Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital heart disease (CHDs) are seen in 6 to 10 per 1000 live births. These include structural defects of the heart, of the great vessels, or both.
During the newborn period, the most important presenting features of CHD are central cyanosis, decreased perfusion to the body, and tachypnea. Cardiac murmurs are frequently heard but have a low sensitivity (44%) and low positive predictive value (54%).
The most useful screening methods to detect CHD include prenatal ultrasound, routine newborn physical examination, and pulse oximetry screening of newborns.
Three types of CHD can present soon after birth, including d-transposition of great arteries with intact ventricular septum and a restrictive foramen ovale, hypoplastic left heart syndrome with an intact atrial septum, and total anomalous venous connections with obstruction.
Congenital heart disease (CHD) is the most common birth defect. Recent incidence estimates for CHD range from 6 to 10 per 1000 live births. One out of every four neonates with CHD has critical CHD , that is, a defect that requires either a surgical or transcatheter procedure within the first year of life. These lesions can be broadly viewed as structural defects of the heart, of the great vessels, or of both. Another way to classify these lesions could be based on the presence or absence of ventricular inflow/outflow abnormalities ( Table 36.1 ). Defects range from relatively simple lesions, which neither cause clinical symptoms nor require therapy, to complex life-threatening lesions, which require emergent intervention in the neonatal period. ,
| Defect | Description |
|---|---|
| With Ventricular Inflow or Outflow Abnormality | |
| Tricuspid valve stenosis or atresia | Stenosis or atresia of tricuspid valve |
| Ebstein anomaly | Inferior displacement of tricuspid valve |
| Pulmonary atresia with intact ventricular septum | Atresia of pulmonary valve |
| Pulmonary stenosis | Subvalvar, valvar, or supravalvar obstruction to pulmonary blood flow |
| Tetralogy of Fallot with pulmonary stenosis or atresia | Anterior malalignment of conal septum leading to variable degree of obstruction to pulmonary blood flow, overriding aorta, VSD, and right ventricular hypertrophy |
| Without Right Ventricular Inflow or Outflow Abnormality | |
| Transposition of great arteries | Aorta arises from right ventricle, and pulmonary artery arises from left ventricle (ventriculoarterial discordance) |
| Truncus arteriosus | Single arterial trunk arises from ventricles, with variable origins of pulmonary arteries from trunk |
| Totally anomalous pulmonary venous connection with obstruction | Abnormal connection of all pulmonary veins to systemic venous system |
Current screening methods to detect CHD include prenatal ultrasound, routine newborn physical examination, and pulse oximetry screening of newborns. Although prenatal diagnosis of CHD is increasing, a significant proportion of babies are not diagnosed as having CHD before birth. , Postnatal diagnosis of CHD in the delivery room or newborn nursery is possible only if symptoms and signs of CHD manifest during the hospital stay or if there is a universal screening protocol using pulse oximetry. , Left-sided obstructive lesions such as coarctation of aorta are more likely to be diagnosed in babies after discharge from the nursery, , whereas those who have cyanotic CHD are more likely to be identified while still in the nursery. Many neonates with serious CHD do not exhibit clinical manifestations of the underlying heart disease before discharge from the nursery.
Although a cardiac murmur is often considered the most common presenting sign of CHD, murmurs have a low sensitivity (44%) and low positive predictive value (54%) in the newborn period. Rather, the three major presenting features of CHD in the newborn period are central cyanosis , decreased perfusion to the body , and tachypnea . The predominant clinical manifestation depends on the type of CHD. Timing and severity of clinical presentation of CHD can vary depending on the nature of the defect.
When cyanosis is restricted to the periphery, that is, in the nail beds or extremities, it is called peripheral cyanosis, a common and often innocuous condition in newborns. Peripheral cyanosis should be differentiated from central cyanosis, a more ominous sign. Unlike peripheral cyanosis, central cyanosis is indicative of hypoxemia .
In patients with CHD, shunting of systemic venous blood into the arterial circuit causes arterial hypoxemia and central cyanosis. Examples of lesions likely to present with central cyanosis include those that involve restriction of blood flow into the lungs, such as pulmonary stenosis, pulmonary atresia, and tetralogy of Fallot (TOF) with pulmonary stenosis ( Fig. 36.1 ). Typically, these defects are diagnosed when constriction of the ductus arteriosus causes further decrease in pulmonary blood flow. Congenital heart defects without restriction to pulmonary blood flow but characterized by the presence of desaturated blood in the aorta may also present with central cyanosis. These include dextro-transposition of the great arteries (d-TGA) ( Fig. 36.2 ) and truncus arteriosus ( Fig. 36.3 ). Table 36.1 lists congenital heart defects, which commonly present with central cyanosis.
