Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Conotruncal anomalies are a group of congenital heart defects involving the outflow tracts of the heart. The conotruncal anomalies include tetralogy of Fallot (TOF), transposition of the great arteries (TGA), double-outlet ventricles, truncus arteriosus, and interrupted aortic arch (IAA) type B.
Conotruncal abnormalities are the result of abnormal division or rotation of the primitive truncus during embryologic development. The common outlet of the embryonic univentricular heart normally undergoes a complex sequence of events to separate into the right ventricular outflow tract (RVOT) and left ventricular outflow tract (LVOT), the aorta, and the main pulmonary artery (see Chapter 62 ). Control by numerous genes and migration of the mesenchymal cells from the embryonic neural crest are required for this development. Mutations in a number of genes have been associated with conotruncal anomalies in humans and animal models.
Echocardiography is the mainstay of clinical diagnosis. Conventional angiography is used primarily to evaluate coronary artery anatomy and aortopulmonary collaterals. Magnetic resonance imaging (MRI) and computed tomography (CT) occasionally are used to clarify anatomic details that are not fully delineated by echocardiography in the preoperative patient; these details often are related to the aortic arch, pulmonary arteries and their supply, and pulmonary veins. MRI and CT more often are requested for routine follow-up of patients with conotruncal anomalies, particularly as they reach their teenage and adult years.
TOF is the most common cyanotic congenital heart defect. The median described incidence of TOF is 356 per 1 million live births in the United States. Classic manifestations include RVOT obstruction, ventricular septal defect (VSD), overriding of the aortic root above the VSD, and right ventricular (RV) hypertrophy ( Fig. 75.1A ). These findings are a result of underdevelopment of the subpulmonary infundibulum, which is associated with anterior deviation of the conal septum. Rather than sitting between the anterior and posterior limbs of the trabecula septomarginalis (a Y -shaped bundle of muscle along the right side of the ventricular septum), the conal septum in TOF typically is fused with the anterior limb, bringing the aorta over the ventricular septum and leading to the malalignment VSD. The septal malalignment and hypertrophy of the trabeculations of the infundibular free wall result in RVOT obstruction ( Fig. 75.1B ). The VSD in TOF is located between the malaligned conal septum superiorly and the muscular septum inferiorly, and typically is large, nonrestrictive, and subaortic.
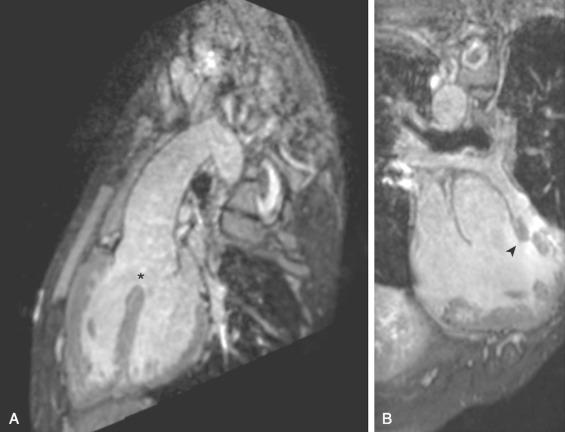
TOF can include pulmonary atresia or dysplastic (absent) pulmonary valve syndrome, in addition to the more common pulmonary stenosis. The extent of RVOT obstruction is variable, ranging from minimal to complete atresia. The pulmonary valve is often thickened with doming leaflets and a hypoplastic annulus causing valvular stenosis. The size of the main and branch pulmonary arteries also varies. Patients with pulmonary valve atresia have pulmonary blood flow supplied by a patent ductus arteriosus, aortopulmonary collateral arteries, or both ( e-Fig. 75.2 ). The central pulmonary arteries can be absent, discontinuous, or diminutive. In TOF with a dysplastic pulmonary valve, congenital severe pulmonary regurgitation occurs, which often is associated with severe dilatation of the central pulmonary arteries and resultant airway compression ( e-Fig. 75.3A and B ).
