Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
About 40% of the body is skeletal muscle, and perhaps another 10% is smooth and cardiac muscle. Some of the same basic principles of contraction apply to all these muscle types. In this chapter, we mainly consider skeletal muscle function; the specialized functions of smooth muscle are discussed in Chapter 8 , and cardiac muscle is discussed in Chapter 9 .
Figure 6-1 shows that skeletal muscles are composed of numerous fibers ranging from 10 to 80 micrometers in diameter. Each of these fibers is made up of successively smaller subunits, also shown in Figure 6-1 , and described in subsequent paragraphs.
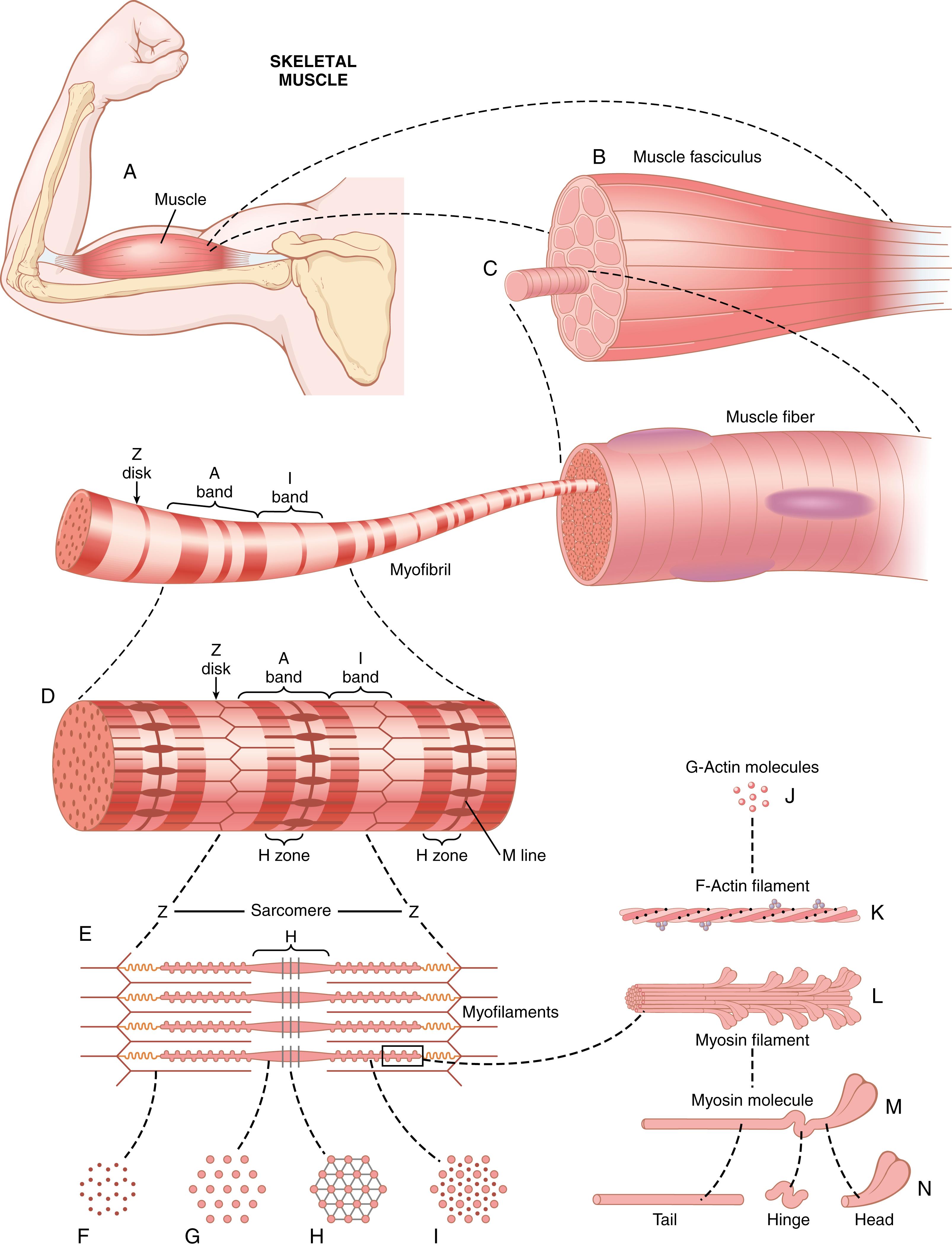
In most skeletal muscles, each fiber extends the entire length of the muscle. Except for about 2% of the fibers, each fiber is usually innervated by only one nerve ending, located near the middle of the fiber.
