Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
The mammalian cell cycle is divided into four phases: mitosis (M), deoxyribonucleic acid (DNA) synthesis (S), and the gap phases G1 and G2 ( Fig. 17.1 ). Mitosis is recognized when cells visibly undergo cell division and chromatin becomes condensed, sequentially progressing through prophase, metaphase, anaphase, and telophase. The G1 phase occurs immediately after mitosis has been completed and ends when DNA synthesis begins. During the S phase, cells duplicate their entire genome by DNA replication. G2 occurs after DNA synthesis has been completed and before chromosomal condensation in mitosis. Although the duration of the S, G2, and M phases is relatively constant for most mammalian cells, there can be a large degree of variability in the duration of G1. Among the earliest observations regarding the generation time for cells, it was shown that by varying the growth conditions, the length of a cell division cycle could change, with the length of G1 responsible for most of this variability. Although cells progress through S, G2, and M phases in relatively invariable time periods, the length of the G1 phase is highly variable, and this variability is dependent at least in part on the presence of growth factors.
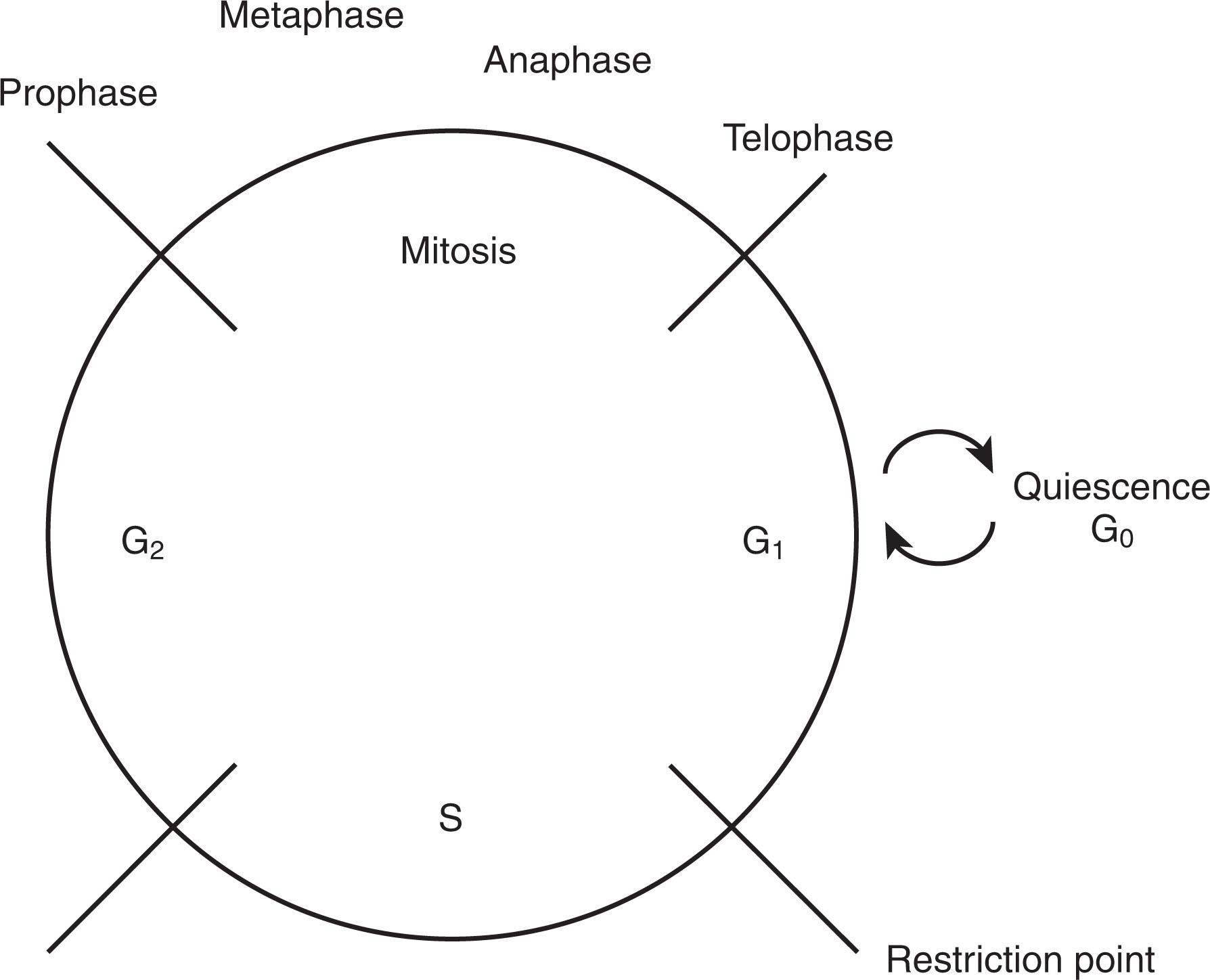
Quiescence (G0) is a non-proliferative state in which viable cells have left the cell cycle and may remain for prolonged periods. Quiescent cells may be difficult to distinguish morphologically from cells in a prolonged G1 phase, but they can be distinguished by different markers. Terminally differentiated cells, such as neutrophilic granulocytes, muscle cells, and neurons, have irreversibly exited the cell cycle during the process of differentiation and are examples of cells that have irreversibly entered G0. Other cells, including stem cells, reversibly enter G0 and may be induced to re-enter the cell cycle with appropriate stimuli, such as growth factors. Differentiation provides the organism with a supply of cells to execute specific and specialized functions. In some cell types, such as muscle and nerve cells, differentiation and proliferation are mutually exclusive fates, and cells undergo “terminal differentiation.” In other cell types, such as those of the hematopoietic lineage, proliferation may continue after cells acquire differentiated characteristics. For example, erythroblasts, myeloblasts, and megakaryoblasts are committed to particular differentiation pathways and possess lineage-specific markers yet continue to proliferate. T and B lymphocytes are fully differentiated and express antigen-specific receptors but can be induced to proliferate when appropriately stimulated.
