Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Consistency of terminology is necessary for optimal communication.
Limb outgrowth and patterning are controlled by specific signaling centers within the developing limb bud via the activation and interaction of molecular messengers.
Anomalies of limb development result from:
spontaneous mutation of genetic material
inheritance of abnormal genes
subtle or gross insult to the limb bud.
The anomaly may be isolated or syndromic.
The International Federation of Societies for Surgery of the Hand (IFSSH) has recommended that the Swanson classification (previously approved by the IFSSH) be replaced by the Oberg, Manske, Tonkin (OMT) classification, which separates malformations from deformations and dysplasias. Malformations are further subdivided according to the axis of limb development that is primarily involved and whether the insult affects the whole of the upper limb or the hand plate only. This classification incorporates our increasing understanding of the molecular basis of limb development.
Assessment and treatment are specific for each child and family but are based on a detailed understanding of the processes of limb development, the psychological and physical effects of limb anomalies, and simple surgical principles.
![]() Access video content for this chapter online at Elsevier eBooks+
Access video content for this chapter online at Elsevier eBooks+
The upper limb bud appears during the fourth week of development as an outgrowth on the sides of the embryo. Cells of the somatic lateral plate mesoderm form the skeletal framework of the limb, while mesoderm from the somite migrates in to form the muscular component and contributes to the limb's vascular network. Over the next 4 weeks, growth and differentiation transform the limb bud into an elegant asymmetric organ that is one of the defining features of the human species. Limb bud growth and differentiation are under the control of signaling centers, specialized areas within the limb that dictate, in concert, the appropriate sequential events of development.
The establishment of signaling centers and their subsequent behavior are under genetic control. The genes and the morphogens (proteins) they encode control the process of limb growth and differentiation and act as messengers between signaling centers and developing cells.
As knowledge of the process of limb development becomes more sophisticated, an understanding of the causation of limb anomalies becomes clearer.
Genetic mutations can disrupt the molecular function of a number of proteins orchestrating limb development, including secreted proteins (ligands), ligand receptors, and transcription factors. The mutation may be inherited or may arise spontaneously. Environmental factors, such as thalidomide, which resulted in an epidemic of limb malformations during the 1960s, radiation, nutritional defects, and infections may affect the molecular pathways of development or be responsible for a more gross insult resulting in tissue hemorrhage and/or necrosis. For example, if such direct damage occurs obliterating the apical ectodermal ridge, an important signaling center that directs limb outgrowth, transverse truncation will result.
Congenital anomalies in the hand demand a reproducible and consistent terminology, a universal language that allows discussion of complex clinical entities, indications for treatment, and comparisons of results.
Swanson's classification was adopted by the IFSSH in 1976 as the standard system by which congenital hand anomalies were described. It is derived from ideas existing in the 1960s regarding limb embryology and is largely based on morphological appearance. Knight and Kay have presented an extended version, attempting to incorporate a list of all congenital anomalies. Regrettably, this classification is intrinsically unsuited to alterations based on causation and etiology at a molecular level.
In 2014, the IFSSH Scientific Committee for Congenital Conditions recommended that the Oberg, Manske, Tonkin (OMT) classification replace that of Swanson. This system utilizes dysmorphological terminology, separating congenital upper limb anomalies into malformations , deformations , and dysplasias . Malformations, the majority of encountered anomalies, are further subdivided according to which axis of formation and differentiation is primarily affected and whether the consequence affects primarily the hand plate alone or the whole upper limb. It is probable that the causation of some anomalies will confound our attempts to assign a specific site in the molecular pathway and/or the anatomical site in the limb bud and the time at which the aberration occurs. However, the OMT system would appear to be a more logical approach to classification and has been adopted by many working in the field of congenital hand surgery.
It is clear that the terminology applied to congenital anomalies should reflect a language common to, and understood by, the geneticist, the anatomist, the pathologist, and the surgeon. For this reason, these authors have chosen to link the terminology of embryology of the limb bud at the molecular level, the terminology of dysmorphology, and the terminology of classification. This consistency would appear to provide less controversy in a rapidly changing field of knowledge. Furthermore, such an approach is of benefit when attempting to explain to parents how the limb normally forms and how any particular anomaly may occur. This process plays a vital role in the assessment of any specific anomaly and leads to a sensible plan of management.
