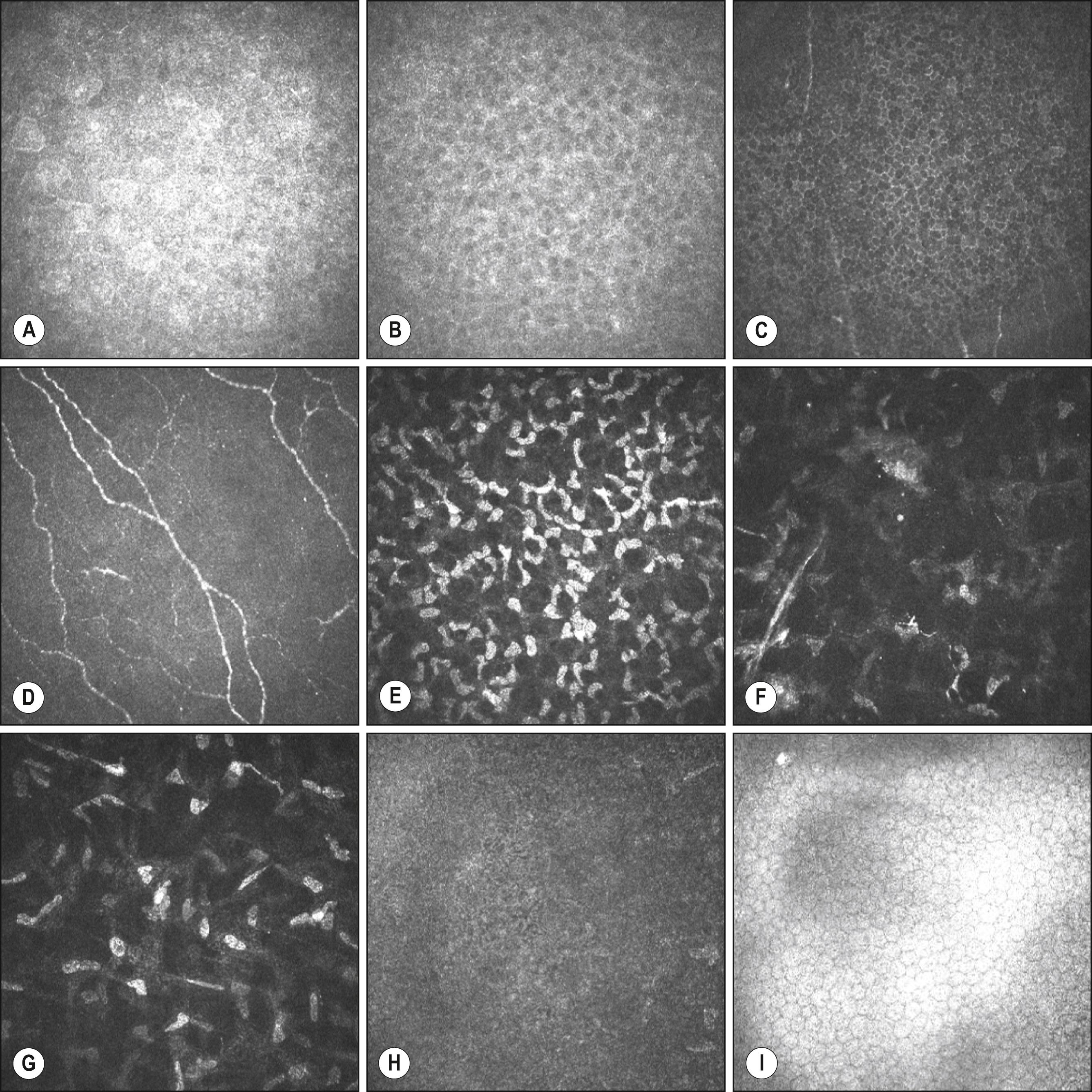
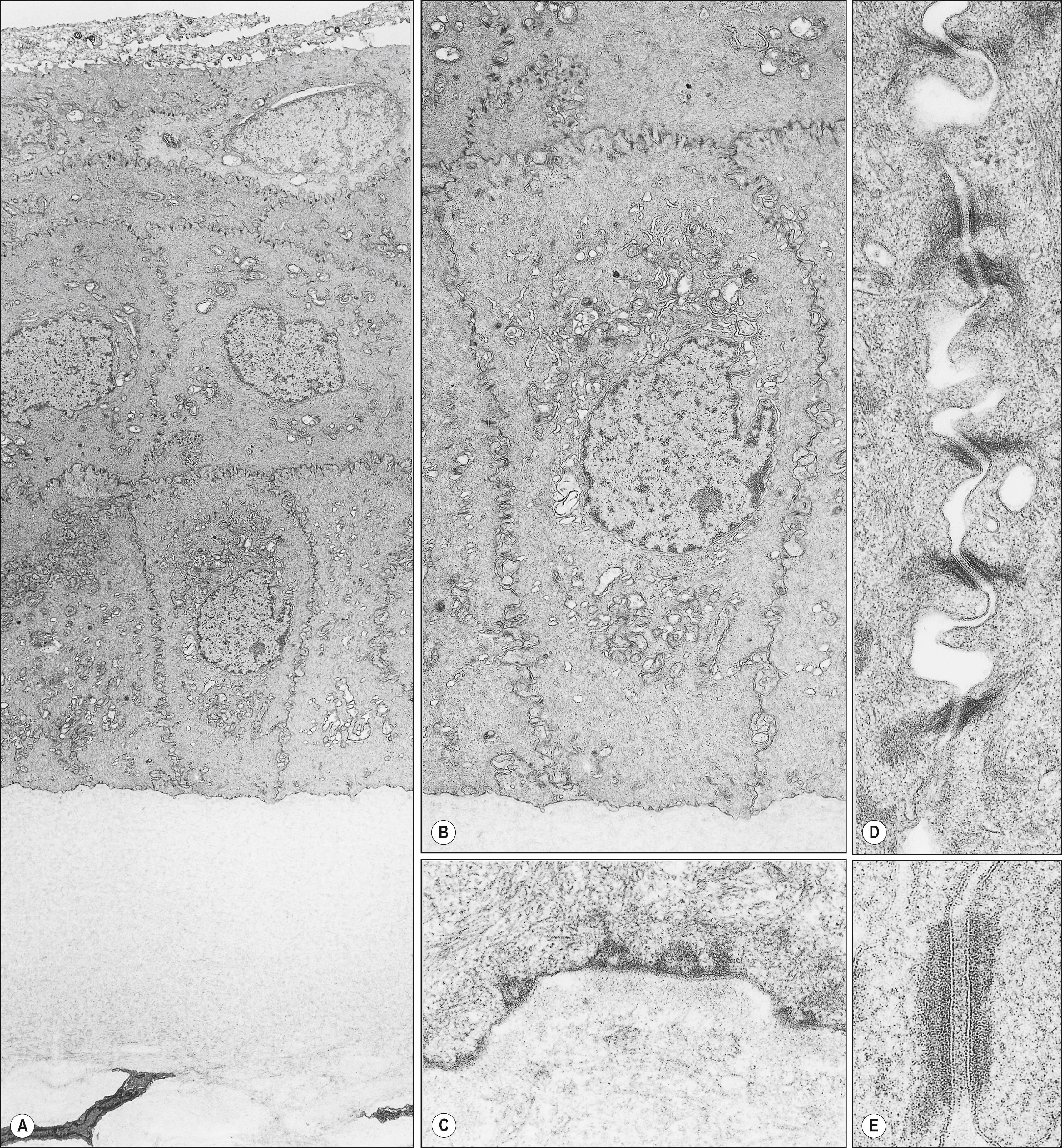
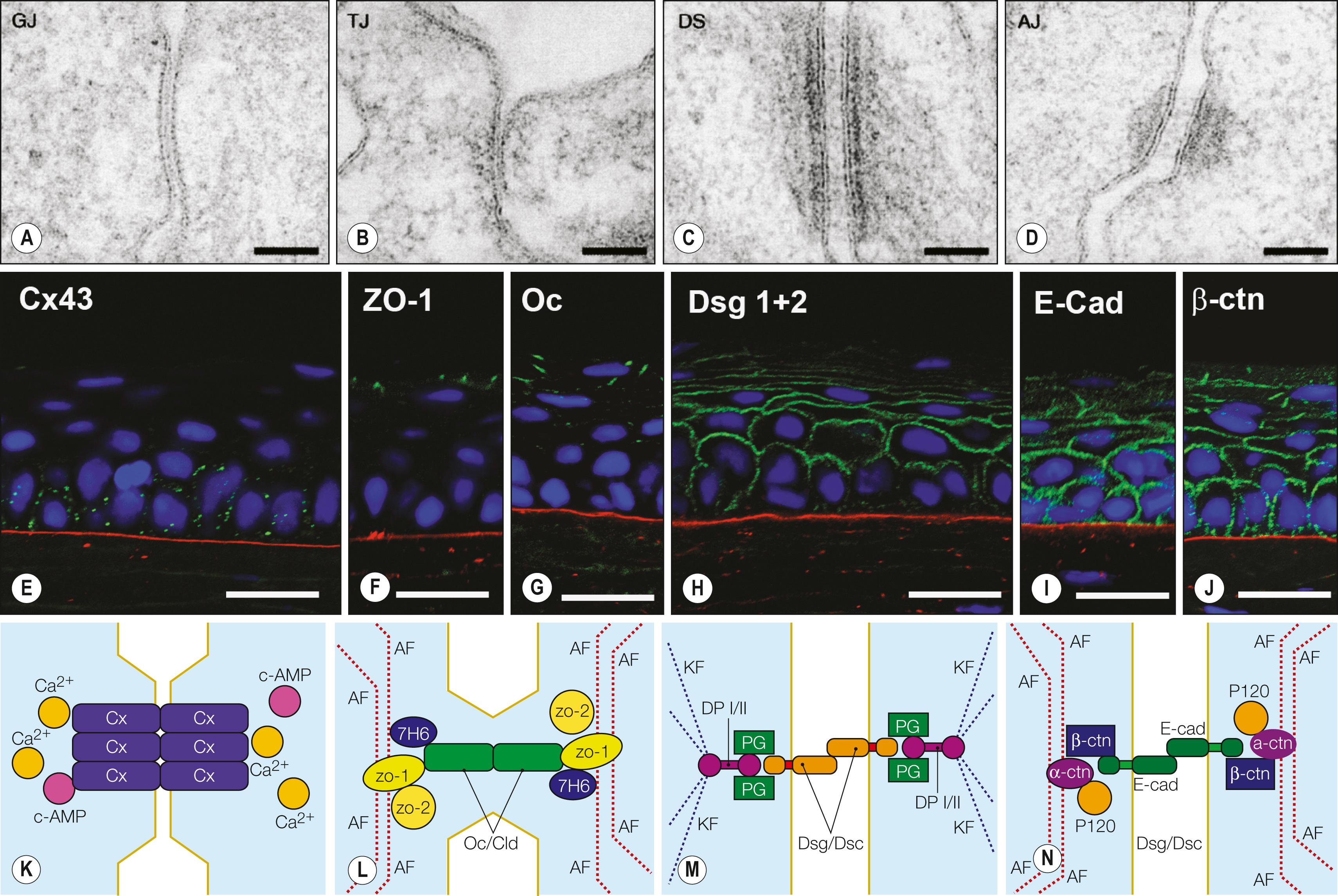
Although their characteristics differ, both corneal and conjunctival epithelia cooperate to provide the biodefense system of the anterior surface of the eye. , , , The characteristics of the different types of junctional complexes present in the corneal epithelium ( Figs. 1.4 and 1.5 ) are summarized in Table 1.1 . Tight junctions (zonula occludens) are present mostly between cells of the superficial cell layers and provide a highly effective barrier to prevent the penetration of tear fluid and its chemical constituents. Desmosomes and adherens junctions are present in all layers of the corneal epithelium, whereas gap junctions, which allow the passage of small molecules between cells, are present in wing cells and basal cells; and hemidesmosomes are localized to basal cells. After damage to the corneal epithelium, actively migrating epithelial cells no longer manifest junctional complexes in the wounded region lacking a basement membrane. Reestablishment of the continuity of the corneal epithelium is accompanied by the synthesis of basement membrane proteins and reconstruction of the basement membrane and by the reassembly of the various types of junctional apparatus, suggesting that the presence of the basement membrane may be required for re-formation of cell-cell junctions in the corneal epithelium (see Fig. 1.5 ).