G1, which occupies the period or gap between M and S phases, is the interval between the completion of one round of cell division and initiation of the next. Its duration is the most variable, can be prolonged depending on the cell type, and is subject to regulation by environmental factors such as the availability of growth factors and nutrients. It is the period of cell growth, and as a first approximation, the amount of time a cell spends in G1 is inversely related to its rate of proliferation. A certain increase in mass usually is required before the cell initiates the next S phase. When conditions are unsuitable for proliferation (e.g., because of insufficient nutrients or absence of mitogens), cells arrest in G1, and those that are already in S, G2, or M phase usually complete the round to which they have been committed and arrest only when they reach G1 again. For example, when the 40 S ribosomal protein S6 is missing, cells stop proliferation. If cell size or mass were not regulated, S phase entry might cause cells to become progressively smaller or be at risk for DNA replication errors because of insufficient substrates. Cell size regulation is intimately linked to ribosome biogenesis and nutrient-sensing systems, central to which are the phosphoinositide 3-kinase (PI3K) and target of rapamycin (TOR) pathways. Notably, when the MYC transcription factor is missing, cells slow their growth and often do not enter the S phase. Myelocytomatosis (MYC) transcription factor regulates cyclin and cyclin-dependent kinase (CDK) genes as well as genes involved in ribosome biogenesis and translation. In aggregate, studies of a variety of cell systems indicate that cell size regulation is linked to cell proliferation, except in specialized cells that undergo endoreplication or in embryos shortly after fertilization, when G1 is virtually undetectable, and there is no cell enlargement. In the latter, the original mass of egg cytoplasm is partitioned among thousands of cells within a few hours without a noticeable increase in size.
S phase is the period of wholesale DNA synthesis during which the cell replicates its genetic content; a normal diploid somatic cell with a 2 N complement of DNA at the beginning of the S phase acquires a 4 N complement of DNA at its end. (Recall that N = 1 copy of each chromosome per cell [haploid]; 2 N = 2 copies [diploid].) The duration of the S phase may vary from only a few minutes in rapidly dividing, early embryo cells to a few hours in most somatic cells. Early embryo cells generally “live off” the accumulated stores of maternal ribonucleic acid (RNA) and proteins present in the egg and are transcriptionally silent, whereas cells in later development and mature organisms must actively transcribe subsets of their genes to survive and maintain specialized functions. The longer time required for the latter to complete the S phase probably allows these cells to coordinate DNA replication with transcription and to preserve higher-order gene and chromatin structural information that influences gene expression for transmission to progeny cells.