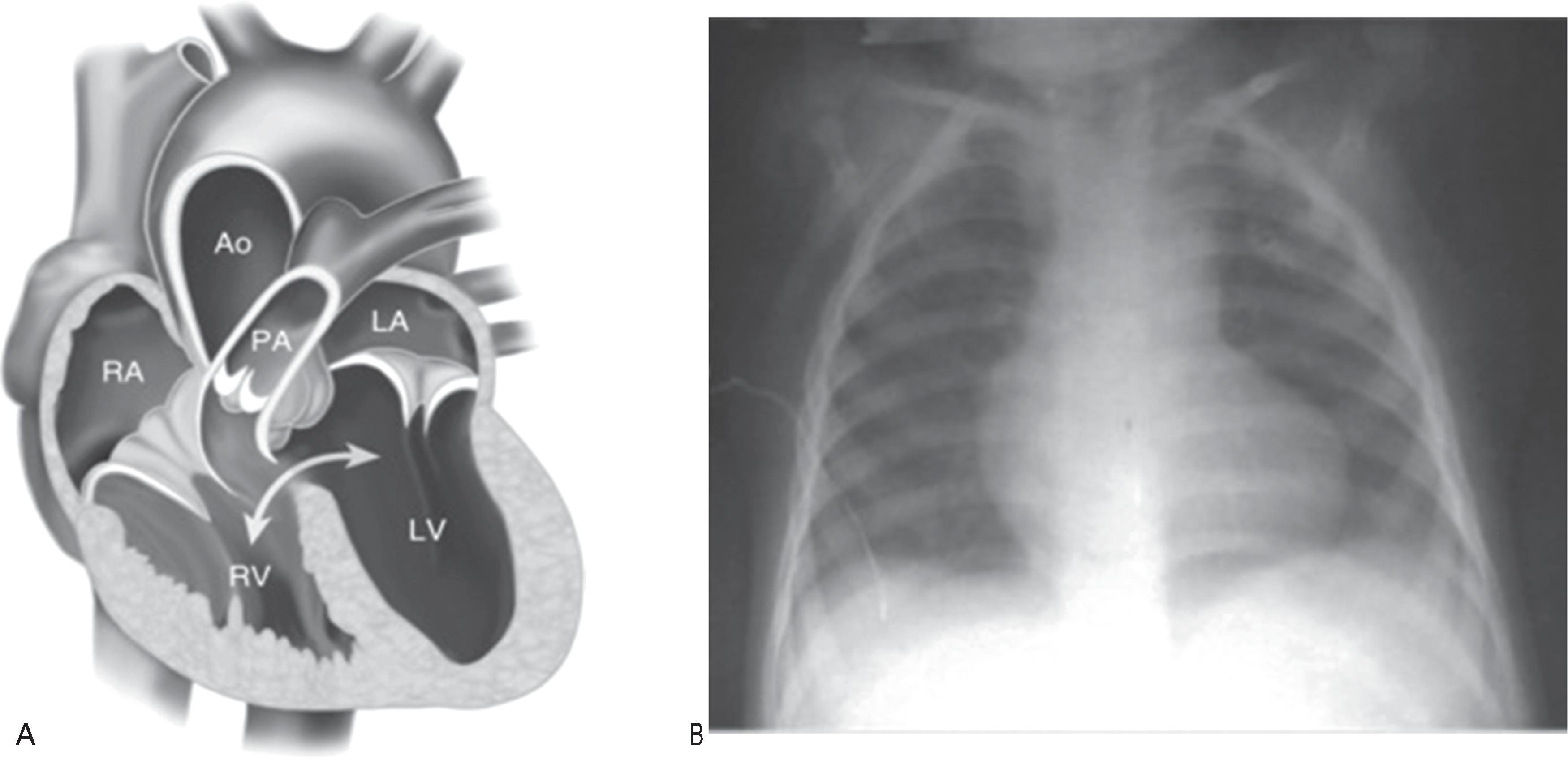
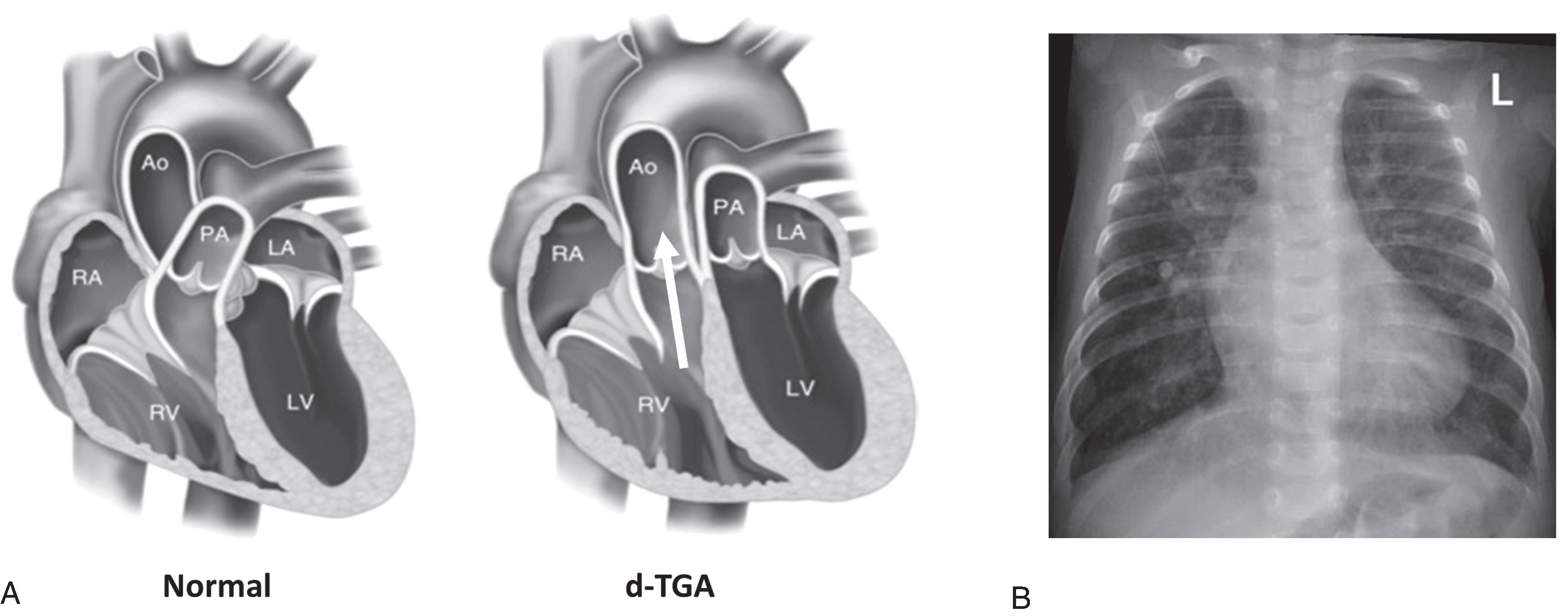
Cyanosis can also be seen in other types of CHD that may cause hypoxemia and systemic hemoglobin oxygen desaturation. In the differential diagnosis, diseases involving lung parenchyma or the pleural space, such as a pleural effusion or pneumothorax, may affect gas exchange and oxygenation. In patients with these conditions, other clinical features suggestive of respiratory disease, such as nasal flaring, grunting, dyspnea, and hypercarbia, often accompany cyanosis. Neonates born with congenital neurologic, muscular, or neuromuscular conditions may present with cyanosis and hypercarbia caused by hypopnea. In neonates with cyanotic CHD, cyanosis is often the sole clinical feature. The absence of respiratory distress and hypercarbia in a cyanotic newborn should raise a strong suspicion of CHD.
On several occasions, infants with cyanotic heart disease may not appear cyanotic. A critical amount of deoxyhemoglobin must be present in the capillary microcirculation for cyanosis to be apparent. Detection of cyanosis may be difficult if deoxy-hemoglobin is less than 3 to 5 g/dL or in babies with darkly pigmented skin. For this reason, serious cyanotic CHD often goes unrecognized unless measurement of oxygen saturation by pulse oximetry is performed.