| Shape | Layers | Size | Mitotic Activity | Interdigitation | Junctional Complexes | Cytoplasmic Organelles | Keratin | Microfilaments (Actin) | Microtubules | |
|---|---|---|---|---|---|---|---|---|---|---|
| Superficial cells | Flat Microvilli Microplicae |
2–4 | 40–60 μm in diameter 4–6 μm thick at the nucleus 2 μm thick at the periphery |
− | Entire surface | Desmosomes Tight junctions Adherens junctions |
Sparse | + | + | ± |
| Wing cells | Wing-like processes | 2–3 | − | Entire surface | Desmosomes Gap junctions Adherens junctions |
Sparse | +++ | + | ± | |
| Basal cells | Columnar | Monolayer | 18–20 μm high 8–10 μm in diameter Flat at posterior surface |
+ | Apical surface | Desmosomes Gap junctions Adherens junctions Hemidesmosomes |
More than superficial cells Large numbers of glycogen granules Prominent mitochondria and Golgi apparatus |
+++ | + | + |
In corneal epithelial cells, intermediate filaments of the cytoskeleton are formed by specific types of acidic (type I) and basic (type II) keratin molecules. Basal cells of the corneal epithelium express keratin 5/14, like basal epidermal cells of the skin. However, keratin 3/12 (64-kDa keratin) is specifically expressed in the epithelium of the cornea, not being found in that of the conjunctiva or in the epidermis, , and it is essential for the normal architecture of corneal epithelial cells. Deletion of the keratin 12 gene results in fragility of the corneal epithelium in mice. In humans, genetic mutation of the keratin 12 gene is responsible for Meesmann dystrophy of the corneal epithelium.
Replacement of most organs or tissues by transplantation from a genetically nonidentical individual is associated with an immune response that may lead to rejection. In contrast, the cornea is “immune privileged,” a characteristic that is critical for the success of corneal transplantation. Dendritic Langerhans cells, specialized macrophages derived from the bone marrow that are implicated in antigen processing, are abundant at the periphery of the corneal epithelium but are not present in the central region of the normal cornea. , These cells express human leukocyte antigen (HLA) class II molecules and are thought to function in the afferent arm of the ocular immune response by presenting antigens to T lymphocytes. , Injury to the central cornea results in the rapid migration of peripheral Langerhans cells to the damaged area.
Corneal epithelial cells are renewed continuously to maintain the normal layered structure of the epithelium in a process characterized by dynamic equilibrium ( Fig. 1.6 ). Only the basal cells of the corneal epithelium proliferate, with the daughter cells instead differentiating into wing cells and subsequently into superficial cells and gradually emerging at the corneal surface. The differentiation process requires approximately 7 to 14 days, after which the superficial cells undergo desquamation into the tear film. Mechanical friction associated with blinking, ultraviolet radiation, and hypoxia induces apoptosis (programmed cell death) and desquamation of corneal epithelial cells. Thoft and Friend proposed that an equilibrium, represented by the equation X + Y = Z, exists between the proliferation of basal epithelial cells and their differentiation into superficial cells (X), the centripetal movement of peripheral epithelial cells (Y), and epithelial cell loss from the corneal surface (Z). This X, Y, Z hypothesis explains well the dynamic equilibrium of epithelial cells in the cornea. Given the continuous desquamation of surface epithelial cells, it is essential that new epithelial cells be supplied not only by mitosis of basal cells but also by the emergence of epithelial cells from the periphery.
