Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenitally corrected transposition of the great arteries is a congenital cardiac anomaly with ventriculoarterial discordant connection (transposition of the great arteries) and atrioventricular (AV) discordant connection, the right atrium connecting to left ventricle and left atrium connecting to right ventricle. 1 Circulatory pathways are therefore in series. The condition occurs in atrial situs solitus and atrial situs inversus. Ventricles may lie in any position.
1 The adjectives left and right used to modify atrium or ventricle always mean morphologically right or left. Position of the chamber is referred to as right-sided or left-sided .
Rokitansky probably was first to describe a case of congenitally corrected transposition of the great arteries (CCTGA) in 1875. After that, pathologists recognized the condition easily but considered it rare. With advent of cardiac surgery, interest and knowledge expanded rapidly, and papers by Anderson and colleagues from the University of Minnesota in 1957 and by Schiebler and colleagues from the Mayo Clinic in 1961 established the clinical syndromes associated with it.
Monckenberg (1913) and later Uher (1936) described the anterior position of the AV node, its usual location in CCTGA. In 1931, Walmsley recognized fundamental differences in cardiac structure in such hearts, including a different coronary arterial pattern and altered morphology in the central fibrous body and conduction system. In 1963, Lev and colleagues again described the anomalous position of the AV node and His bundle. Clinicians, however, remained unaware of these observations until Anderson and colleagues confirmed the unusual position of the AV node and extended knowledge of the pathway of the bundle of His.
First repairs of a cardiac malformation associated with CCTGA were reported in 1957 by Anderson, Lillehei, and Lester from the University of Minnesota. This repair and others reported from the Mayo Clinic resulted in the morphologic right ventricle serving the systemic circulation. In 1990, Ilbawi and colleagues introduced the double switch concept in which the morphologic left ventricle serves the systemic circulation.
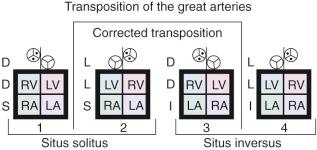
In atrial situs solitus, the most common arrangement of CCTGA is ventricular L-loop and L-malposition of the aorta (S,L,L; see “Symbolic Convention of Van Praagh” under Terminology and Classification of Heart Disease in Chapter 1 ) ( Fig. 55-1 ). The left ventricle (LV) usually lies to the right side and right ventricle (RV) to the left side. The mitral valve then lies to the right side and tricuspid valve to the left side. The LV is usually slightly posterior and inferior to the RV. Mirror-image relationships pertain when there is atrial situs inversus (I,D,D). In rare cases, unusual twisting of the heart along its long axis occurs, resulting in atypical topology (crisscross heart) . For example, in atrial situs solitus, there may be a discordant AV connection, but the ventricles are D-loop; similarly, discordant AV connection in atrial situs inversus will have L-loop ventricles.
Usually there is fibrous continuity in the right-sided LV between the right-sided mitral and pulmonary valves and a well-developed left-sided RV infundibulum separating left-sided tricuspid and aortic valves. However, rare cases have been described with bilateral conus or bilaterally deficient conus.
The LV outflow tract beneath the pulmonary valve lies between the septal (pulmonary) leaflet of the mitral valve on the right and muscular ventricular septum on the left ( Fig. 55-2 ). In its anterior part, there is often a prominent recess. Ventricular outflow tracts do not cross, and ascending aorta and pulmonary trunk are parallel.

In atrial situs solitus, the apex of the heart is usually to the left and is formed by the RV. Dextrocardia exists in about 25% of cases, and occasionally mesocardia. In atrial situs inversus, there is nearly always dextrocardia.
Other bizarre rotational anomalies occasionally occur in this and other hearts with AV discordant connections (see Morphology in Section II later in this chapter).
The pulmonary valve lies in a transverse plane and arises from the right-sided LV in a wedged position between the mitral and tricuspid valves. Wedging of the pulmonary valve is said to be more marked in corrected than in complete transposition (see Morphology in Chapter 52 ) and more marked than that of the aorta in the normal heart (see Chapter 1 ). The pulmonary valve lies to the right and posterior to the aortic valve. Axis of the AV valves is partway between transverse and sagittal planes as in the normal heart.
The long axis of pulmonary outflow from the right-sided LV is obliquely oriented and potentially restrictive, particularly when there is LV hypertrophy. Obstruction is organic in about half the hearts, and in at least 25% it is hemodynamically important.
Pulmonary valve cusps may be thickened and fused or occasionally bicuspid or unicuspid. When valve stenosis is present, the pulmonary trunk may be narrowed by valve tethering, as in tetralogy of Fallot (see “Pulmonary Valve” under Morphology in Section I of Chapter 38 ). There may be pulmonary atresia with or without confluence between right and left pulmonary arteries. There may be subvalvar narrowing due either to a membrane that is adherent on its right (laterally) with the right-sided anterior mitral leaflet (see Fig. 55-2 ) or to an aneurysmal bulging of the membranous septum into the posterior part of the outflow tract with or without a ventricular septal defect (VSD) ( Fig. 55-3 ). Less severe obstruction is usually due to fibrous tags (valvar excrescences) attached to the LV–pulmonary trunk junction, membranous septum, or right-sided mitral valve or due to valvar excrescences projecting through a VSD from the left-sided tricuspid valve leaflet. In about 1% of cases, pulmonary atresia is associated with arborization abnormalities of the branch pulmonary arteries and presence of major aortopulmonary collateral arteries.

Atrial and ventricular septa are malaligned except where pulmonary, mitral, and tricuspid valves lie in close proximity and are joined by the right fibrous trigone. Elsewhere, atrial septal attachment to the fibrous skeleton of the heart is moved to the right of ventricular septal attachment. These alignment differences are usually severe enough in hearts with atrial situs solitus to prevent the normally positioned (regular) AV node (known as posterior, inferior, or lateral node ) from reaching the underlying ventricular septum.