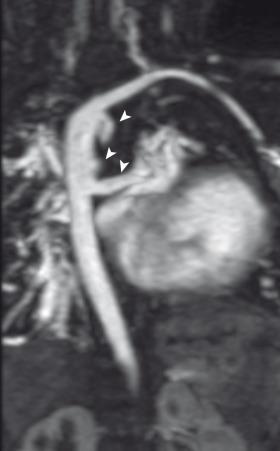
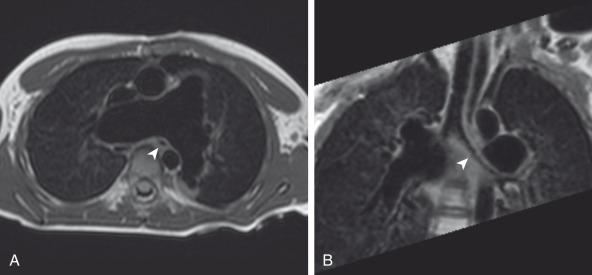
Genetic abnormalities such as chromosome 22q11 deletion, which also leads to DiGeorge or velocardiofacial syndrome, may play an important role in some patients with TOF. Many cases are sporadic, without any specific genetic abnormality identified.
Clinical manifestations are variable. Most patients have adequate pulmonary blood flow at birth, and increasing cyanosis develops early in life. If RVOT obstruction is severe, right-to-left shunting occurs, resulting in cyanosis. When the obstruction is less severe, the shunting is predominantly left to right; patients with this condition can present with congestive heart failure. If patients who have TOF with pulmonary atresia are dependent on the patent ductus arteriosus, an infusion of prostaglandin E 1 is necessary to maintain ductal patency until a more stable pulmonary blood flow can be established. In patients who have TOF with dysplastic pulmonary valve syndrome, presentation may be with tracheobronchomalacia and air trapping, as well as cyanosis.
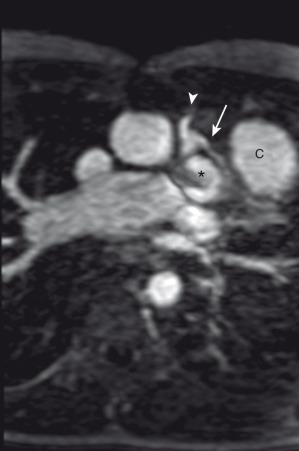
Other congenital heart anomalies can accompany TOF, including right aortic arch (25%) and coronary artery anomalies such as abnormal origin of the left anterior descending (LAD) coronary artery arising from the right coronary artery (5%–6%) or dual LAD coronary arteries. When the LAD coronary artery arises from the right coronary artery, it passes over the RVOT before supplying its usual territory ( e-Fig. 75.4 ).
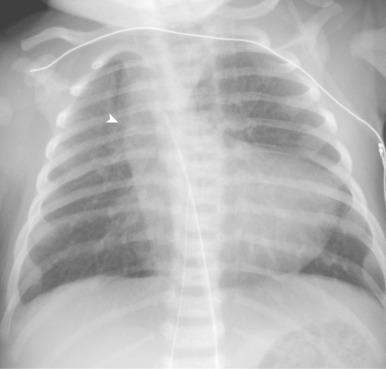
RV hypertrophy causes uplifting of the cardiac apex. Concavity is present at the location of the main pulmonary artery because of underdevelopment, causing a “wooden shoe “or “boot shape” appearance of the heart (in French, Coeur en sabot) on frontal chest radiographs, a classic sign for TOF ( Fig. 75.5 ). The shadow of the main pulmonary artery is absent, and pulmonary vascularity is decreased. Rarely, dilatation of the pulmonary artery occurs as a result of an aneurysm, or asymmetric pulmonary vascularity is present as a result of differential pulmonary artery stenosis and collateralization. In the absence of a thymus, the possibility of DiGeorge syndrome should be considered. A right-sided aortic arch also can be seen on the frontal chest radiograph (see Fig. 75.5 ).