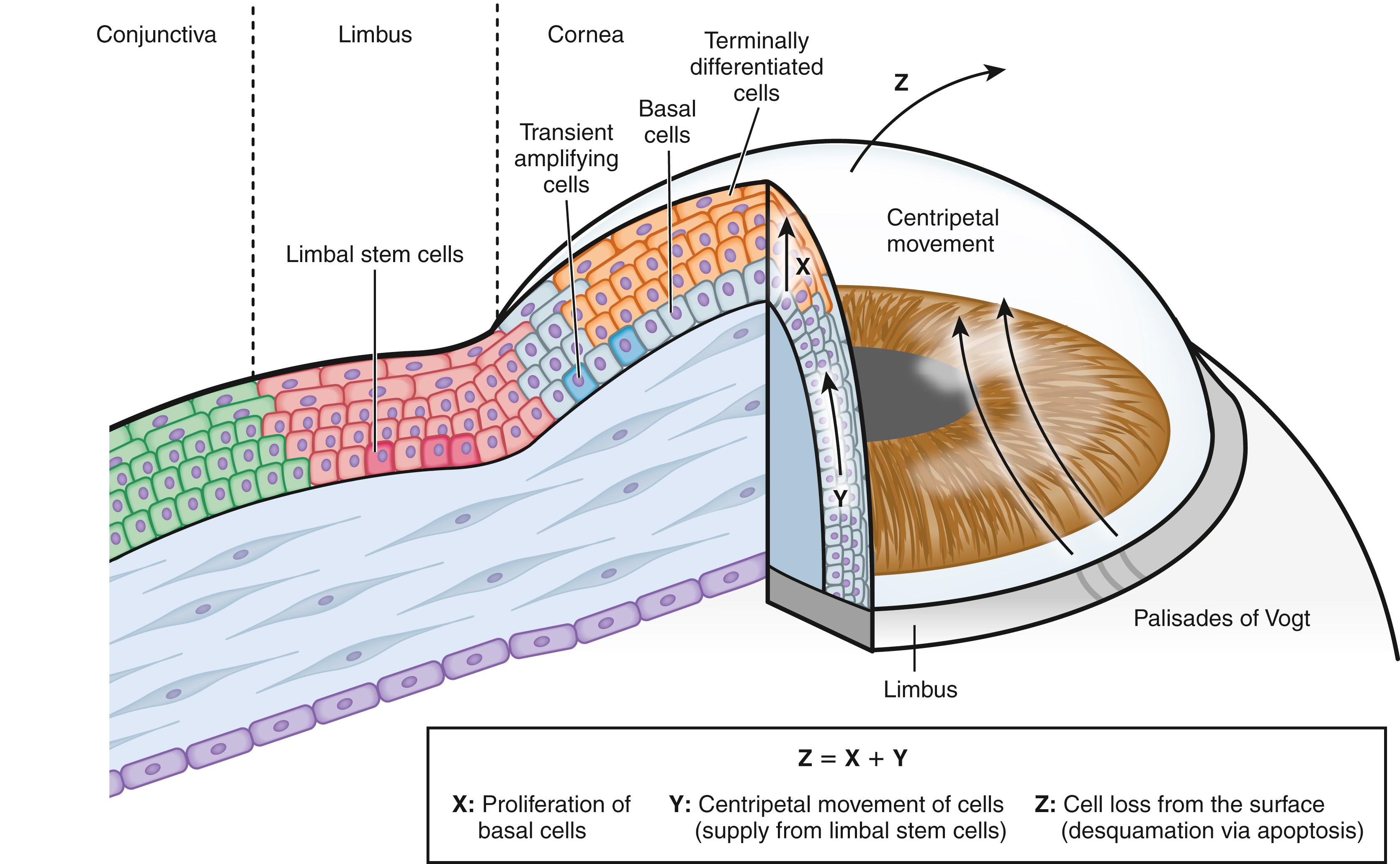
The function of stem cells is to replenish cells lost in normal or damaged tissue. The asymmetric division of each stem cell generates a new stem cell and a transit amplifying cell that initially proliferates and then gives rise to terminally differentiated cells. As in other tissues, the existence of stem cells to maintain homeostasis of the corneal epithelium has been postulated. Although keratin 3/12 (64-kDa keratin) is expressed in all layers of corneal epithelial cells, it is present only in the suprabasal epithelial cells at the limbus. The presence of slowly cycling cells in the basal cell layer at the limbus was demonstrated by cell labeling with [ 3 H]thymidine, and basal cells at the limbus were found to have a higher mitotic potential than those of the central cornea in vitro. Centripetal movement of corneal epithelial cells has also been well documented. These observations suggested that stem cells for corneal epithelial cells reside at the limbus, the transitional zone between the cornea and conjunctiva. , ,
The palisades of Vogt, richly vascularized papillae at the transition zone between the cornea and conjunctiva, have been identified as the likely location of limbal stem cells for corneal epithelial cells. , These structures are able to provide a protective environment for stem cells, as well as to supply them with growth factors, extracellular matrix (ECM), and neural signals to maintain their nature as stem cells. Limbal epithelial crypts, anatomic structures that extend from the palisades of Vogt, have been proposed to be the actual site of the stem cell niche. The putative stem cells in the basal layer of the limbus have unique characteristics in that they cycle slowly and therefore retain DNA labels such as [ 3 H]thymidine, they are poorly differentiated with primitive cytoplasm, they have a high proliferative potential without undergoing maturation, they are small with a high nucleus-to-cytoplasm ratio, they are capable of generating a large number of differentiated progeny, and they reside in close contact with a subset of mesenchymal niche cells. ,
Although many markers for limbal stem cells have been proposed, , there is no single positive marker that distinguishes these cells. The expression of p63 (a marker of cell proliferative ability), α-enolase, keratin 19, and the hepatocyte growth factor (HGF) receptor has been shown to be higher in the limbal epithelium than in the corneal epithelium. The transporter protein ABCG2 is also expressed specifically in the basal layer of the limbal epithelium. , Although no direct evidence for the existence of limbal stem cells has been obtained to date, ABCG2 appears to be the most promising surface marker for the identification of such cells.