The sarcolemma consists of a true cell membrane, called the plasma membrane, and an outer coat made up of a thin layer of polysaccharide material that contains numerous thin collagen fibrils. At each end of the muscle fiber, this surface layer of the sarcolemma fuses with a tendon fiber. The tendon fibers, in turn, collect into bundles to form the muscle tendons that then connect the muscles to the bones.
Each muscle fiber contains several hundred to several thousand myofibrils , which are illustrated in the cross-sectional view of Figure 6-1 C . Each myofibril ( Figure 6-1 D and E ) is composed of about 1500 adjacent myosin filaments and 3000 actin filaments , which are large polymerized protein molecules that are responsible for the muscle contraction. These filaments can be seen in longitudinal view in the electron micrograph of Figure 6-2 and are represented diagrammatically in Figure 6-1 E through L . The thick filaments in the diagrams are myosin , and the thin filaments are actin.

Note in Figure 6-1 E that the myosin and actin filaments partially interdigitate and thus cause the myofibrils to have alternate light and dark bands, as illustrated in Figure 6-2 . The light bands contain only actin filaments and are called I bands because they are isotropic to polarized light. The dark bands contain myosin filaments, as well as the ends of the actin filaments, where they overlap the myosin, and are called A bands because they are anisotropic to polarized light. Note also the small projections from the sides of the myosin filaments in Figure 6-1 E and L . These projections are cross-bridges. It is the interaction between these cross-bridges and the actin filaments that causes contraction ( ).