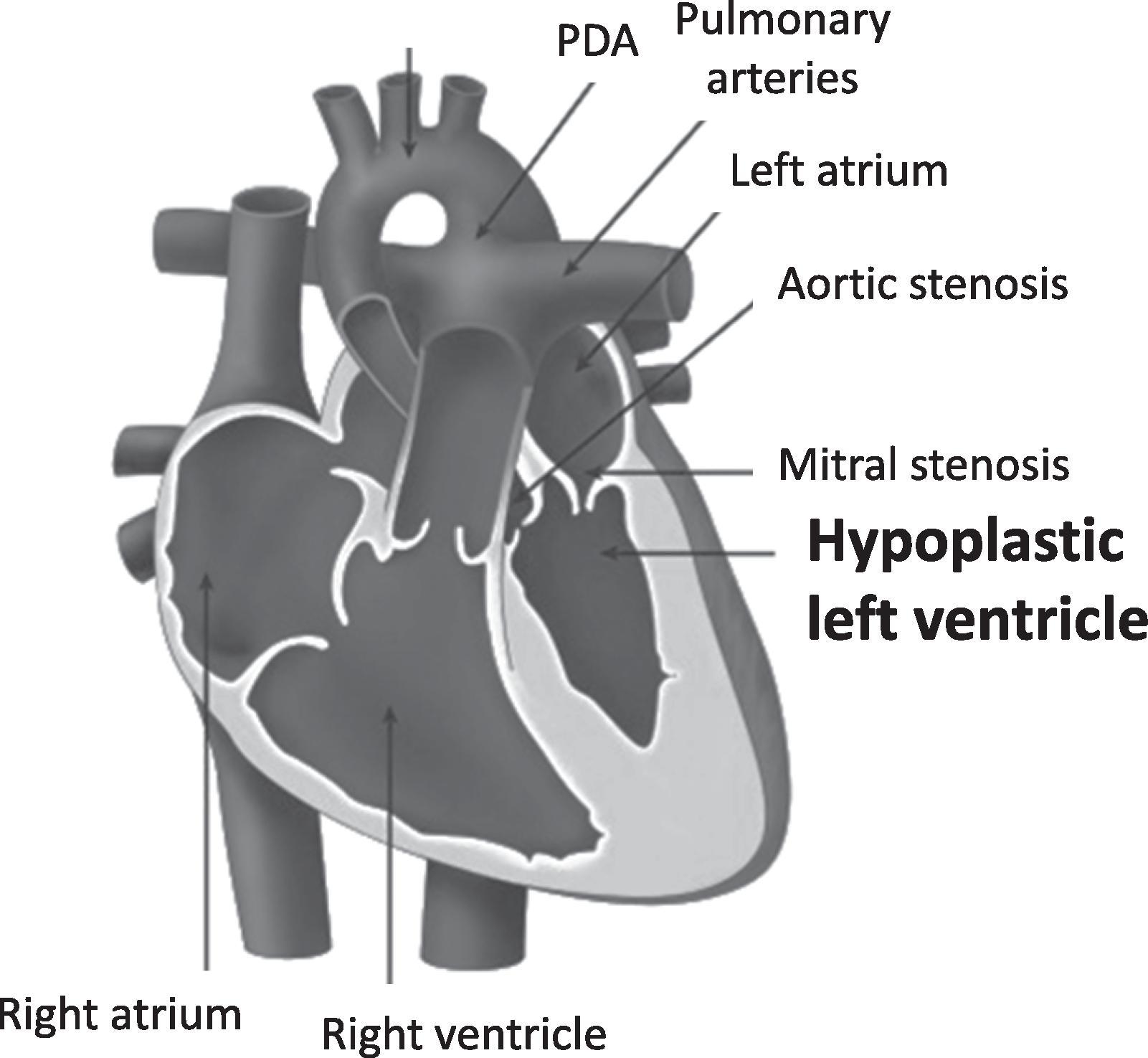
Lesions that involve obstruction to blood flow from the left side of the heart present with decreased systemic perfusion ( Table 36.2 ). , These defects range in severity from obstruction limited to the aortic isthmus, such as coarctation of the aorta ( Fig. 36.4 ), to those causing severe hypoplasia of the left-sided structures of the heart, such as hypoplastic left heart syndrome (HLHS; Fig. 36.5 ). , Despite the obstruction, systemic blood flow is maintained before birth through a patent ductus arteriosus (PDA). In infants with critical left-sided obstructive lesions, signs of compromised systemic perfusion become evident with ductal constriction in the first few days after birth. Pulses become diminished, perfusion worsens, and capillary refill time increases. Metabolic acidosis ensues, and rapid progression into circulatory failure leads to severe end organ ischemia and death. It is not unusual for babies with left-sided obstructive lesions to manifest these symptoms after discharge from the nursery. The initial symptoms caused by left-sided heart obstruction are subtle but often progress rapidly. These