Limbal stem cell deficiency has been recognized as a complex corneal disorder resulting from functional or structural loss of the limbus. Deficiency of limbal stem cells has been suggested to lead to impairment of corneal epithelial homeostasis in individuals with aniridia, inflammatory disorders of the ocular surface such as Stevens-Johnson syndrome, or severe alkali burn of the ocular surface. No medical treatment for limbal stem cell deficiency is currently available. Transplantation of stem cells is a potential approach to the treatment of limbal stem cell deficiency. However, such an approach requires sorting of limbal stem cells from explants of limbus tissue, given that the stem cells are thought to constitute less than 1% of cells in the basal layer of the limbus. The lack of a definitive stem cell marker has thus impeded the sorting process. Sorting based on the presence of stem cell–associated markers (ABCG2, vimentin, keratin 19) and the absence of differentiation markers (keratin 3/12, connexin 43, involucrin) might be the best current approach.
The surface of the corneal epithelium contains two to four layers of terminally differentiated superficial cells. In contrast to the epidermis of the skin, the corneal epithelium is not normally keratinized, although it may become so under pathologic conditions such as vitamin A deficiency. These cells are flat and polygonal with a diameter of 40–60 μm and a thickness of 2–6 μm (see Table 1.1 ). Their surface is covered with microvilli. Given that superficial cells are well differentiated, they do not proliferate.
Numerous glycoprotein (mucin) and glycolipid molecules are embedded in the cell membrane of corneal epithelial cells. Mucins include both membrane-bound and secreted molecules, with the former in humans including MUC1, MUC4, and MUC16, all of which have been detected in superficial epithelial cells of the cornea and conjunctiva. In mice, MUC16 is expressed in the conjunctiva but not in the cornea. These glycoproteins and glycolipids form floating particles in the cell membrane that are collectively termed the glycocalyx and which confer hydrophilic properties on the anterior surface of the superficial epithelial cells. The glycocalyx interacts with the mucinous layer of the tear film and helps to maintain the layered structure of the latter. Loss either of the glycocalyx of corneal epithelial cells or of goblet cells in the conjunctival epithelium results in tear film instability and the mucin-deficiency form of dry eye.
Beneath the superficial cells lie two or three layers of wing cells, so called because of their characteristic wing-like shape. Wing cells are in an intermediate state of differentiation between basal and superficial cells and are rich in intracellular tonofilaments composed of keratin (see Table 1.1 ). The cell membranes of adjacent wing cells are interdigitated (see Fig. 1.5 ).
The single layer of columnar basal cells of the corneal epithelium rests on the basement membrane. Basal cells, unlike superficial and wing cells, possess mitotic activity, and they differentiate consecutively into wing and superficial cells (see Table 1.1 ). Neighboring basal cells interdigitate laterally and are joined by desmosomes, gap junctions, and adherens junctions (see Fig. 1.5 ). The posterior surface of basal cells is flat and abuts the basement membrane.
Basal cells adhere to the basement membrane via hemidesmosomes that are linked to anchoring fibrils of type VII collagen (see Fig. 1.4 ). The anchoring fibrils penetrate the basement membrane and course into the stroma, where they form anchoring plaques together with type I collagen, a major component of the stroma. The adherens junctions are present at the lateral surface of the basal cells of the corneal epithelium and are thought to mediate cell-cell interaction.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here