Around day 26 (4 weeks) after fertilization (Carnegie stage 12), the upper limb bud appears as an oblong ventrolateral bulge on the body wall between somites 9–12 (C5–8) ( Fig. 32.1 ). The emerging limb bud is composed of somatic lateral plate mesoderm covered by ectoderm. Subsequent limb bud growth and differentiation are described in terms of three coordinate axes: proximodistal, dorsoventral (dorsovolar), and anteroposterior (or radioulnar) ( Fig. 32.2 ), each controlled by distinctive regions – signaling centers.
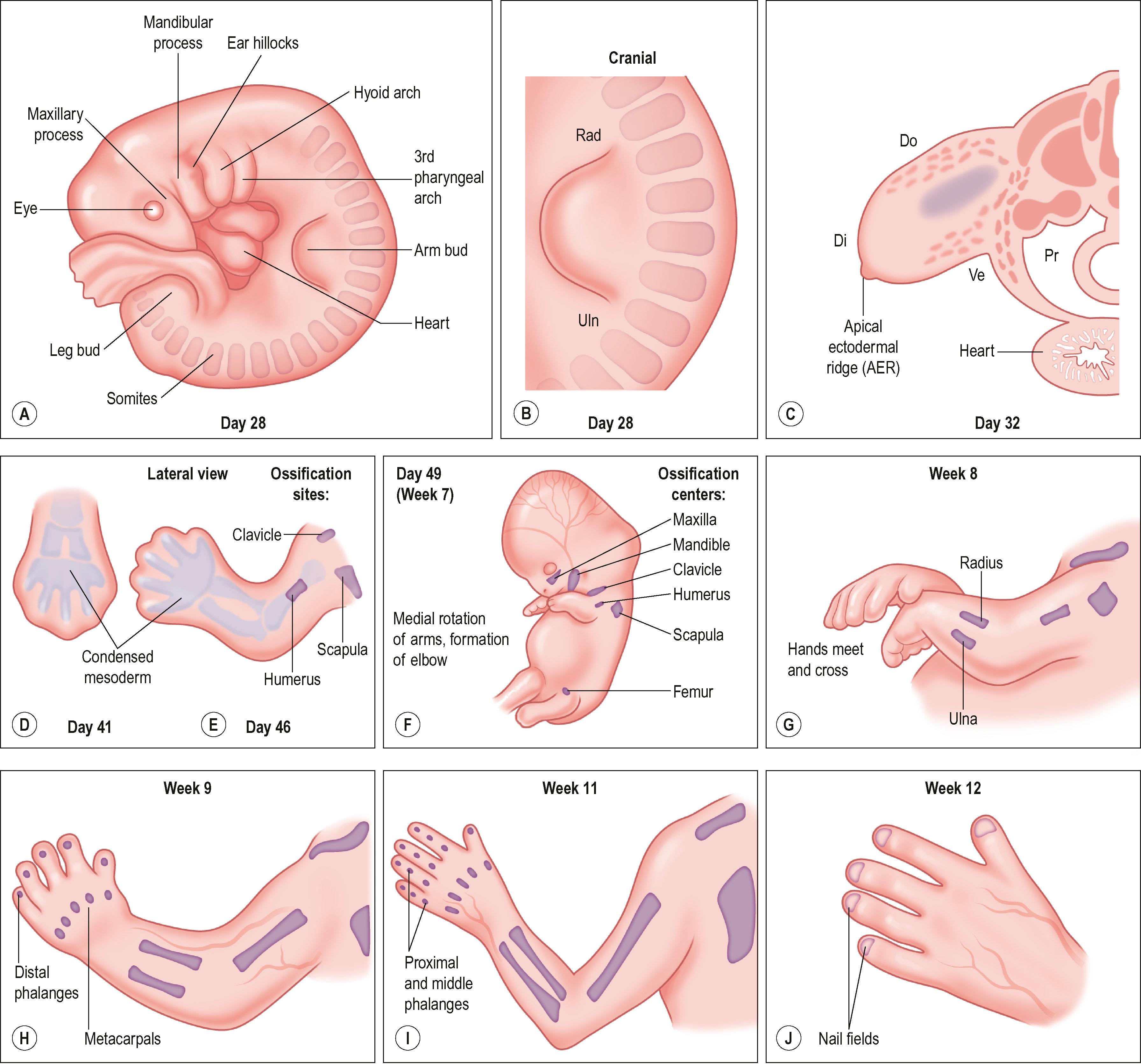
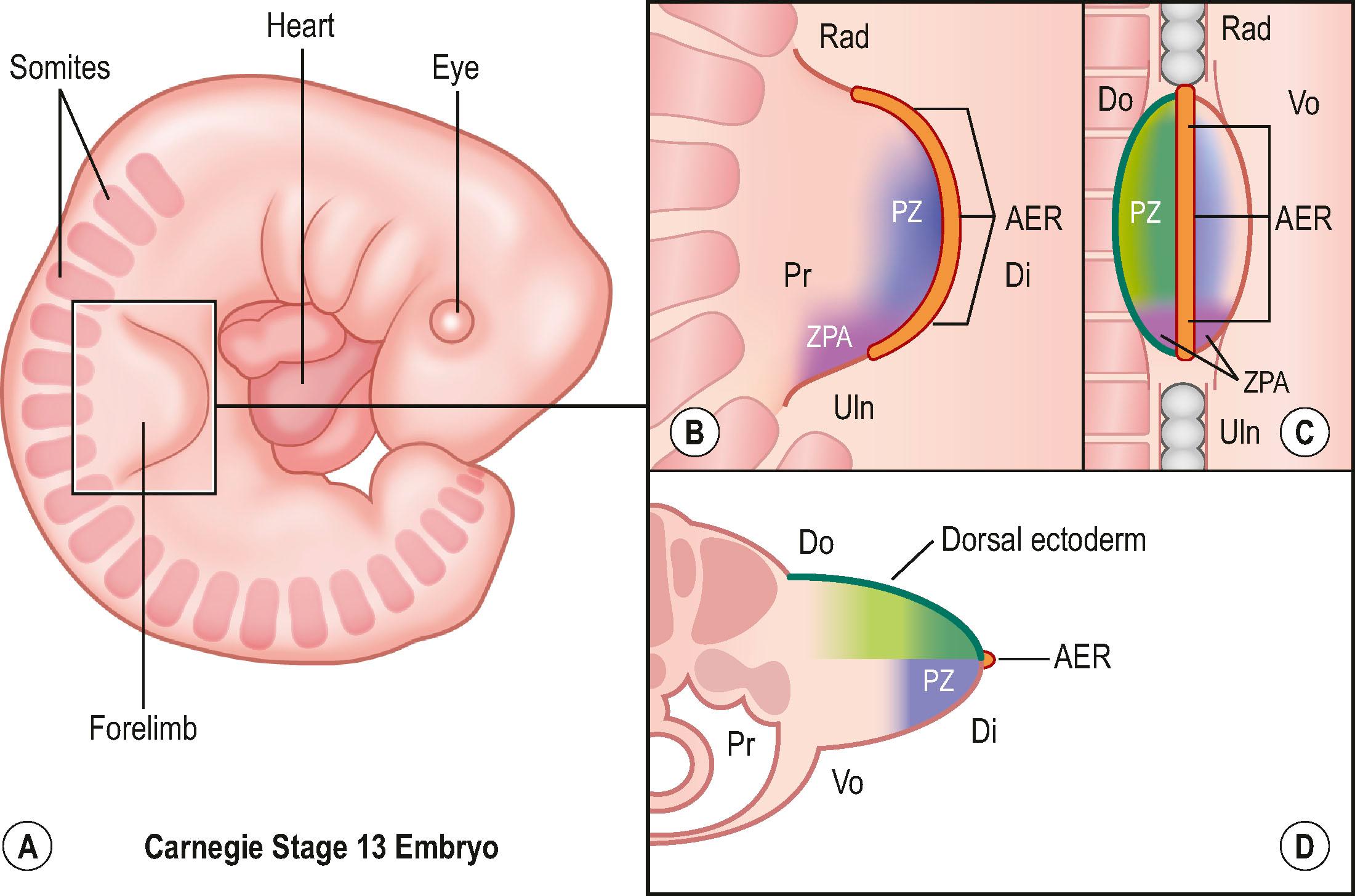
The ectoderm overlying the distal edge of the limb bud at the dorsoventral boundary thickens and forms a distinct ridge of stratified ectoderm, the apical ectodermal ridge (AER). The thickened AER is critical for proximodistal outgrowth and also adds mechanical rigidity to the distal rim of the limb bud, flattening it along the dorsoventral axis. Excision of the AER in chicken embryo limb buds prevents further proximodistal outgrowth and results in limb truncation. Underlying the AER, the distal undifferentiated mesoderm has been termed the progress zone (PZ). Cells in the PZ ultimately differentiate into specific cell types and are directed to specific positions within the limb.