Figure 6-1 E also shows that the ends of the actin filaments are attached to a Z disk. From this disk, these filaments extend in both directions to interdigitate with the myosin filaments. The Z disk, which is composed of filamentous proteins different from the actin and myosin filaments, passes crosswise across the myofibril and also crosswise from myofibril to myofibril, attaching the myofibrils to one another all the way across the muscle fiber. Therefore, the entire muscle fiber has light and dark bands, as do the individual myofibrils. These bands give skeletal and cardiac muscle their striated appearance.
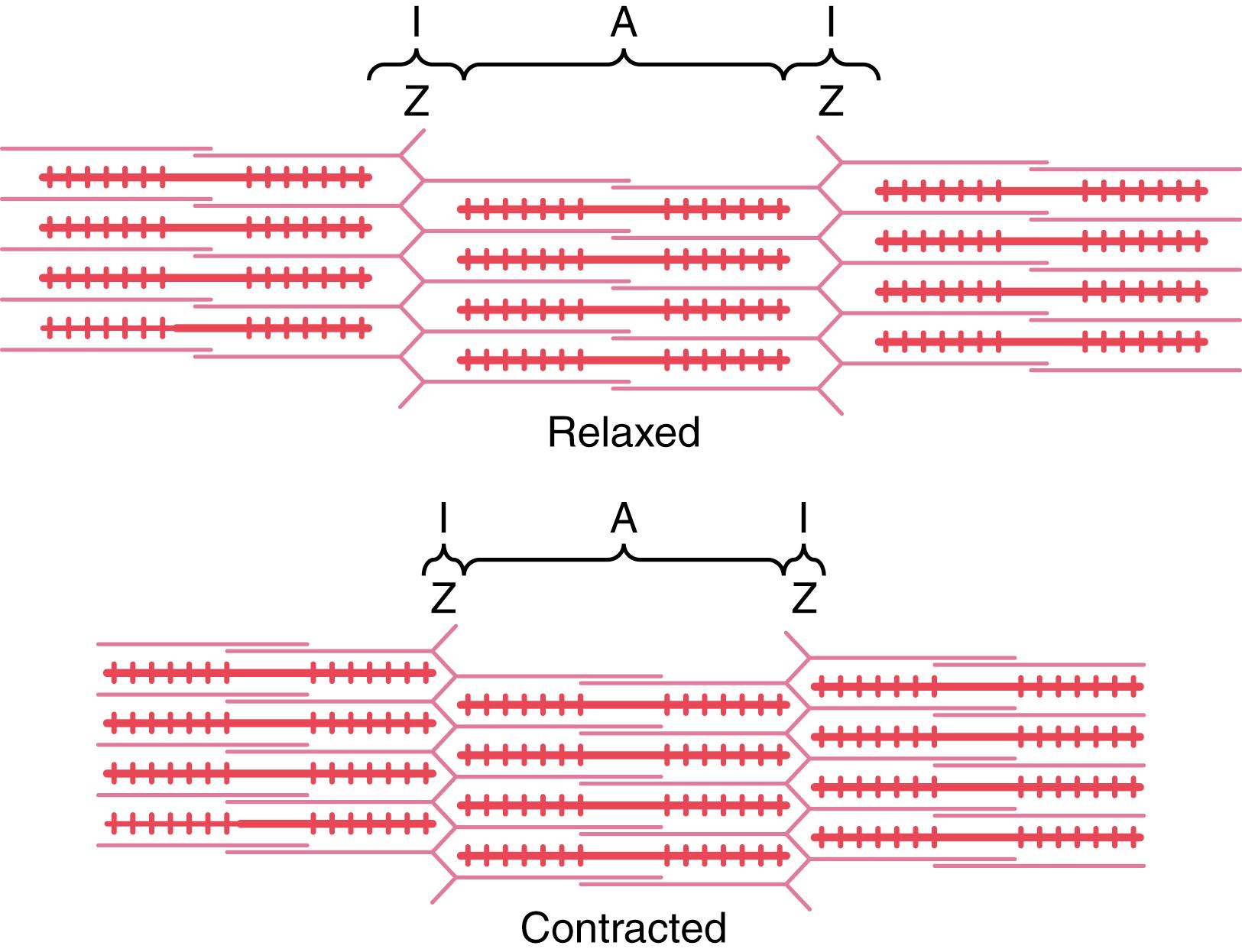
The portion of the myofibril (or of the whole muscle fiber) that lies between two successive Z disks is called a sarcomere. When the muscle fiber is contracted, as shown at the bottom of Figure 6-5 , the length of the sarcomere is about 2 micrometers. At this length, the actin filaments completely overlap the myosin filaments, and the tips of the actin filaments are just beginning to overlap one another. As discussed later, at this length, the muscle is capable of generating its greatest force of contraction.
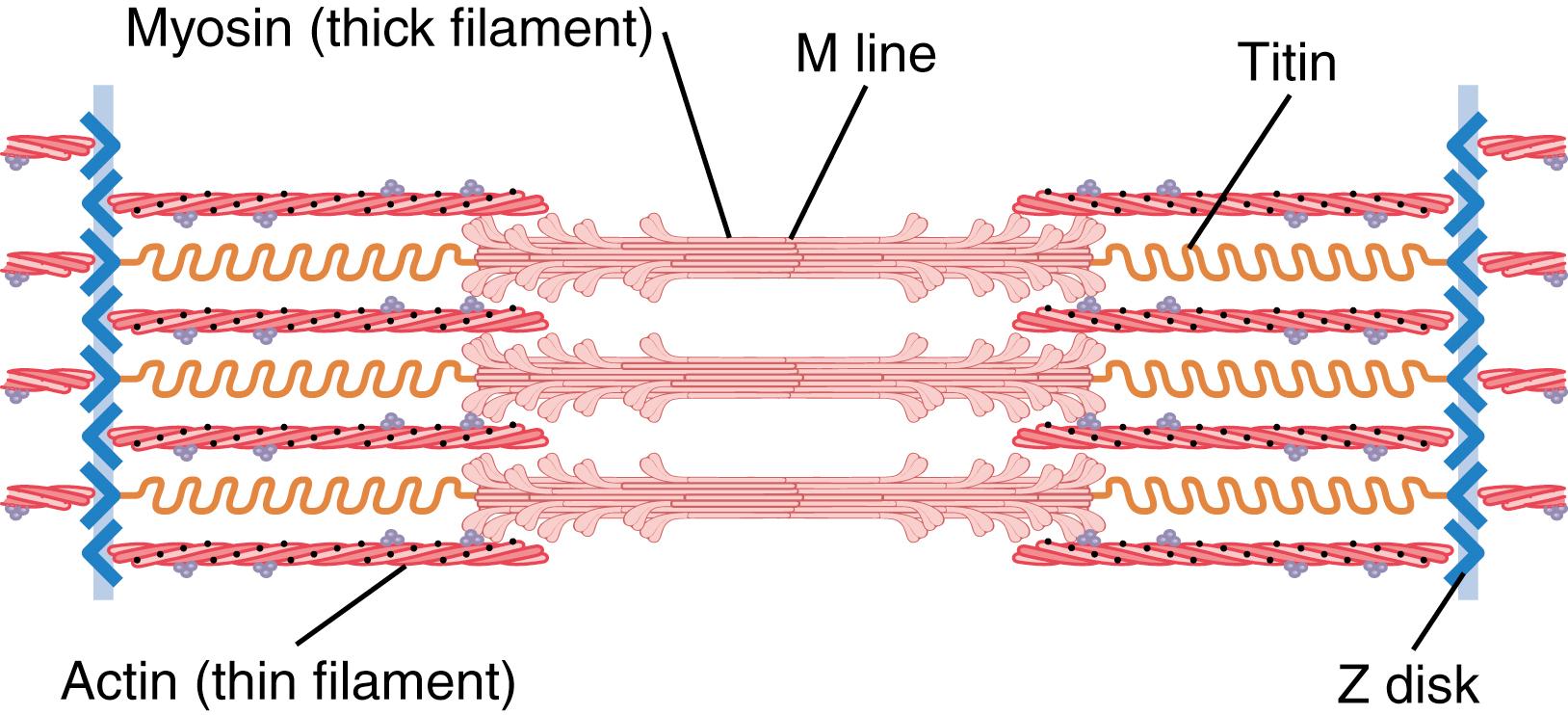
The side-by-side relationship between the myosin and actin filaments is maintained by a large number of filamentous molecules of a protein called titin ( Figure 6-3 ). Each titin molecule has a molecular weight of about 3 million, which makes it one of the largest protein molecules in the body. Also, because it is filamentous, it is very springy. These springy titin molecules act as a framework that holds the myosin and actin filaments in place so that the contractile machinery of the sarcomere will work. One end of the titin molecule is elastic and is attached to the Z disk, acting as a spring and changing length as the sarcomere contracts and relaxes. The other part of the titin molecule tethers it to the myosin thick filament. The titin molecule may also act as a template for the initial formation of portions of the contractile filaments of the sarcomere, especially the myosin filaments.