patients may initially present to the pediatrician’s office with mild tachypnea, difficulty or frequent interruptions in feeding, diaphoresis, and poor weight gain. Critical left-sided heart obstruction must be suspected when the infant presents in extremis to the emergency department. ,
| Defect | Description |
|---|---|
| Aortic valve stenosis or atresia | Stenosis or atresia of aortic valve |
| Hypoplasia of aortic arch | Varying degrees of underdevelopment of ascending aorta, transverse arch, or both |
| Interruption of aortic arch | Complete separation or interruption of aortic arch; blood supply distal to interruption is via ductus arteriosus |
| Coarctation of aorta | Narrowing of aorta, usually at isthmus in juxta ductal region |
| Hypoplastic left heart syndrome | Underdevelopment of entire left-sided structures of heart |
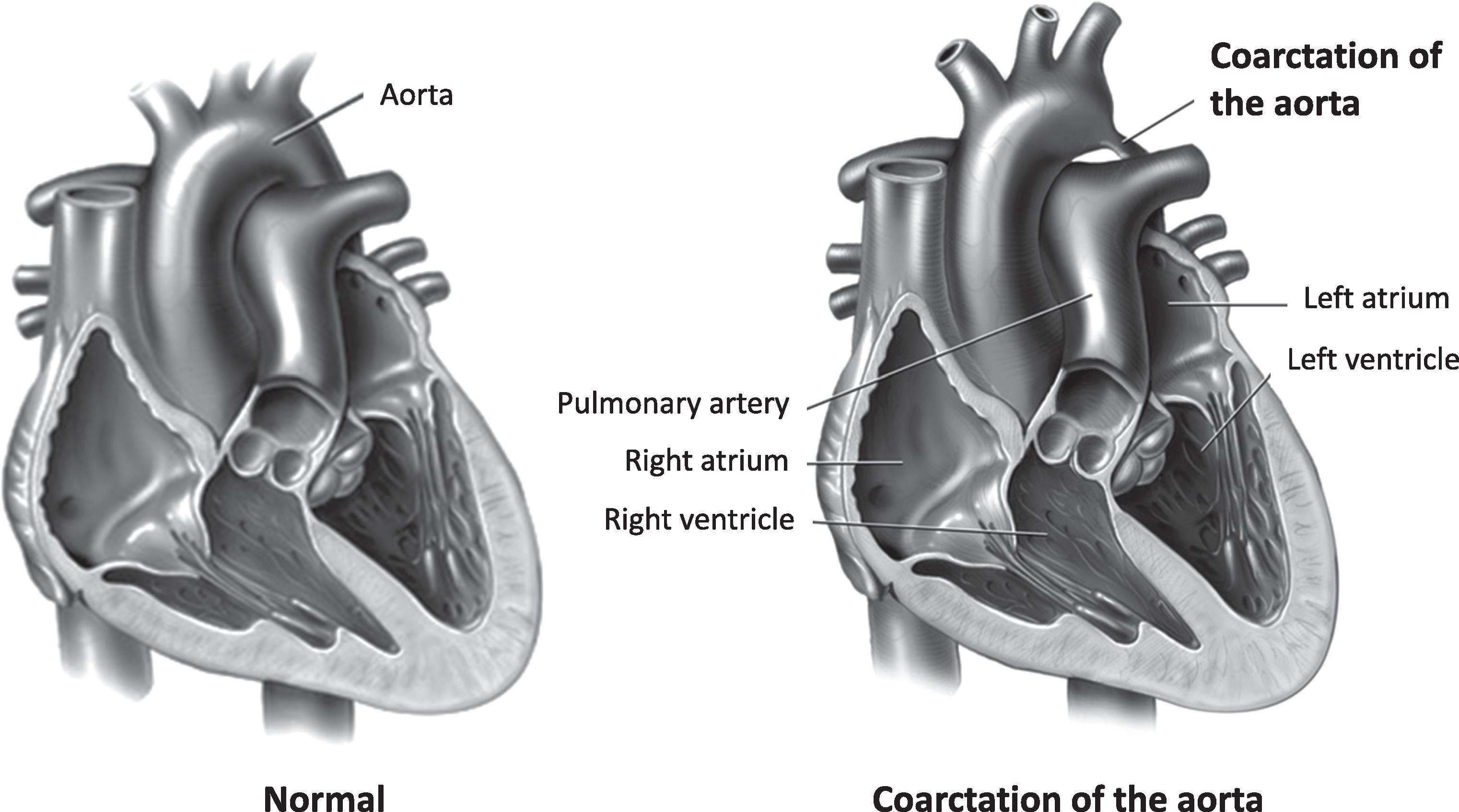
Infants with heart lesions characterized by excessive pulmonary blood flow present with increased work of breathing. Lesions can range from defects of the atrial or ventricular septum, or both, to abnormal communications at the venous or great artery level ( Table 36.3 ).