The right-sided mitral valve lies at the entrance to the right-sided LV. Because of the wedged position of the pulmonary valve, the mitral anulus extends anterior to the pulmonary anulus so that the pulmonary valve is tucked beneath (to the left of) the septal mitral valve leaflet (see Fig. 55-2 ). The mitral valve is rotated so that its usual septal leaflet, which is in fibrous continuity with the pulmonary valve and can therefore be called the pulmonary leaflet, is posterior and its mural leaflet anterior (see Fig. 55-3 ). The smaller papillary muscle arises from the anterolateral free wall of the ventricle, where it can be damaged by left ventriculotomy. Its position is frequently marked by direct coronary artery branches crossing the front of the LV from the anterior descending coronary artery. The larger papillary muscle arises from the posterolateral LV free wall. Mitral valve abnormalities are common, having been found in 55% of an autopsy series ( Fig. 55-4 ).

The aortic valve, usually normal, is over the RV infundibulum, and it and the aorta are usually in a leftward and anterior position (S,L,L). Occasionally the aorta lies to the right and anterior to the pulmonary artery (S,L,D), associated with infundibular rotation in this direction. In atrial situs inversus, the aorta is virtually always to the right (I,D,D).
Subaortic obstruction rarely occurs in the left-sided RV outflow tract.
The left-sided tricuspid valve lies at the entrance to the left-sided RV, which has usual coarse trabeculations, a trabecula septomarginalis (septal band), and an infundibular septum. The valve is positioned almost in a sagittal plane and has the usual three leaflets but with the septal leaflet more medial and anterior than normal. According to some, it is nearly always structurally abnormal (90% of cases according to Allwork et al. ). Others report fewer structural abnormalities, ranging from 23% to 43%. In most instances, there is leaflet dysplasia with abnormal thickened chordal attachments of the septal and posterior leaflets, and in a minority there is a true Ebstein anomaly with downward displacement of origins of septal and posterior leaflets. Ebstein anomaly often differs from that in a heart with normal connections in three respects :
The anterior leaflet is normal in size rather than large and sail-like.
The anulus is not dilated.
The RV sinus is not enlarged.
In about 30% of hearts, morphologic changes make the tricuspid valve regurgitant or, rarely, stenotic. There may be a thinned, dilated atrialized portion of the RV with a variable degree of hypoplasia.
The AV node and bundle of His in CCTGA (and in most, if not all, hearts with atrial situs solitus and AV discordant connection) differ from normal. Although a regular (posterior) AV node is present in front of the coronary sinus ostium in the apex of the triangle of Koch, the penetrating bundle of His usually does not extend from it because of septal malalignment. In exceptions in which the regular (posterior) AV node gives rise to the penetrating bundle, septal malalignment is mild. Degree of septal malalignment is influenced by size of the pulmonary trunk. Thus, presence of either pulmonary atresia or severe pulmonary stenosis results in less septal malalignment and an increased chance that the posterior AV node will align with the penetrating bundle.
In contrast, in atrial situs inversus, the penetrating bundle of His most commonly extends from the regular (posterior) node. The bundle of His arising from the regular AV node then lies adjacent to the posteroinferior margin of the VSD. An anterior AV node is generally also present but without a connection to a bundle of His. It has been suggested that in those cases of situs inversus in which pulmonary atresia or severe pulmonary stenosis has been present and the conduction system studied, there was only a minor degree of septal malalignment; therefore, this biased case selection may be the reason the penetrating bundle is said to “always” arise from the regular AV node in atrial situs inversus. Indeed, there are many exceptions to the rule that the AV node and penetrating bundle are abnormal in situs solitus CCTGA and normal in situs inversus CCTGA.
In situs solitus, the second anterior (superior) node is located adjacent to the right AV orifice beneath the ostium of the right atrial appendage at its junction with the anterior atrial wall where the anterior horn of the limbus of the atrial septum joins the AV anulus (see Fig. 55-4 ). It is from this node that the penetrating bundle of His most commonly arises. Immediately beneath the node is the right fibrous trigone through which the penetrating bundle passes to lie immediately inferior (caudad) to the pulmonary anulus in the anterior LV free wall (see Fig. 55-2 ). It then passes over the anulus and descends away from it onto the anterior part of the infundibular septum. The bundle descends for some distance before branching, lying between membranous and muscular portions of the septum. It is subendocardial in position and frequently visible as a pale ridge of tissue (see Fig. 55-2 ). A cordlike right branch penetrates across the crest of the muscular septum to reach the left-sided RV septal surface near the origin of the papillary muscle of the conus and passes downward on the surface of the septal band to reach the moderator band. The sheetlike left bundle branch continues downward on the LV septal surface from the branching bundle. Occasionally, penetrating bundles pass from both regular and anterior AV nodes to form a sling of conducting tissue surrounding the pulmonary valve orifice.
The encircling portion of the AV bundle is prone to fibrosis in older people, a feature that may explain spontaneous occurrence of complete heart block. Occasionally, when there is congenital complete heart block, anatomic discontinuity has been demonstrated between the node and either the bundle of His or a sling of conduction tissue in the ventricular septum. In cases with Wolff-Parkinson-White syndrome, accessory pathways are present (see Section III in Chapter 16 ).
In the absence of positional anomalies, most of the muscular sinus septum lies in a sagittal plane and is therefore profiled in the anteroposterior view (rather than left anterior oblique) on cineangiography. However, the left-sided RV cavity is circular in cross-section, and the lower-pressure LV wraps around it.