Many myofibrils are suspended side by side in each muscle fiber. The spaces between the myofibrils are filled with intracellular fluid called sarcoplasm , containing large quantities of potassium, magnesium, and phosphate, plus multiple protein enzymes. Also present are tremendous numbers of mitochondria that lie parallel to the myofibrils. These mitochondria supply the contracting myofibrils with large amounts of energy in the form of adenosine triphosphate (ATP) formed by the mitochondria.
Also, in the sarcoplasm surrounding the myofibrils of each muscle fiber, is an extensive reticulum ( Figure 6-4 ), called the sarcoplasmic reticulum. This reticulum has a special organization that is extremely important in regulating calcium storage, release, reuptake and therefore muscle contraction, as discussed in Chapter 7 . The rapidly contracting types of muscle fibers have especially extensive sarcoplasmic reticula.
The initiation and execution of muscle contraction occur in the following sequential steps.
An action potential travels along a motor nerve to its endings on muscle fibers.
At each ending, the nerve secretes a small amount of the neurotransmitter acetylcholine.
Acetylcholine acts on a local area of the muscle fiber membrane to open acetylcholine-gated cation channels through protein molecules floating in the membrane.
The opening of acetylcholine-gated channels allows large quantities of sodium ions to diffuse to the interior of the muscle fiber membrane. This action causes a local depolarization that in turn leads to the opening of voltage-gated sodium channels, which initiates an action potential at the membrane.
The action potential travels along the muscle fiber membrane in the same way that action potentials travel along nerve fiber membranes.