The dorsal ectoderm is a critical signaling center for directing formation of dorsal and volar characteristics of the limb. Transplantation of dorsal ectoderm to the ventral limb cause the formation of dorsal structures on the ventral limb. Another collection of mesodermal cells at the distal ulnar (posterior) margin is called the zone of polarizing activity (ZPA). Although not morphologically distinct, these cells direct radioulnar patterning and coordinate asymmetric limb patterning with the other signaling centers during development. Removal of the ZPA in animal models leads to loss of the ulna and the ulnar digits. Conversely, transplantation of these cells to the anterior (radial) aspect of limb buds of chicken embryos will result in formation of a mirrored complement of ulnar digits radially.
Over the next week, the limb bud expands and elongates, particularly along the proximodistal axis ( Table 32.1 ). By day 33 of development (Carnegie stage 14), differential growth and programmed cell death transform the distal portion into a paddle-shaped hand plate. There is also progressive mesodermal condensation along the proximodistal axis, starting with the pectoral girdle (shoulder) adjacent to the axial skeleton, then a tripartite appendage skeleton composed of a proximal section, the stylopod (humerus), a middle section, the zeugopod (radius and ulna), and a distal section, the autopod (hand). The joints become fully evident about day 51 (Carnegie stage 20) when the elbow and wrist joints flex and by day 56 (end of eighth week, Carnegie stage 23), the major morphologic features of the limb are complete (see Fig. 32.1 ).
| Time after fertilization | Hand development |
|---|---|
| 27 days | Development of arm bud |
| 28–30 days | Further development of arm bud |
| 34–36 days | Elongation of arm bud |
| 34–38 days | Formation of hand paddle |
| 38–40 days | Early separation of digits |
| 44–46 days | Digits separated |
| Week 9–10 | Formation of fingernails begins |
During early embryonic development, Hox transcription factors set up a segmental body plan along the cranial–caudal axis. By the fourth week of development the presumptive limb fields are established, triggering the expression of Tbx5 in the upper limb field and Tbx4 in the hind limb field to initiate limb bud outgrowth and patterning. Tbx5 is also expressed in the heart and Tbx5 mutations cause Holt–Oram syndrome, characterized by heart and upper limb abnormalities. In the hindlimb, Islet1 and Pitx1are also involved in the induction of Tbx4 and in Leibenberg syndrome, misexpression of Pitx1 in the upper limb field causes homeotic leg to arm transformations.
In the upper limb, Tbx5 upregulates Fgf10 in the limb mesoderm and Wnt3 in the distal ectoderm. Mesodermal Fgf10, in conjunction with ectodermal Wnt3, TP63, Dlx5/6, and radical fringe (R-fng) transform the distal ectodermal at the dorsovolar boundary into the thickened AER. Formation of the AER initiates the expression of AER-related Fgf proteins (including Fgf2/4/8/9/17), which, in turn, act on the mesoderm just underneath the AER to sustain Wnts and Fgf10 expression in the PZ. This reciprocal loop of ectodermal and mesodermal Fgf/Wnt proteins promotes progressive proximodistal outgrowth ( Fig. 32.3 ). In the absence of Wnt3 or Fgf10, limbs fail to develop and tetramelia results. As noted above, removal of the AER causes truncation defects, but application of Fgfs to the distal AER-excised chick limb bud will reactivate mesodermal FGF10 and recover proximodistal limb outgrowth. Within the PZ, mesodermal cells persist in an undifferentiated or receptive state, allowing the signaling centers to direct their fates.