| Defect | Description |
|---|---|
| Ventricular septal defect | Single or multiple defects in ventricular septum |
| Atrial septal defect | Single or multiple defects in atrial septum |
| Common atrioventricular canal | Defects of atrial and ventricular septa, along with a common atrioventricular valve |
| Patent ductus arteriosus | Pathologic patency of ductus arteriosus |
| Aortopulmonary window | Defect between aorta and main pulmonary artery |
| Truncus arteriosus | Single arterial trunk arises from ventricles, with variable origins of pulmonary arteries from trunk |
| Transposition of great arteries with ventricular septal defect | Aorta arises from right ventricle, and pulmonary artery arises from left ventricle (ventriculoarterial discordance); one or many defects in ventricular septum may also be present |
| Unobstructed total or partial anomalous pulmonary venous connection | Abnormal connection of some or all pulmonary veins to systemic venous system |
Abnormal communication between the pulmonary and systemic circuit allows left-to-right shunting of blood and an increase in pulmonary blood flow. Because the pulmonary vascular resistance is still elevated, symptoms of the underlying heart defect are usually not present at birth. , As the resistance in the pulmonary vascular circuit declines, left-to-right shunting of blood increases and symptoms emerge. Clinical manifestations usually appear at 4 to 6 weeks of life, when the low pulmonary vascular resistance and physiologic anemia of infancy contribute to increased left-to-right shunt. Earlier presentation is likely in preterm infants or those with multiple defects or if communication is at the arterial level (e.g., truncus arteriosus or aortopulmonary window). Newborns with isolated atrial septal defects (ASDs) do not usually exhibit symptoms in the neonatal period or even in infancy; diagnosis of an ASD is often missed unless an echocardiogram is obtained for evaluation of a murmur.
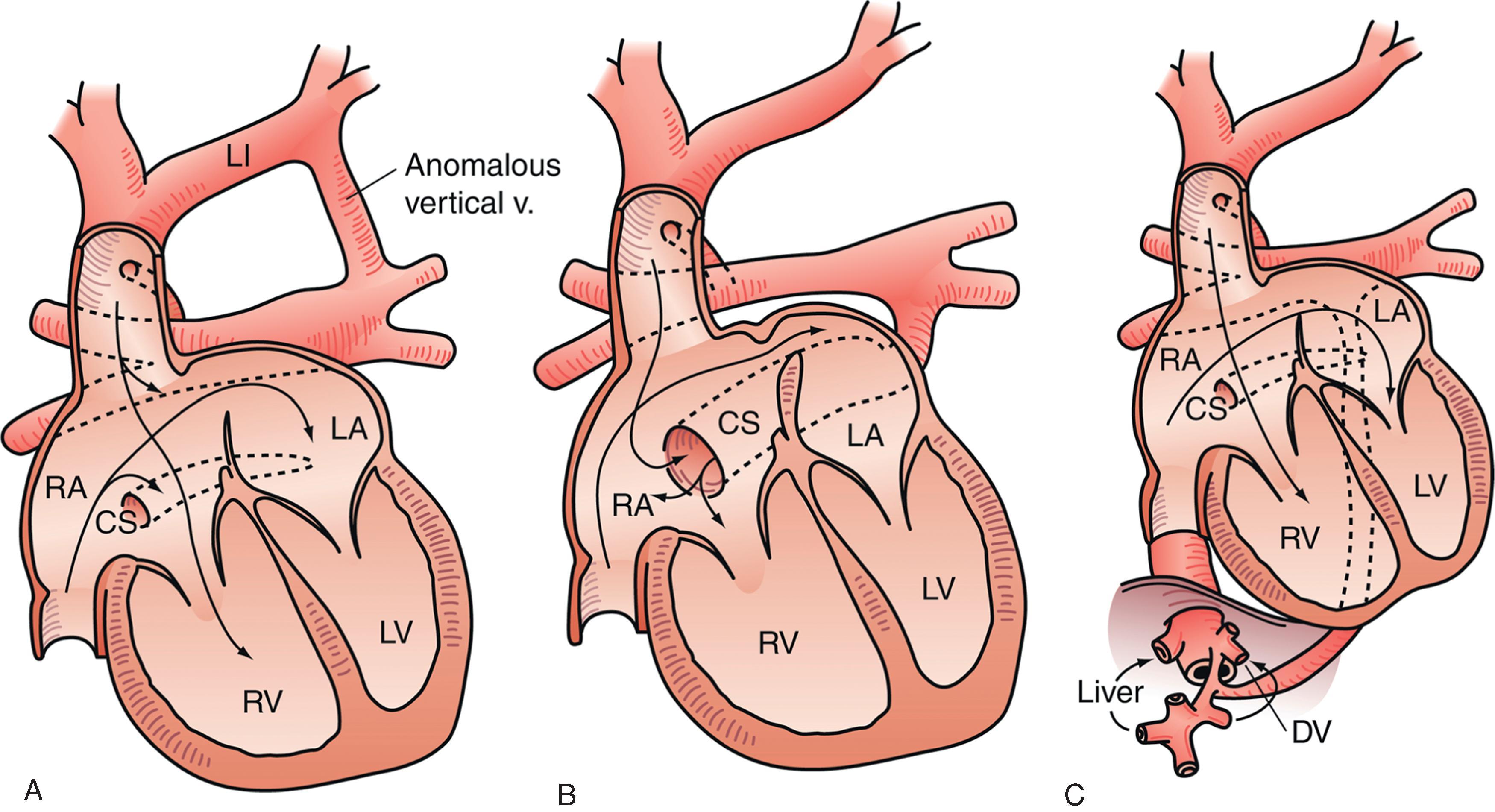