The action potential depolarizes the muscle membrane, and much of the action potential electricity flows through the center of the muscle fiber. Here it causes the sarcoplasmic reticulum to release large quantities of calcium ions that have been stored within this reticulum.
The calcium ions initiate attractive forces between the actin and myosin filaments, causing them to slide alongside each other, which is the contractile process.
After a fraction of a second, the calcium ions are pumped back into the sarcoplasmic reticulum by a Ca 2+ membrane pump and remain stored in the reticulum until a new muscle action potential comes along; this removal of calcium ions from the myofibrils causes the muscle contraction to cease.
We now describe the molecular machinery of the muscle contractile process.
Figure 6-5 demonstrates the basic mechanism of muscle contraction. It shows the relaxed state of a sarcomere (top) and the contracted state (bottom). In the relaxed state, the ends of the actin filaments extending from two successive Z disks barely overlap one another. Conversely, in the contracted state, these actin filaments have been pulled inward among the myosin filaments, so their ends overlap one another to their maximum extent. Also, the Z disks have been pulled by the actin filaments up to the ends of the myosin filaments. Thus, muscle contraction occurs by a sliding filament mechanism.
But what causes the actin filaments to slide inward among the myosin filaments? This action is caused by forces generated by interaction of the cross-bridges from the myosin filaments with the actin filaments. Under resting conditions, these forces are inactive, but when an action potential travels along the muscle fiber, this causes the sarcoplasmic reticulum to release large quantities of calcium ions that rapidly surround the myofibrils. The calcium ions, in turn, activate the forces between the myosin and actin filaments, and contraction begins. However, energy is needed for the contractile process to proceed. This energy comes from high-energy bonds in the ATP molecule, which is degraded to adenosine diphosphate (ADP) to liberate the energy. In the next few sections, we describe these molecular processes of contraction.
Each of the myosin molecules, shown in Figure 6-6 A , has a molecular weight of about 480,000. Figure 6-6 B shows the organization of many molecules to form a myosin filament, as well as interaction of this filament on one side with the ends of two actin filaments.
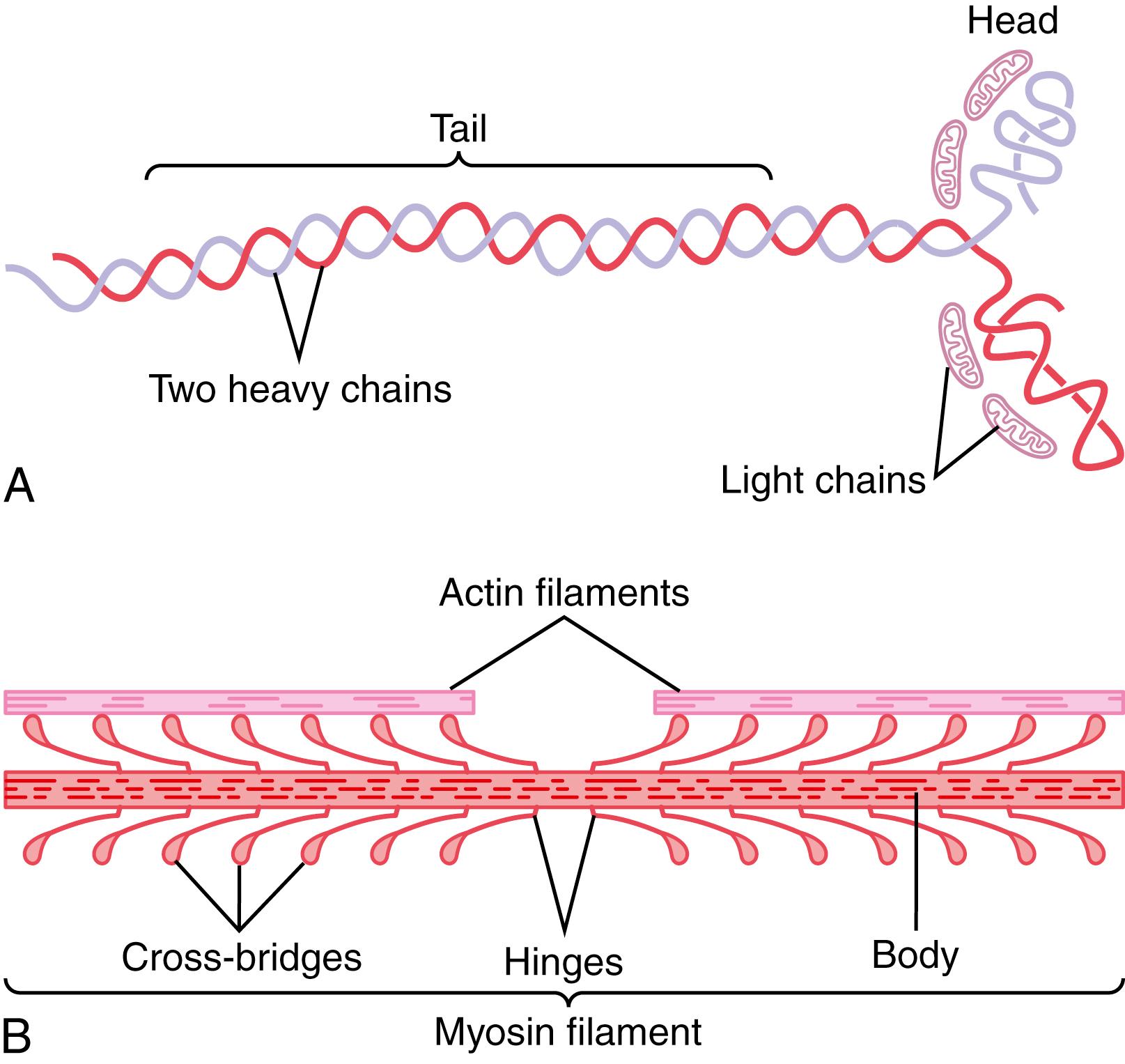