Complete anatomic diagnosis in a neonate with TOF usually is made by echocardiography, with an infrequent need for cross-sectional imaging. CT or MRI typically is requested to determine pulmonary artery anatomy and sources of pulmonary blood flow, including the central pulmonary arteries, patent ductus arteriosus, and aortopulmonary collaterals. CT angiography is an effective modality for delineating pulmonary artery and collateral anatomy in these patients but has the disadvantage of using ionizing radiation. MRI also can accurately describe these anatomic details without the risks of ionizing radiation. Turbo spin echo techniques can display vessels clearly and demonstrate airway anatomy. The mainstay of MRI for these anatomic questions is three-dimensional, gadolinium contrast-enhanced magnetic resonance angiography (MRA), which is highly accurate compared with diagnostic catheterization.
Current management of TOF in most large centers is early single-stage reconstructive surgery, typically performed at 3 to 6 months of age. Staged reconstruction can be required if significant hypoplasia of the central pulmonary arteries is present; a palliative shunt is placed from the systemic to the pulmonary circulation to provide pulmonary blood flow. When pulmonary supply is from multiple aortopulmonary collaterals, a staged approach of unifocalization of collaterals to either a shunt or the central pulmonary arteries is utilized, eventually bringing the major vessels into continuity with the RV. VSD closure often is not tolerated until later in life in this subgroup of patients.
The goal of surgical repair of TOF is to close the VSD and relieve the RVOT obstruction, thus providing unobstructed flow to the pulmonary vessels from the RV. The approach depends on the severity of the anatomy. The entire repair can be performed transatrially, including VSD closure and division of muscle bundles within the RVOT to relieve obstruction, with no right ventriculotomy. If the pulmonary valve annulus is hypoplastic, a limited transannular patch may be needed to relieve the obstruction. This procedure inevitably results in severe pulmonary regurgitation. In the past, this operation was performed with a large right ventriculotomy, which now usually is avoided. In patients who have TOF with pulmonary atresia, a limited transannular patch may be sufficient to relieve RVOT obstruction. With a longer segment atresia, an RV to pulmonary artery conduit may be required. In patients with an LAD coronary artery arising from the right coronary artery and crossing the RVOT, an RV to pulmonary artery conduit occasionally is necessary to avoid damaging the vessel (see e-Fig. 75.4 ).
In patients with a transannular patch, a pulmonary valve replacement may be indicated later in life to remove the volume load of pulmonary regurgitation from the RV ( ). The appropriate timing of this valve replacement is the subject of intense interest. In patients who require an RV to pulmonary artery conduit, a conduit replacement will be needed in time because of somatic growth of the patient. A percutaneous pulmonary valve is also available for placement within a conduit to relieve both stenosis and regurgitation ( ).
Repaired TOF is a frequent referral diagnosis for cardiac MRI. Chronic, severe pulmonary regurgitation can lead to RV pathology. Cardiac MRI is widely considered the gold standard for assessment of RV size and function, making it particularly useful in this patient population ( ). Other questions in this patient population include anatomy of the RVOT ( ), quantification of pulmonary regurgitation ( and Fig. 75.6A ), assessment of branch and segmental pulmonary artery anatomy, measurement of branch pulmonary artery flow, anatomy of aortopulmonary collaterals, assessment of the left ventricle (LV), and aortic valve and root pathology.