Septal malalignment and separation result in enlargement of the membranous septum and filling of the gap between atrial, ventricular, and infundibular septa. The degree of septal malalignment is thought to be influenced by size of the pulmonary trunk. The AV part of the membranous septum lies between left atrium and LV (rather than right atrium and LV as in the normal heart), and its interventricular portion lies beneath the posterior part of the pulmonary anulus. An aneurysm of this portion of the septum is common with or without a VSD (see Fig. 55-3 ) and can be a cause of LV outflow tract obstruction. When a VSD is present, the aneurysm lies along its superior margin.
VSD is the most common coexisting anomaly and is present in about 80% of hearts. Usually it is large, subpulmonary, and associated with virtual absence of the membranous septum (infundibulum). The pulmonary valve commonly overrides the VSD to arise in part from the left-sided RV. As viewed from the right (LV) side ( Fig. 55-5 ), the VSD is bounded superiorly by the pulmonary anulus or pulmonary valve itself, depending on degree of overriding. There may be membranous septal remnants along this margin (see Fig. 55-3 ). Posteriorly, it is bounded by that part of the right-sided mitral anulus from which the septal leaflet arises, and anteriorly and inferiorly by infundibular and muscular interventricular septa, respectively. Its posteroinferior margin may extend to the mitral anulus with a zone of mitral-pulmonary-tricuspid fibrous continuity. It is frequently narrowed or nearly closed by an aneurysm of the membranous septum (see Fig. 55-3 ) or valvar excrescences from the left-sided tricuspid valve (see Section IV in Chapter 38 ).

Viewed from the RV (left) side, this perimembranous VSD lies, as usual, within the Y of the trabecula septomarginalis and beneath the infundibular septum; the VSD, in other words, is infundibular in type and is often accompanied by some malalignment of the infundibular septum that contributes to subpulmonary stenosis. The bundle of His courses along its anterior margin on the LV (right) side in a subendocardial position and bifurcates at its anteroinferior angle with the right bundle branch crossing this angle of the defect to reach the RV.
In about 10% of cases (more often in Japanese patients), the VSD lies within the infundibular septum; when it completely replaces it, it is immediately below both great arteries (doubly committed, juxta-arterial) ( Fig. 55-6 ). Uncommonly, it is muscular, lying in the sinus (trabecular) septum. A large, typical inlet septal VSD may uncommonly occur. Also, there may be multiple VSDs.

Coronary arteries demonstrate anatomy appropriate to their ventricles. Thus, the right-sided left coronary artery (coronary artery to right-sided LV) with its left anterior descending and circumflex branches supplies the LV, and the right coronary artery and its conal and posterior descending branches supplies the RV. Aortic origins are, however, peculiar to the malformation. The anterior sinus is the noncoronary one; the right-sided left coronary artery arises from the right posterior sinus and passes directly in front of the pulmonary valve to divide into left anterior descending and circumflex branches, the latter passing in front of the right atrial appendage in the AV groove; the left-sided right coronary artery arises from the left posterior sinus and runs in the AV groove and in front of the left atrial appendage, terminating posteriorly as the posterior descending artery. Lev and Rowlatt used the terms “right sided” and “left sided” to describe these vessels. The most common major variation from this arrangement is for a single coronary artery to arise from the right sinus and divide into right and left main branches; this occurs in less than 10% of cases. Other minor variations occur.
Only 1% to 2% of hearts with CCTGA have no coexisting anomalies. Coexisting anomalies other than those described in the preceding text include a supravalvar left atrial ring, which may be a cause of left-sided (tricuspid) valve stenosis, and coarctation of the aorta in association with a VSD. Coarctation may be particularly common when severe forms of Ebstein anomaly are present. A patent ductus arteriosus is sometimes present, as is a true atrial septal defect in about 20% of cases.
Overriding or straddling of AV valves is more common when there are positional anomalies, as is hypoplasia of one or other ventricle. The left-sided tricuspid valve may override or straddle a VSD, which is at times associated with hypoplasia of the left-sided RV and at times with superior-inferior ventricles (see “ Positional Anomalies ” under Ventricular Position and Rotation in Section II of this chapter and “Cardiac and Arterial Positions” under Terminology and Classification of Heart Disease in Chapter 1 ). The left-sided tricuspid valve straddles the posterior part of the ventricular septum, which is then prevented from reaching the crux, and the conduction tissue passes anterior to the pulmonary anulus. More rarely, the right-sided mitral valve may behave similarly (as it does at times in AV discordant connection with double outlet right ventricle [see Section II ]); invariably this is associated with LV hypoplasia and superior-inferior ventricles. The mitral valve straddles the anterior part of the septum so that it does not extend to the crux, and a regular posterior node only may be present, with the bundle passing posterior to the pulmonary anulus.
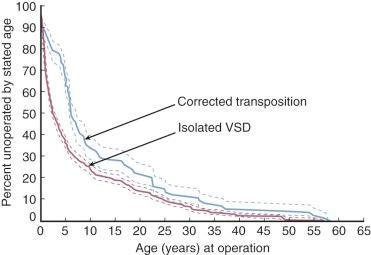
Clinical features depend solely on the presence and combination of associated cardiovascular lesions. The rare patient with no associated lesions will be asymptomatic for years or decades and may present with left-sided RV failure after several decades or more. More commonly, clinical features are dominated by a large VSD associated with some restriction of pulmonary blood flow attributable to morphology of the subpulmonary LV outflow tract. Therefore, symptoms from sequelae of large pulmonary blood flow occur in only about 30% of cases ( Fig. 55-7 ), in contrast to an isolated large VSD (see “Clinical Features and Diagnostic Criteria” in Section I of Chapter 35 ).