The myosin molecule (see Figure 6-6 A ) is composed of six polypeptide chains—two heavy chains , each with a molecular weight of about 200,000; and four light chains, with molecular weights of about 20,000 each. The two heavy chains wrap spirally around each other to form a double helix, which is called the tail of the myosin molecule. One end of each of these chains is folded bilaterally into a globular polypeptide structure called a myosin head. Thus, there are two free heads at one end of the double-helix myosin molecule. The four light chains are also part of the myosin head, two to each head. These light chains help control the function of the head during muscle contraction.
The myosin filament is made up of 200 or more individual myosin molecules. The central portion of one of these filaments is shown in Figure 6-6 B , displaying the tails of the myosin molecules bundled together to form the body of the filament, while many heads of the molecules hang outward to the sides of the body. Also, part of the body of each myosin molecule hangs to the side along with the head, thus providing an arm that extends the head outward from the body, as shown in the figure. The protruding arms and heads together are called cross-bridges. Each cross-bridge is flexible at two points called hinges —one where the arm leaves the body of the myosin filament and the other where the head attaches to the arm. The hinged arms allow the heads either to be extended far outward from the body of the myosin filament or brought close to the body. The hinged heads, in turn, participate in the contraction process, as discussed in the following sections.
The total length of each myosin filament is uniform, almost exactly 1.6 micrometers. Note, however, that there are no cross-bridge heads in the center of the myosin filament for a distance of about 0.2 micrometer because the hinged arms extend away from the center.
Now, to complete the picture, the myosin filament is twisted so that each successive pair of cross-bridges is axially displaced from the previous pair by 120 degrees. This twisting ensures that the cross-bridges extend in all directions around the filament.
Another feature of the myosin head that is essential for muscle contraction is that it functions as an adenosine triphosphatase (ATPase) enzyme. As explained later, this property allows the head to cleave ATP and use the energy derived from the ATP’s high-energy phosphate bond to energize the contraction process.
The backbone of the actin filament is a double-stranded F-actin protein molecule , represented by the two lighter-colored strands in Figure 6-7 . The two strands are wound in a helix in the same manner as the myosin molecule.