Increased blood flow into the pulmonary vascular bed and interstitial edema decrease lung compliance and cause an increase in work of breathing. Babies with large left-to-right shunts exhibit tachypnea, easy fatigability during feeding, and diaphoresis. , Decreased oral intake and increased respiratory effort lead to poor weight gain and failure to thrive. Cyanosis is usually absent, unless there is a concomitant mixture of desaturated blood escaping into the arterial circuit, such as with unobstructed total anomalous pulmonary venous connection (TAPVC; Fig. 36.6 ) or truncus arteriosus. , In patients with these lesions, oxygen saturation levels measured by pulse oximetry are lower than usual. Clinical manifestations of large left-to-right shunts are usually not apparent at birth or prior to discharge from the nursery. These symptoms are often first reported to the pediatrician during routine office visits in the first 2 months of life.
Respiratory symptoms caused by a left-to-right shunt should be differentiated from those caused by primary lung disease. Underlying CHD should be suspected when symptoms described above are present, if there is an active precordium or a murmur on examination, or if cardiomegaly with prominent pulmonary vascular markings is noted on a chest radiograph.
The age when symptoms or signs of CHD appear varies. It can range from immediate and life-threatening presentation at birth to subtle symptoms of heart failure several weeks after birth.
Three types of CHD can present soon after birth. These lesions include d-TGA with intact ventricular septum and a restrictive foramen ovale , HLHS (with mitral atresia) with intact or restrictive atrial septum , and total anomalous pulmonary venous connection (TAPVC) with obstruction . Babies with d-TGA, intact ventricular septum, and a restrictive foramen ovale are profoundly hypoxemic and cyanotic in the first minutes to hours after birth. Babies with HLHS and intact or restrictive foramen ovale have severe cyanosis, respiratory distress, and signs of low cardiac output that are evident within minutes after birth. Survival depends on urgent creation of an atrial-level communication by interventional cardiologists. This procedure can be performed only in cardiac centers; hence, early diagnosis and transfer are critical to survival. Babies with obstructed TAPVC present with profound cyanosis and respiratory distress within the first day of life. Temporary medical stabilization is possible, but very difficult, and urgent surgical repair is necessary for survival.