It is uncommon for pulmonary stenosis to be severe enough to require a shunting procedure in the first year of life. Friedberg and Nadas found only 30% of their patients presented in the first year of life with cyanosis, although cyanosis was present at some time during the course of the disease in two thirds of patients.
Most often, presentation is in childhood or in the second decade because of growth failure and exercise intolerance from a left-to-right shunt or, if there is important pulmonary stenosis, mild or moderate cyanosis or effort intolerance. Left-sided tricuspid valve regurgitation may complicate the other anomalies present or occur as an isolated finding. Regurgitation seems to worsen with time, and therefore patients with it occasionally present first in the third, fourth, or fifth decade of life; however, occasionally a neonate or infant will present in heart failure with severe left-sided tricuspid valve regurgitation.
The clinical feature bringing some patients to medical attention is bradycardia from congenitally complete heart block (present from birth or soon after), which occurs in 10% to 30% of cases. Complete heart block at times is episodic and induced temporarily by cardiac catheterization, anesthesia, exercise, or sternotomy. First- or second-degree heart block is found in an additional 20% to 30% of patients, many of whom earlier had normal AV conduction, and this degree of heart block may be a prelude to developing complete heart block (see Natural History later in this section). Wolff-Parkinson-White syndrome, either type A or B, coexists occasionally (see Section III in Chapter 16 ).
Physical findings are generally not diagnostic, but finding a loud second heart sound at the second left intercostal space is suggestive because it may represent closure of the leftward and anterior aortic valve.
Although chest radiography may suggest that a congenital cardiac malformation has AV discordant connection by an ascending aortic shadow appearing along the left upper cardiac silhouette, this is not diagnostic because there are many other anomalies with the aorta in L-malposition (see Clinical Features and Diagnostic Criteria in Chapter 57 ). Electrocardiography (ECG) may suggest a correct diagnosis when there is reversal of precordial Q-wave pattern with deep Q waves in leads V 2 and aVR, and QS complexes in leads V 3 and aVF in right precordial leads. Congenital or developing complete heart block is also suggestive of CCTGA.
Echocardiography provides accurate diagnosis of CCTGA. When spatial orientation of the ventricular septum is abnormal, the left-sided AV valve inserts more toward the apex than the right-sided one and has direct chordal attachments to the inlet septum. There is also continuity between the right-sided AV valve and the posterior (pulmonary) semilunar valve. Additional findings of transposed great arteries with aorta anterior and to the left, and a left-sided ventricle containing a coarsely trabeculated endocardial surface and moderator band, help confirm the diagnosis ( Fig. 55-8 ). Presence of VSDs, valve function, and venous connections can all be defined.

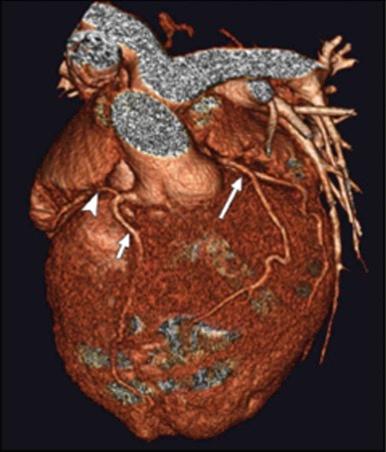
Computed tomography (CT) and magnetic resonance imaging (MRI) provide excellent delineation of the morphology of CCTGA ( Fig. 55-9 ), but in neonates and infants, these imaging modalities add little to echocardiography with respect to making the diagnosis and identifying associated cardiac anomalies. These additional studies can be extremely helpful, however, in providing additional information that may be important in complex management decisions that eventually must be made for many of these patients. MRI can be particularly helpful in quantitating ventricular volumes and valve regurgitant fraction ( Fig. 55-10 ). It can also be helpful in quantitating LV mass and determining the ventricular septal position in cases being evaluated for a double switch procedure ( Fig. 55-11 ). Volume-rendered CT imaging is capable of showing excellent spatial resolution of the coronary arteries and is particularly helpful in demonstrating the interrelationships between the coronary arteries and adjacent structures ( Fig. 55-12 ).



Cardiac catheterization and biplane cineangiography provide confirmatory diagnostic data ( Fig. 55-13 ). Pressure and flows are measured to quantify severity of pulmonary stenosis and any intracardiac shunt. Angiographic views must profile the ventricular septum and establish morphology of various chambers and sites of systemic and pulmonary venous connection and, thus, cardiac connections present. They can define location and number of VSDs, nature of the pulmonary stenosis and of tricuspid valve function, and other associated anomalies. Catheterization is rarely used in modern practice to define morphology, because echocardiography, CT, and MRI adequately provide these details in essentially all cases. Catheterization is indispensable if pulmonary vascular resistance, shunt fractions, or ventricular end-diastolic pressure is required for decision making.

About 5% to 10% of infants with CCTGA or other types of AV discordant connection (see Section II ) have complete heart block at birth. This proportion slowly increases at about 2% per year to reach a prevalence of about 10% to 15% by adolescence and 30% by adulthood. Block may be in the AV node or in single or multiple sites more distally. In some infants born with complete heart block, bundle of His potentials are not recordable, and morphologic evidence of a connection between an AV node and more distal parts of the His bundle cannot be found.
At least 40% to 50% of patients with AV discordant connection are born with first- or second-degree AV block. As time passes, prolongation of the PR interval often develops, even in those with originally normal intervals. Thus, Gillette and colleagues found normal AV conduction at age 6 to 7 years in only 38% (CL 29%-47%) of 40 patients with CCTGA. Progressive prolonging of the PR interval may eventuate in episodic or permanent complete heart block. However, about 40% of patients with AV discordant connection retain normal PR intervals and QRS durations throughout their lives.