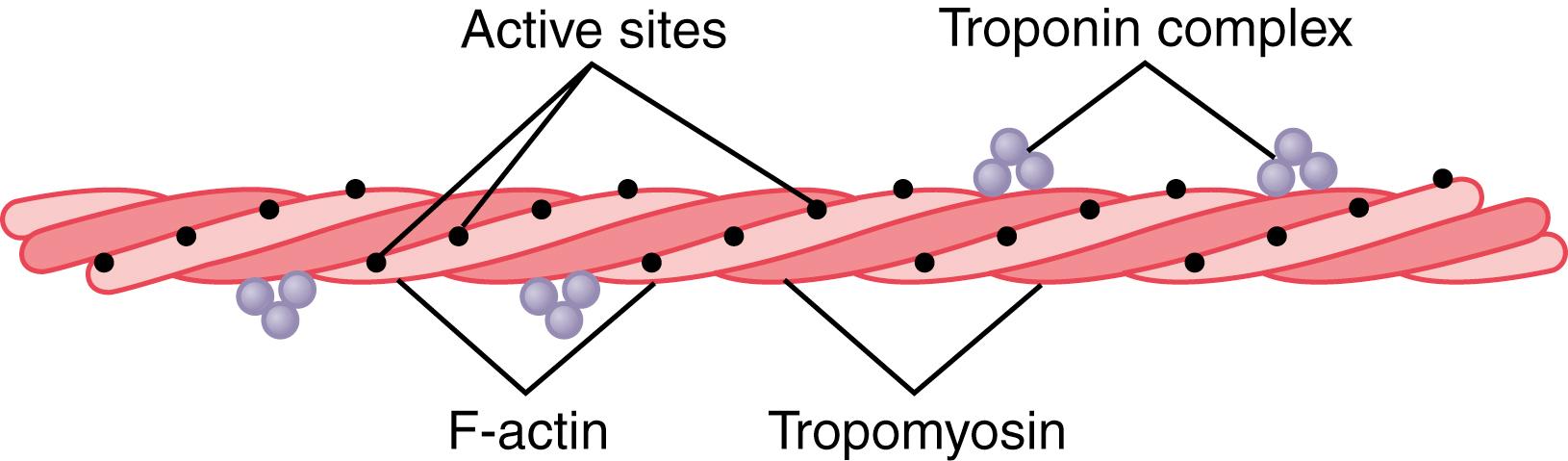
Each strand of the double F-actin helix is composed of polymerized G-actin molecules , each having a molecular weight of about 42,000. Attached to each one of the G-actin molecules is one molecule of ADP. These ADP molecules are believed to be the active sites on the actin filaments with which the cross-bridges of the myosin filaments interact to cause muscle contraction. The active sites on the two F-actin strands of the double helix are staggered, giving one active site on the overall actin filament about every 2.7 nanometers.
Each actin filament is about 1 micrometer long. The bases of the actin filaments are inserted strongly into the Z disks; the ends of the filaments protrude in both directions to lie in the spaces between the myosin molecules, as shown in Figure 6-5 .
The actin filament also contains another protein, tropomyosin. Each molecule of tropomyosin has a molecular weight of 70,000 and a length of 40 nanometers. These molecules are wrapped spirally around the sides of the F-actin helix. In the resting state, the tropomyosin molecules lie on top of the active sites of the actin strands so that attraction cannot occur between the actin and myosin filaments to cause contraction. Contraction occurs only when an appropriate signal causes a conformation change in tropomyosin that “uncovers” active sites on the actin molecule and initiates contraction, as explained later.
Attached intermittently along the sides of the tropomyosin molecules are additional protein molecules called troponin. These protein molecules are actually complexes of three loosely bound protein subunits, each of which plays a specific role in controlling muscle contraction. One of the subunits (troponin I) has a strong affinity for actin, another (troponin T) for tropomyosin, and a third (troponin C) for calcium ions. This complex is believed to attach the tropomyosin to the actin. The strong affinity of the troponin for calcium ions is believed to initiate the contraction process, as explained in the next section.
A pure actin filament without the presence of the troponin-tropomyosin complex (but in the presence of magnesium ions and ATP) binds instantly and strongly with the heads of the myosin molecules. Then, if the troponin-tropomyosin complex is added to the actin filament, the binding between myosin and actin does not take place. Therefore, it is believed that the active sites on the normal actin filament of the relaxed muscle are inhibited or physically covered by the troponin-tropomyosin complex. Consequently, the sites cannot attach to the heads of the myosin filaments to cause contraction. Before contraction can take place, the inhibitory effect of the troponin-tropomyosin complex must itself be inhibited.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here