G2 is the period or gap between S and M phases when cells have finished replicating their DNA, are preparing to divide, and have a 4 N DNA content. For most cells entering the S phase, passage through G2 is “automatic,” and the duration of G2 is fixed, except under unusual circumstances. For example, G2 duration can be extremely short and is essentially undetectable in rapidly proliferating, early embryonic cells.
Mitosis, or M phase, is the period of actual nuclear and cell division during which the duplicated chromosomes are divided equally between two progeny cells. It is obvious microscopically as the period of chromosome condensation and segregation, nuclear division (karyokinesis), and physical separation of the two daughter cells (cytokinesis). A cell entering the M phase has a 4 N DNA content and finishes as two cells, each with an identical 2 N complement of DNA. The complex sequence of changes that take place allows mitosis to be subdivided into prophase, prometaphase, metaphase, anaphase, and telophase. Prophase is the period of chromatin/chromosome condensation, centrosome separation/migration to opposite poles, and nuclear membrane breakdown. The centrosomes are microtubule organization centers that eventually give rise to the bipole mitotic spindle apparatus that will separate the sister chromatids of each duplicated chromosome. During prometaphase , chromosomes attach to microtubules of the mitotic spindle so that sister chromatids attach to opposite poles. In metaphase , the condensed chromosomes align at the equatorial plate. The cohesive “bond” between sister chromatids of duplicated chromosomes is dissolved, allowing anaphase , the period of sister chromatid separation, to proceed. On reaching their poles, nuclear membranes form to envelop each of the two separated sets of chromosomes, which also begin to decondense, marking telophase and karyokinesis. This is immediately followed by cytokinesis, during which an actin-myosin ring at the cell membrane contracts to move inwards until a midbody structure is formed. The abscission of the midbody marks the exit from mitosis. Following mitosis, cells re-enter G1, and for approximately 3 hours, they are capable of leaving the cell cycle into quiescence when growth factors and nutrients are missing. Once past this point, cells are no longer sensitive to mitogen withdrawal and can commit to another round of cell division.