General outlines of the truth about systemic (morphologic right) and pulmonary (morphologic left) ventricular function are gradually becoming apparent, although many of the details are missing (see Special Situations and Controversies later in this section). Ventricular function is not normal but is sufficiently good that a large proportion of patients maintain essentially normal functional status well into adult life. In 12 adults with CCTGA, many with associated anomalies, followed longitudinally for 10 years, ventricular ejection fraction did not change. However, systemic ventricular function (function of the RV) tends to gradually deteriorate during and after the second decade of life ; isolated reports of survival into the seventh, eighth, and ninth decades do exist.
In the unusual circumstance of CCTGA without other cardiac anomalies, an adequate cardiac index is usually sustained during exercise, but increase in heart rate accounts for this, and stroke volume is not increased. Response of the systemic (right) ventricular ejection fraction to exercise is variable, the ejection fraction increasing in some patients but not in others. Systemic (right) ventricular end-systolic and end-diastolic volumes also behave variably during exercise, but on the average do not change, whereas in normal individuals, systemic (left) ventricular end-systolic volume decreases with exercise.
Pulmonary (left) ventricular ejection fraction usually increases with exercise in patients with CCTGA without other cardiac anomalies. Other indices do not change systematically with exercise, as is also the case in normal individuals.
Etiology of ventricular dysfunction is poorly understood. Myocardial perfusion plays a role. Perfusion defects at rest are common in the morphologic RV in CCTGA, and their extent correlates inversely with ejection fraction.
Women of childbearing age seem to tolerate pregnancy and delivery moderately well, with some increased risk of maternal complications and fetal loss.
Because of the high prevalence of coexisting cardiac anomalies, survival free from cardiac intervention is less than 30% at 36 months after birth. Even without coexisting anomalies, the likelihood of developing heart block and reduced systemic (right) ventricular function probably adversely affects natural history; coexisting cardiac anomalies further affect it. A multicenter study involving 182 patients confirms these points: By age 45 years, 25% of patients without coexisting anomalies developed heart failure, and 67% of those with associated anomalies did. Other studies link heart failure in this setting to increased risk of death.
The natural history of CCTGA patients whose only coexisting lesion is a large VSD tends to be slightly better than that of patients with isolated large VSDs (see Fig. 55-7 ); this may be because their VSDs are smaller than in patients presenting with isolated VSDs. (This difference was not apparent in the report of Friedberg and Nadas, possibly related to their inclusion of patients with univentricular AV connection [single ventricle] in their study.) Chronic symptoms of effort intolerance and growth failure are common in patients with CCTGA in the first 2 decades of life, but death is infrequent. Although estimates of survival are unavailable, presumably death from chronic heart failure occurs with increasing frequency during the third, fourth, and fifth decades of life.
When important pulmonary stenosis coexists with VSD, cyanosis appears in early life, and the natural history may be similar to that of tetralogy of Fallot (see Natural History under Section I of Chapter 38 ). However, compared with tetralogy of Fallot, lack of a subpulmonic infundibulum in CCTGA may substantially alter the likelihood of a dynamic muscular component of pulmonary obstruction.
The natural history of the left AV valve in patients with CCTGA and other types of AV discordant connection is unclear. Occasionally the valve may be importantly regurgitant from early in life, but more commonly there is little or no regurgitation initially, and then its prevalence and magnitude increase progressively during the second through fifth decades. The exception is Ebstein anomaly of the left-sided tricuspid valve, when regurgitation is commonly present from birth.
In atrial situs inversus, VSD and pulmonary stenosis are more likely to be present than in atrial situs solitus. However, there is less likelihood of developing spontaneous complete heart block, because of the considerably higher prevalence of the penetrating bundle connecting to a normally positioned AV node.
Preparations for operation, median sternotomy, and placing pericardial stay sutures are as usual (see “Preparation for Cardiopulmonary Bypass” in Section III of Chapter 2 ). Placing purse-string sutures for aortic cannulation and cannulation itself are more difficult than usual because of aortic L-malposition. These procedures are facilitated by grasping aortic adventitia with one or two small curved hemostats and retracting them inferiorly and rightward. Usual purse-string sutures are placed for aortic cannulation and the cardioplegic catheter; caval tapes and purse-string sutures are also placed. In atrial situs solitus and dextrocardia, right atrium and venae cavae are hidden behind the ventricle, making cannulation difficult, although difficulty of direct caval cannulation is not increased.
Cardiopulmonary bypass (CPB) is established in the usual manner, as are cardioplegia and controlled reperfusion (see “Cold Cardioplegia, Controlled Aortic Root Reperfusion, and [When Needed] Warm Cardioplegic Induction” in Chapter 3 ). The right atrium is opened through an oblique incision ( Fig. 55-14 ). A pump-oxygenator sump sucker is introduced through the right superior pulmonary vein across the left-sided AV valve into the left-sided ventricular chamber.

The VSD is examined through the right-sided mitral valve ( Fig. 55-15, A ). Although it does not permit quite as free access to the interior of the ventricle as the normal right-sided tricuspid valve, in most cases the VSD can be repaired through the intact mitral valve. When exposure is suboptimal, an incision is made in the base of the mitral valve septal leaflet near the superior commissure and through the base of the commissural tissue into the mural leaflet ( Fig. 55-15, B-C ). The technique is similar to that used occasionally for inlet septal VSD. Working through the aperture created, VSD repair can be accomplished nicely ( Fig. 55-15, D-E ). However, the alternative of repairing the VSD through the aorta should be considered when approach through the right atrium is not optimal (see text that follows).