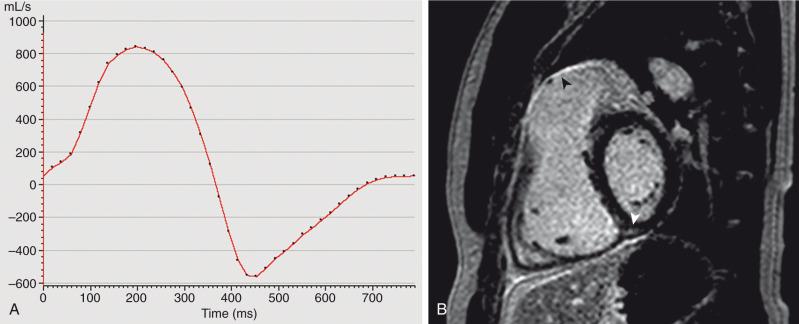
These studies typically are performed with balanced steady-state free-precession (bSSFP) imaging in the vertical and horizontal long-axis planes and short axis of the ventricles, as well as parallel to the RVOT. Gadolinium-enhanced three-dimensional MRA offers high-resolution assessment of the distal pulmonary arteries and can evaluate for aortopulmonary collaterals. Velocity-encoded phase-contrast imaging assesses the ratio of pulmonary to systemic flow, differential pulmonary blood flow, and valve regurgitation. Late gadolinium enhancement imaging reveals scarring or fibrosis in the heart ( Fig. 75.6B ).
CT for patients with repaired TOF is used for evaluation of pulmonary artery anatomy and aortic size, particularly in patients in whom MRI is contraindicated (e.g., patients with an internal defibrillator). Electrocardiographic-gated multidetector array CT can provide RV size and systolic function, although with less temporal resolution than MRI.
TGA is defined by discordant ventriculoarterial relations; the aorta is connected to the RV and the pulmonary artery is connected to the LV. The most common type of TGA has {S,D,D} cardiac segmental anatomy—that is, visceral and atrial situs solitus (S), ventricular D loop (D), and dextroposition of the aortic valve (D) (see Chapter 63 ). The aortic valve is side-by-side or anterior and rightward of the pulmonary valve ( Fig. 75.7A ) and usually is separated from the tricuspid valve by conal tissue. “D-looped TGA” also is acceptable terminology. {S,D,D} TGA is the second most common cyanotic congenital heart disease. The median described incidence of {S,D,D} TGA is 303 per 1 million live births.
TGA usually is not associated with extracardiac anomalies or syndromes. It is associated with VSD in approximately 40% to 45% of cases. Other anomalies that can be seen in patients with TGA are LVOT obstruction, aortic coarctation or interrupted aortic arch, tricuspid valve abnormalities, or, less commonly, mitral valve abnormalities, leftward juxtaposition of the atrial appendages, and RV hypoplasia.
Patients present with cyanosis as a result of parallel systemic and pulmonary circulations. Deoxygenated blood returns to the right side of the heart via systemic veins, then circulates back to the aorta. Pulmonary venous blood returns to the left atrium and LV, then back to the pulmonary artery. Survival depends on communication between the systemic and pulmonary circulations, typically via an atrial septal defect, patent ductus arteriosus, and/or VSD.
The radiographic appearance is variable. The classic finding of TGA by chest radiography is the “egg on a string” sign ( Fig. 75.7B ), which is caused by a narrow mediastinum and the cardiac shadow. The narrow mediastinum is a result of stress-related thymic atrophy and the parallel position of the great vessels, with the pulmonary artery obscured by the aorta. The heart size varies from normal to enlarged.
Cross-sectional imaging is seldom used for preoperative evaluation of TGA, but it can be helpful for specific questions that are unanswered by echocardiography, typically complex associated abnormalities of the aorta or pulmonary arteries. MRI often is preferred instead of CT to avoid the risks of ionizing radiation. In some centers, CT may be preferred because it offers greater accessibility and decreases the need for anesthesia or sedation as a result of shorter scanning times. CT scans should be performed with parameters optimized to minimize exposure of the patient to ionizing radiation.
In newborn infants with {S,D,D} TGA, adequate communication between the pulmonary and systemic circulations is critical. Infusion of prostaglandin E 1 maintains ductal patency. If the atrial septum is restrictive, urgent cardiac catheterization for balloon atrial septostomy is often required. In this procedure, a catheter is passed across the atrial septum into the left atrium. The balloon is inflated, and the catheter is sharply pulled back, fracturing the septum and enlarging the opening, allowing for greater mixing of oxygenated and deoxygenated blood.