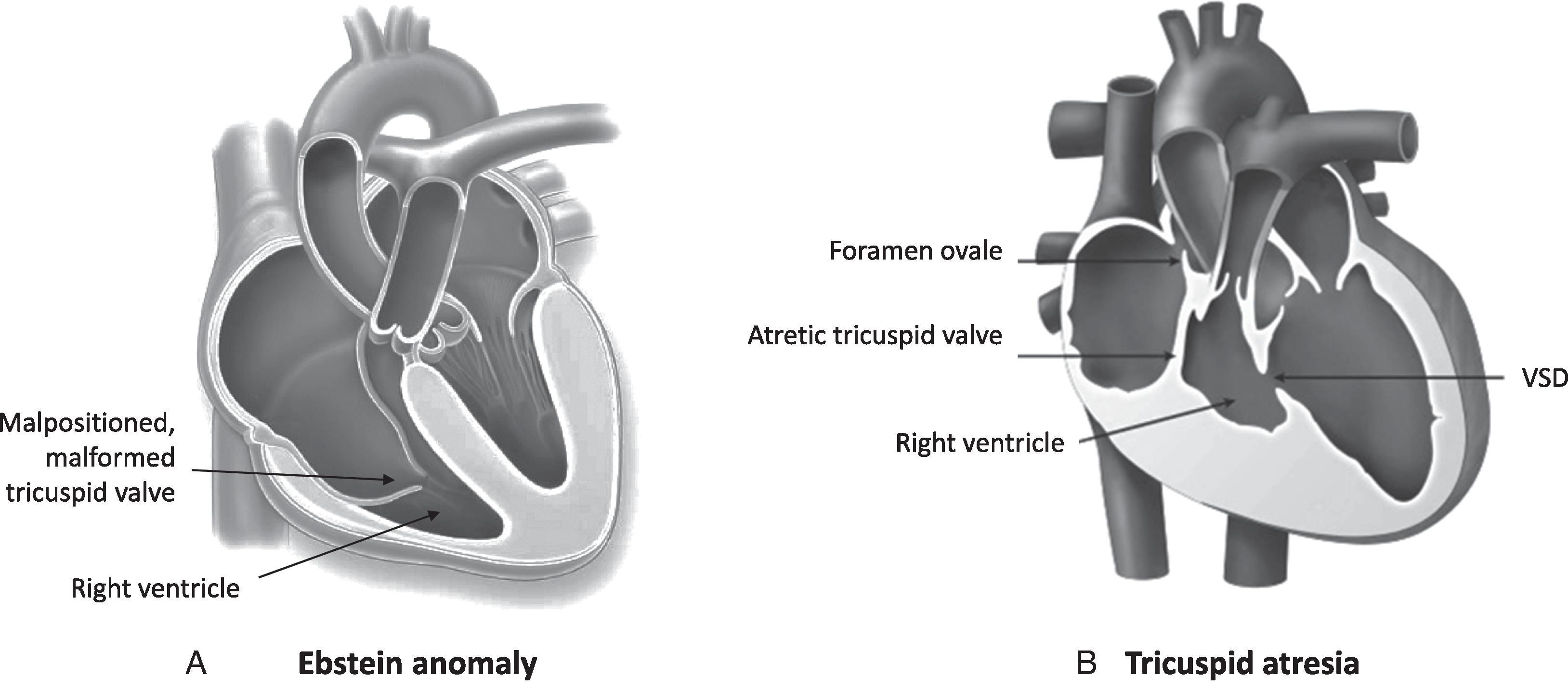
Lesions likely to be diagnosed in the nursery are those that usually present with cyanosis or murmur in the first couple days of life. Cyanotic lesions, including those with restriction to blood flow into or out of the right ventricle (see Table 36.1 ), are likely to be diagnosed in the nursery, as are d-TGA with intact ventricular septum and TAPVC with obstruction. Pathologic murmurs that present soon after birth are usually caused by turbulent flow across ventricular septal defects (VSDs) or stenotic semilunar valves, for example, aortic or pulmonary valvar stenosis. Often, the tricuspid regurgitation murmur of Ebstein anomaly ( Fig. 36.7 ) may be recognized in the first few days of life. In absent pulmonary valve syndrome, turbulent antegrade and retrograde flow across a functionally incompetent pulmonary valve creates a to-and-fro murmur.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here