Margins of the VSD are studied (see Fig. 55-15, A and C ). Location of the anterior AV node and bundle of His arching over the subpulmonary outflow tract and passing anterior to the VSD are conceptualized (in fact, the bundle often can be seen as a thin, pale line as shown in Fig. 55-14 ). Electrophysiologic mapping is unnecessary. The left-sided tricuspid valve can usually be seen through the VSD, and some of its chordae often attach to the inferior VSD border.
VSD repair is made by sewing into place a properly sized patch of either glutaraldehyde-treated autologous pericardium or double-velour knitted polyester, keeping sutures on the left (RV) side of the defect anterosuperiorly, anteriorly, and as much as possible inferiorly (see Fig. 55-15, A and D ). Chordae from the left-sided tricuspid valve, often attached to the inferior edge of the VSD, limit this possibility inferiorly. Continuous polypropylene suture, ranging from 6-0 to 4-0 depending on the size of the patient, is used, or interrupted pledgeted mattress sutures when exposure is difficult because of overlying chordal structures. After VSD repair is complete, if a circumferential incision has been made in the mitral leaflets, this incision is closed with continuous polypropylene suture using previously placed fine stay sutures to keep the closure properly oriented so valve distortion is avoided ( Fig. 55-15, E ). If a patent foramen ovale or atrial septal defect is present, it is closed.
The subpulmonary (LV outflow) tract is examined. Unless the pulmonary valve itself is stenotic or valvar excrescences obstruct the subvalvar area, little can be done to improve the variable degree of narrowing usually present (see Results ). Only placing an LV–pulmonary trunk valved extracardiac conduit provides good relief. However, if pulmonary blood flow has been large (>2.0 preoperatively), even a 50-mmHg gradient does not necessarily indicate need for a conduit. With elimination of left-to-right shunt by closing the large VSD, right-sided LV pressure usually decreases appreciably.
The usual de-airing procedures are accomplished (see “De-airing the Heart” in Section III of Chapter 2 ). The aortic clamp is removed, and the right atrial incision is closed with a continuous polypropylene suture. The remainder of the procedure, including placing temporary atrial and ventricular pacing wires, is carried out in the usual manner (see “ Placing Epicardial Pacemaker Leads ” later in this section and “Completing Cardiopulmonary Bypass” in Section III of Chapter 2 ).
An attractive alternative approach is closing the VSD through the aorta, which allows the patch to be sutured into place from the RV (left-sided) aspect of the septum. Experience of Russo and colleagues suggests this may reduce the prevalence of perioperative complete heart block. This approach is more attractive than that through the pulmonary trunk, which some advocate.
When isolated dextrocardia complicates CCTGA and VSD, the VSD can be repaired through a left-sided incision in the usually large left-sided left atrium. Exposure through the left-sided tricuspid valve usually allows good exposure, and surgically induced heart block should be avoidable because suturing is all on the RV (left) side of the septum.
The main decision-making challenge is determining whether satisfactory repair can be accomplished without a valved extracardiac conduit. When the pulmonary valve is stenotic, it is approached through a pulmonary arteriotomy during moderately hypothermic CPB and cold cardioplegia, and valvotomy is performed as for isolated pulmonary valve stenosis (see Fig. 39-10 in Chapter 39 ). The pulmonary arteriotomy is best closed with a patch and continuous suture. Obstructing fibrous subvalvar tags are excised, bearing in mind the His bundle position (see Fig. 55-14 ). A subvalvar fibrous membrane can be excised with utmost care if it is at the anteroinferior angle. Aneurysm of the membranous ventricular septum is excised and the deficiency closed as part of VSD repair. If other excrescences are present, they are first examined to ensure they are not functioning parts of the AV valves or subvalvar mechanisms; then they are sharply resected.
Muscle must never be removed from the rightward (medial) aspect of the right-sided LV outflow tract or from the anterior part adjacent to the pulmonary anulus, because the His bundle lies there. Then Hegar dilators are used to measure the resulting orifice and the z value estimated (see “General Plan and Details of Repair Common to All Approaches” under Technique of Operation in Section I of Chapter 38 ). Valvotomy may be inadequate because of a bicuspid valve, supravalvar pulmonary trunk narrowing (tethering) at the level of commissural attachment, or (most commonly) a narrow subpulmonary LV outflow tract. However, if the z value is greater than −1, the pulmonary trunk is repaired, usual de-airing and other procedures carried out, and CPB discontinued.
Pressures are measured before removing the cannulae. Relationship between the postvalvotomy left-to-right ventricular pressure (P LV/RV ) in the operating room and that the next morning and late postoperatively is not known. However, it has seemed reasonable not to revert to CPB and place a valved extracardiac conduit if P LV/RV in the operating room is less than about 0.85, considering that the right-sided ventricle and valve are a morphologic LV and mitral valve. On the other hand, pulmonary stenosis usually represents a fixed resistance, and the LV-to–pulmonary trunk gradient will increase with exercise.
A polyvinyl catheter is placed in the right-sided LV and, if possible, threaded into the pulmonary trunk. LV pressure is remeasured the next morning in the intensive care unit with this catheter, and if calculated P LV/RV is less than about 0.7, the patient is not returned to the operating room for placing a valved extracardiac conduit.
When pulmonary stenosis is so severe that the patient is cyanotic preoperatively, or when simple procedures to relieve the stenosis are unsatisfactory (see earlier) or postrepair P LV/RV is too high, a valved extracardiac conduit is used. After VSD repair, working through the right atrium, a site is chosen for attaching the conduit to the right-sided LV by examining the LV interior through the mitral valve. A site is chosen on the anterior wall, but rather inferior and away from any papillary muscles and major coronary artery branches. Left ventriculotomy is then made. If there is reasonable flow across the native LV–pulmonary trunk outflow tract, it can be left intact, creating an end-to-side anastomosis of conduit to pulmonary trunk. This results in LV ejection via two routes: native tract and conduit. More commonly when a conduit is required, obstruction is severe; therefore the pulmonary trunk is transected at the valve level, the proximal stump oversewn, and the conduit connected end to end to the distal pulmonary trunk ( Fig. 55-16 ).