In the first decades of surgical repair of TGA, the atrial switch was used (i.e., “Mustard” or “Senning” procedures). In these procedures, an intraatrial baffle is created with use of pericardium or native atrial tissue, which directs systemic venous return to the morphologic LV and pulmonary artery, and pulmonary venous return to the morphologic RV and aorta ( Fig. 75.8A and B ).
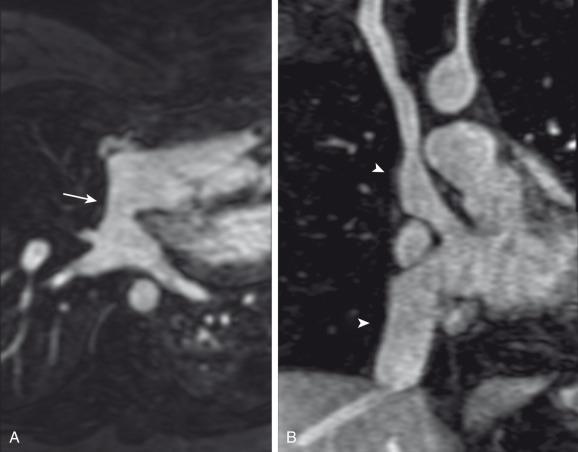
The atrial switch has been replaced by the arterial switch operation, which has been used widely in the United States since the mid to late 1980s. In this operation, the ascending aorta and pulmonary artery are transected at the sinotubular junction and anastomosed to the concordant ventricle, after the pulmonary artery is relocated anterior to the aorta (the “Lecompte maneuver”) ( Fig. 75.9A ). The coronary arteries are relocated from the native aorta to the neoaorta ( Fig. 75.9B ).
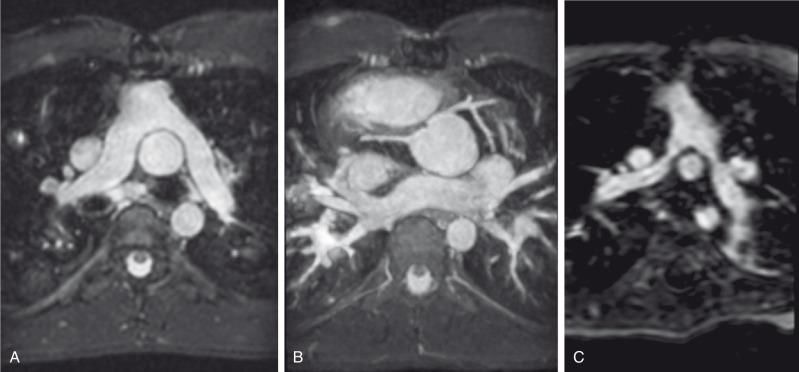
In the presence of a VSD and LVOT obstruction, a Rastelli repair can be performed, with baffling of the LV through the VSD to the native aortic valve, along with placement of an RV to pulmonary artery conduit ( e-Fig. 75.10 ) .
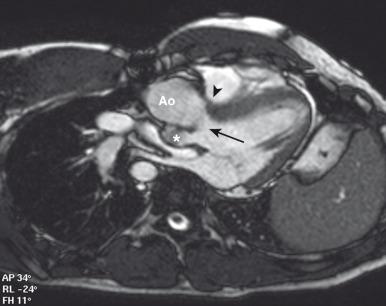
Each of these procedures can be associated with early and late complications. For the atrial switch procedure, important early and mid-term complications include systemic baffle obstruction, most commonly affecting the superior limb of the baffle at the junction of the right atrium with the superior vena cava, baffle leaks (in approximately 20% of patients) ( e-Fig. 75.11 ), and pulmonary venous obstruction (less common) ( e-Fig. 75.12 ). The most important complication after the atrial switch procedure, early or late, is failure of the systemic RV and tricuspid regurgitation. Less common complications include conduction and rhythm disturbances, which can require mechanical pacing or cause sudden death.