To decide whether to proliferate, cells scan their environment with the help of cell surface receptors such as receptor tyrosine kinases (RTKs) that bind extracellular ligands and activate signaling pathways. RTKs become activated by ligand-stabilized dimerization that allows autophosphorylation. The intracellular phosphotyrosine residues are recognized by SH2 (Src homology 2) or PTB (phosphotyrosine-binding) protein domains, leading to the recruitment of signaling effectors and formation of signaling complexes. In turn, these complexes permit activation of signaling pathways, such as rat sarcoma virus (RAS)–mitogen-activated protein kinase (MAPK), PI3K, and phospholipase Cγ (PLCγ). Together, these signaling pathways stimulate cell proliferation ( Fig. 17.2 ).
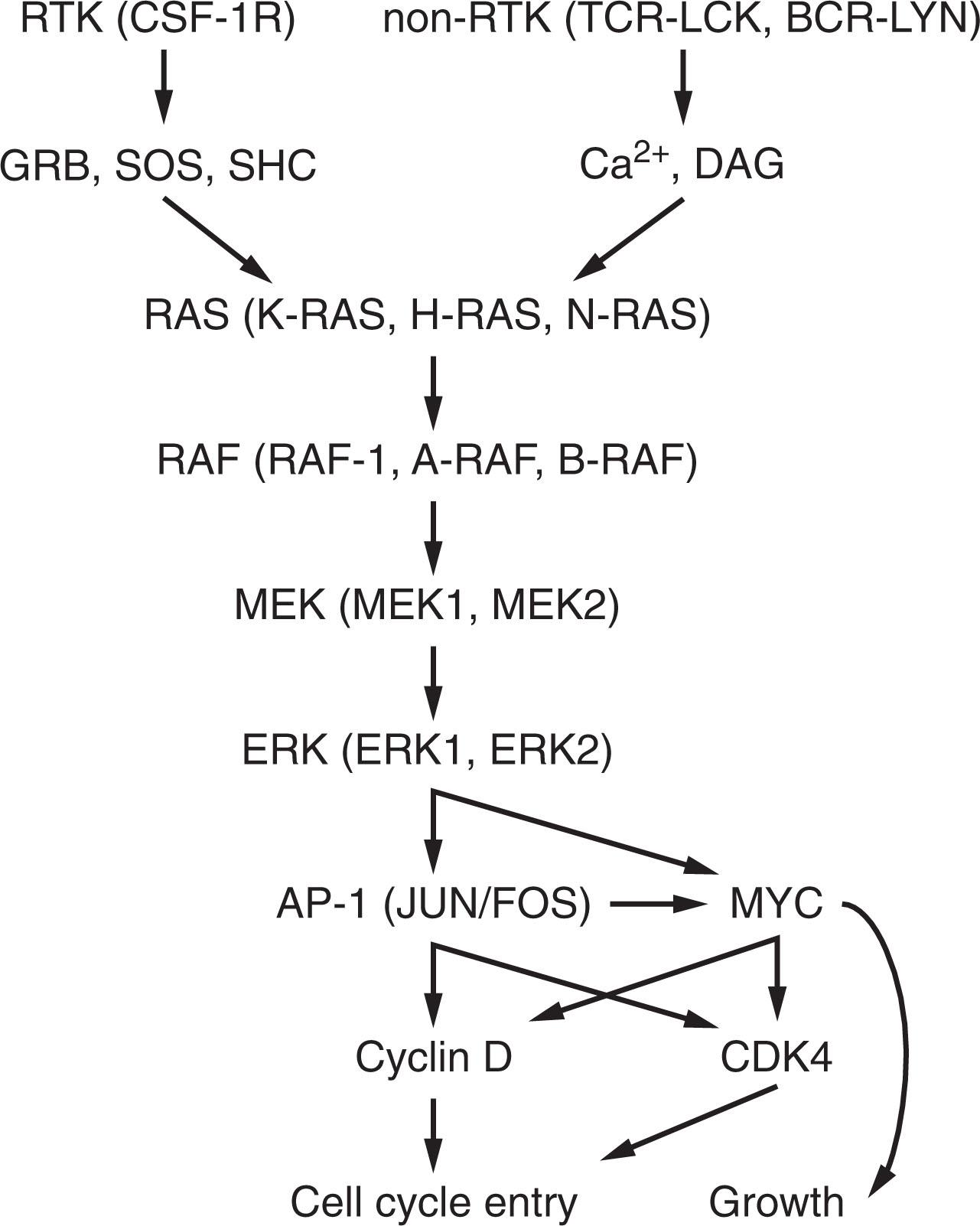
Activation of T cells occurs upon ligation of the T-cell receptor (TCR) by major histocompatibility complex (MHC) molecules in antigen-presenting cells. The TCR and its coreceptors CD4 and CD8 have no intrinsic enzymatic activity. Instead, the associated non–RTK LCK (lymphocyte-specific protein tyrosine kinase) binds to cytoplasmic domains of TCR coreceptors CD4 and CD8, and their activation leads to phosphorylation of immunoreceptor tyrosine-based activation motifs (ITAMs) in CD3. Phosphorylated CD3 recruits the tyrosine kinase zeta-chain-associated protein kinase 70 (ZAP70), leading to a cascade of phosphorylation events that in turn activates LAT (linker for activation of T cells) complexes. The LAT signalosome triggers the release of intracellular Ca 2+ and the production of diacylglycerol (DAG). The latter activates RAS-MAPK and protein kinase C (PKC)–nuclear factor κB (NF-κB) signaling pathways.
Every normal B cell has a unique B-cell receptor (BCR) consisting of pairs of immunoglobulin heavy (IgH) and light (IgL) chains. Each IgH and IgL has a unique variable region that allows the BCR to recognize and bind to diverse antigens, both soluble and on the surface of antigen-presenting cells. Antigen-induced aggregation of BCR leads to phosphorylation of ITAMs by the Src family tyrosine kinase LYN. This phosphorylation event initiates the assembly of intracellular signaling molecules, including SYK, PLCγ2, Bruton tyrosine kinase (BTK), VAV, and the adaptor B-cell linker (BLNK). The BCR coreceptor CD19 is also phosphorylated by LYN, leading to the recruitment of PI3K, BTK, and AKT. Together, this signaling leads to the release of intracellular Ca 2+ and production of DAG, and ultimately leads to activation of RAS-MAPK and PKC–NF-κB signaling pathways.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here