Reconstruction can be accomplished in a number of ways. An allograft-valved conduit has previously been prepared by extending it proximally with a woven polyester tube (see Fig. 55-16 ). Alternatively, proximal extension may be with an aortic allograft, or the allograft aortic valve and ascending aorta may be left long distally, and proximal anastomosis augmented with a pericardial hood (see “Placement of Valved Conduit” in Section II of Chapter 38 ). These extensions are particularly necessary in this situation because the conduit must be of sufficient length to prevent kinking, and the valve must lie away from the LV so that it is not distorted. Estimating length and lie of the conduit is important to avoid its compression by the sternum.
The conduit is trimmed to size, cutting the distal end square but leaving more of the ascending aorta beyond the aortic valve than in the case of tetralogy, because this facilitates a smooth conduit contour and limits length of the polyester extension. The proximal polyester end of the conduit is trimmed to make a cobra head, the distal allograft end anastomosed end to end to the distal pulmonary trunk, and the proximal polyester end anastomosed to the ventriculotomy. The conduit most commonly is placed to the right around the right atrium and atrial appendage (see Fig. 55-16 ), although extreme deviations in cardiac position may influence conduit position, occasionally making a left-sided placement appropriate. Aeba and colleagues describe placing the conduit from the apex of the LV to the pulmonary trunk to avoid the well-known problem of sternal compression.
Doty and colleagues proposed using a posteriorly placed transanular patch across the pulmonary valve anulus in this situation. However, average gradient across the repair was 40 mmHg.
When important left-sided tricuspid valve regurgitation coexists, repair and anuloplasty are only occasionally successful but should be attempted if it seems feasible. If replacement is required, the same considerations apply to the replacement device as in ordinary left-sided mitral valve replacement (see “Choice of Device for Valve Replacement” in Section I of Chapter 11 ). Valve replacement is the same as for a left-sided mitral valve, including choice of venous cannulae and approach through the right side of the left atrium (see “Mitral Valve Replacement” in Section I of Chapter 11 and Fig. 11-19 ). The replacement device is either sewn in with interrupted pledgeted mattress sutures or simple interrupted sutures. A continuous suture technique is not desirable when there is absence of a well-defined anulus in some areas, as may occur when there is downward displacement into the ventricle of some of the left-sided tricuspid valve leaflets, as in Ebstein anomaly.
Because of concern over long-term fate of the morphologic RV and tricuspid valve in the systemic circulation, some suggest placing the morphologic LV and mitral valve into the systemic circulation. This requires switching both venous return and arterial outflow (double switch) by one of several procedures, all of which are technically substantially more complex than those already described. The double switch concept was originally suggested by Ilbawi and colleagues for patients with CCTGA, VSD, and pulmonary stenosis. In this setting, the morphologic LV is connected to the aorta by creating an intraventricular baffle (which also closes the VSD); an extracardiac conduit is placed from morphologic RV to pulmonary trunk, and a Mustard or Senning intraatrial transposition of venous return is performed. The concept was subsequently applied to CCTGA without pulmonary stenosis, with or without VSD. In this setting, an arterial switch is performed to correct the ventriculoarterial discordant connection. (All of these reconstructive procedures used in combination in the double switch procedures are individually described in detail in Chapter 52 .) Aortic translocation has also been used for selected cases of CCTGA with VSD and pulmonary stenosis.
If these procedures are being considered for patients without VSD in whom the morphologic LV is working at low pressure, the same considerations must be addressed as in simple transposition of the great arteries (TGA) with unprepared LV (see “Simple Transposition of the Great Arteries Presenting after Age 30 Days” under Indications for Operation in Chapter 52 ).
Both major double switch procedures are performed using a standard median sternotomy incision, CPB with moderate hypothermia using bicaval venous cannulation through purse-string sutures placed directly on the venae cavae, aortic cannulation at the base of the brachiocephalic artery, and venting of the systemic ventricle by way of a cannula introduced through the right upper pulmonary vein. Multiple doses of cold cardioplegic solution are used for these extensive procedures (see “Methods of Myocardial Management” under Neonates and Infants in Chapter 3 ). Individual components of the two major double switch procedures are as follows:
The atrial baffle procedure, which is performed exactly as for simple TGA (see “Mustard Technique” and “Senning Technique” under Technique of Operation in Chapter 52 ). It is not uncommon in CCTGA of both the S,L,L and the I,D,D types for cardiac positioning abnormalities such as mesocardia or apicocaval juxtaposition to be present. In these cases, the free wall of the systemic venous atrium is likely to be deficient, making the Mustard technique preferable to the Senning. Atrial baffle placement may be more difficult than in simple TGA, because the left-sided tricuspid valve is positioned much more posteriorly across the atrial septum in relation to the vena cavae.
For patients with VSD and pulmonary stenosis, the morphologic LV–to-aortic intracardiac baffle procedure, which is accomplished through a subaortic incision in the infundibulum of the morphologic RV as described under “Intraventricular Repair” in Chapter 52 , for S,D,D transposition with VSD and pulmonary stenosis or atresia. The morphologic RV-to–pulmonary trunk conduit is placed as described under “Rastelli Operation” in Chapter 52 . This procedure is shown in Fig. 55-17, A to E .

For patients without pulmonary stenosis, the arterial switch procedure is performed using the same techniques described under “Arterial Switch Operation” in Chapter 52 for simple TGA. If a VSD is present, it is closed as described earlier under “Repair of Coexisting Ventricular Septal Defect.” This procedure is shown in Fig. 55-18, A to D .