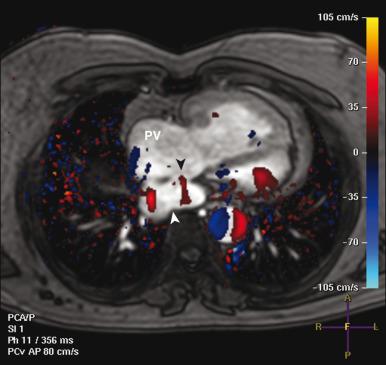
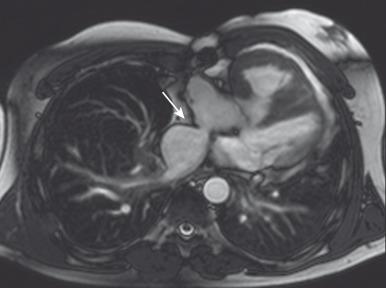
MRI is frequently used for assessment after the atrial switch procedure and is tailored for assessment of the aforementioned complications. Specifically, MRI is useful for the following assessments:
Evaluation of the size and function of the ventricles ( )
Evaluation of the systemic and pulmonary venous pathways ( e-Fig. 75.13 )
Assessment of tricuspid valve regurgitation
Evaluation of the LVOT and RVOT
Late gadolinium enhancement for evaluation of fibrosis in the RV
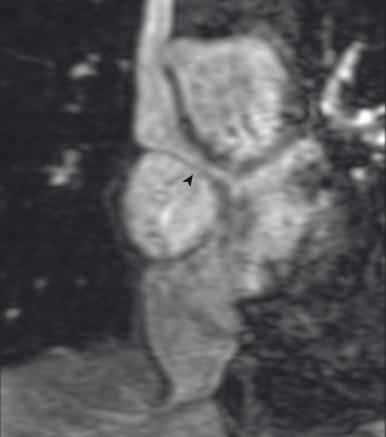
The arterial switch procedure uses the LV as the systemic ventricle and therefore is favored. Potential complications after the arterial switch procedure is performed can include RVOT obstruction, stenosis at the arterial anastomotic sites (most commonly pulmonary stenosis), branch pulmonary artery obstruction (see Fig. 75.9C ), aortic root dilatation, neoaortic valve regurgitation, and coronary artery ostial stenosis.
MRI is used frequently for assessment of patients who have undergone an arterial switch procedure, with clinical questions dictated by the aforementioned potential complications :
RV and LV size and function
Evaluation of the RVOT and LVOT
Evaluation of the great arteries
Evaluation of the semilunar valves
Evaluation of the coronary arteries
The Rastelli repair includes a conduit that can become stenotic and/or regurgitant. In addition, the baffle from the LV to the aorta can become obstructed, often at the level of the VSD, resulting in subaortic stenosis. After a Rastelli-type repair, answers to the following primary questions may be sought with MRI:
LV and RV size and systolic function
Anatomy and potential obstruction of the LV to aorta pathway
Stenosis and regurgitation of the RV to pulmonary artery conduit
MRI protocols are similar to those previously described for TOF, although additional sequences often are needed. For example, an axial bSSFP image stack can help define post-Lecompte pulmonary artery anatomy and the venous baffles of the atrial switch. Oblique bSSFP imaging along the LVOT is performed to rule out obstruction of the Rastelli pathway.
CT is used postoperatively in selected cases to answer anatomic questions, particularly when MRI is contraindicated. CT is particularly useful for assessment of extracardiac anatomy such as repaired aortic coarctation, arterial anastomotic sites, and branch pulmonary arteries after an arterial switch procedure is performed ( e-Fig. 75.14 ) and venous baffles after an atrial switch procedure is performed. Functional evaluation of the heart is limited compared with MRI.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here