Bidirectional superior cavopulmonary anastomosis may provide substantial advantages in the setting of both double switch procedures. It reduces complexity of the intraatrial procedure, because only the inferior vena cava is baffled to the tricuspid valve. This reduces myocardial ischemia time because (1) the cavopulmonary anastomosis can be performed during rewarming after the aortic clamp is removed and myocardial reperfusion is established, and (2) the simplified inferior vena cava baffling can be accomplished much more quickly than a full Mustard or Senning procedure. An additional advantage is that recognized complications of the full Mustard or Senning procedure (e.g., superior caval obstruction, pulmonary venous obstruction, sinus node dysfunction) are eliminated.
Other advantages exist specifically in CCTGA with VSD and pulmonary stenosis. If an extracardiac conduit is necessary owing to severe pulmonary stenosis, its longevity will be extended because of reduced volume of flow it carries. This may be particularly pertinent in a small growing child. Additionally, if the morphologic RV size is reduced, either because of intrinsic reasons or as a result of a large morphologic LV–aortic baffle occupying part of its cavity, the bidirectional superior cavopulmonary anastomosis may provide superior hemodynamics. Finally, in patients with positional abnormalities such as situs solitus with mesocardia or dextrocardia, or with situs inversus, simplicity of the “hemi-Mustard” procedure makes it preferable to the full Mustard or Senning procedure.
For these reasons, bidirectional superior cavopulmonary anastomosis with hemi-Mustard procedure has become the preferred technique for performing the atrial component of the operation in both forms of the double switch for at least one of the authors. The procedure is performed as described in Chapter 41 under “Bidirectional Superior Cavopulmonary Shunt.” The double switch procedure, inferior vena cava–to–tricuspid valve atrial baffle (hemi-Mustard), and bidirectional superior cavopulmonary anastomosis are shown in Fig. 55-19 .

When complete heart block has been present intermittently or permanently preoperatively, or when it has developed intraoperatively, permanent epicardial atrial and ventricular pacemaking leads are placed, and a permanent pacemaker pulse generator is placed subcutaneously (see “Technique of Intervention” in Section I of Chapter 16 ).
Patients are managed with protocols generally used after cardiac surgery (see Chapter 5 ). In patients with AV discordant connection such as these, particular attention is paid to the cardiac rhythm. When complete heart block is present, AV sequential pacing augments cardiac output and is therefore used routinely.
When operation is performed for CCTGA and VSD, hospital mortality has been 5% to 10%. When performed for CCTGA with coexisting VSD and important pulmonary stenosis, it has been 10% to 20%. When performed for coexisting left-sided tricuspid valvar regurgitation requiring valve replacement, it has been 15% to 25%. Reducing hospital mortality is surely possible and has been documented in studies showing a reduction from 21% (17/82; CL 16%-26%) for operations performed prior to 1987 to 3.4% (1/29; CL 0.6%-11%) for those performed between 1987 and 1996.
The 1-month and 1-, 5-, 10-, and 20-year survivals after repair of important coexisting cardiac anomalies in heterogeneous groups of patients with CCTGA repaired over the past 35 years have been about 88%, 80%, 76%, and 46%, respectively, including hospital deaths. In more recent experience, close to 90% 10-year survival has been demonstrated in a risk-unadjusted population of patients undergoing surgery for CCTGA. When a late-rising phase of hazard will become evident in patients operated on in the current era is not yet known.
Hrasksa and colleagues suggest that time-related survival is best when a Fontan procedure is performed (100% at 5 years), with lower survival in septated patients undergoing VSD closure (75% at 5 years) and even lower survival in septated patients undergoing tricuspid valve surgery (55% at 5 years). It should be emphasized that their patients underwent surgery between 1963 and 1996. Hörer and colleagues found similar long-term outcomes in patients undergoing septation and those undergoing a Fontan procedure.
Early outcomes appear to be as good or better with more complicated double switch procedures (“anatomic repair”) that assign the morphologic LV to the systemic circulation than with the “physiologic” procedures just described. Jahangiri and colleagues reported no mortality in the anatomic repair group (0 of 19 patients; 0%; CL 0%-10%), and 7% mortality in the physiologic repair group (5 of 70 patients; CL 4%-12%), P = .3. Yeh and colleagues reported equivalent outcomes.
In a group of patients with structurally abnormal tricuspid valves, mortality was 11% (1 of 9 patients; CL 1%-33%) following anatomic repair and 33% (5 of 15 patients; CL 19%-50%) following physiologic repair ( P for difference = .2). Results reported from 10 single institutional experiences between 1993 and 2002 reveal that early mortality for procedures placing the morphologic LV in the systemic circulation compares favorably with that of simpler, more classic repairs. In these 10 studies, early mortality ranged from 0% to 14%. Each experience was small, with a combined total of 150 patients with 11 early deaths (7.3%; CL 5.1%-10%).
Reports between 2002 and 2010 have larger numbers of patients and confirm that early mortality can be low. In three recent large single-institution studies, early mortality was 0% (46 patients; CL 0%-4.0%), 2.1% (1 of 48 patients; CL 0.3%-6.8%), and 6.8% (3 of 44 patients; CL 3.0%-13%). These studies included both the arterial switch type and the Rastelli type of double switch. In another study focusing on 20 patients with the arterial switch type of double switch only, there was no early mortality. Other large series report early mortality of about 15%. Most studies show similar early mortality of the two major double switch operations, but Gaies et al. found a difference, with 94% early survival (33 of 35 patients; CL 87%-98%) in the arterial switch type and 77% survival (23 of 30 patients; CL 66%-85%) in the Rastelli type.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here