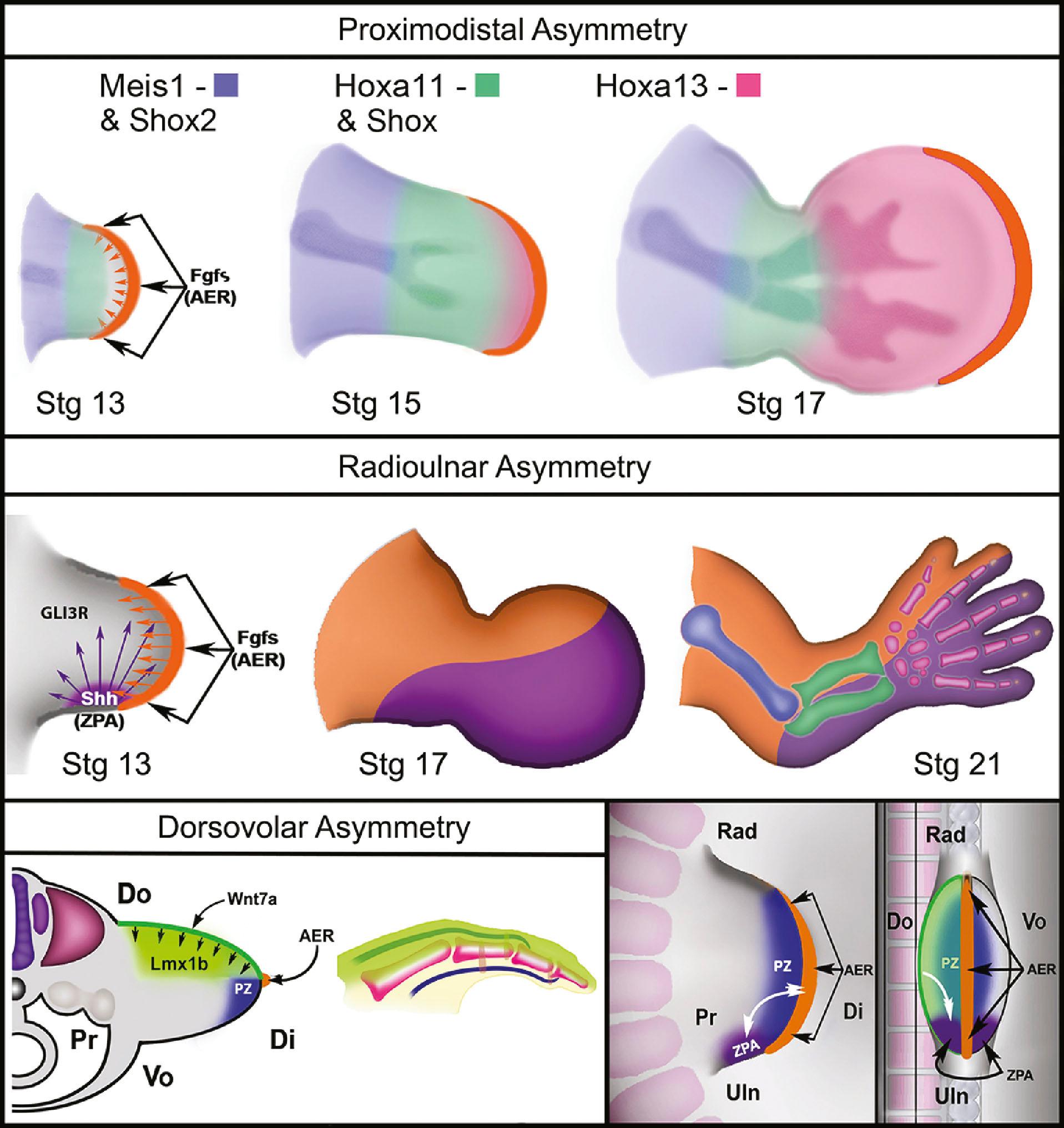
The ZPA in the posterior (ulnar) limb mesoderm secretes a potent morphogen, sonic hedgehog (Shh), that regulates radioulnar patterning (see Fig. 32.3 ). Shh induces posterior (ulnar) proliferation of the limb bud expanding its width. Moreover, Shh posteriorizes (ulnarizes) the developing forearm and defines the identity of the ulnar four digits. In a naturally occurring chicken mutant, limb-specific Shh expression is lacking and the upper limbs develop without ulnas and without digits. Application of exogenous Shh to the posterior (ulnar) aspect of the limb bud can fully recover normal limb morphology. Furthermore, application of Shh to the anterior margin produces mirrored duplication of ulnar digits radially.
Expression of En1 in the ventral ectoderm restricts the expression of Wnt7a to the dorsal ectoderm. Secretion of Wnt7a from the dorsal ectoderm induces the homeodomain transcription factor Lmx1b in the underlying mesoderm and asymmetrically dorsalizes the developing limb (see Fig. 32.3 ).
Patterning is coordinated by communication between all three axes. Distally, between Wnt7a and En1 expression, the dorsoventral boundary is established where the AER forms. The AER and the ZPA are also closely linked by a reciprocal feedback loop that maintains Shh expression at the distal posterior (ulnar) border of the limb bud adjacent to the AER during progressive outgrowth. Removal of the AER causes regression of Shh expression and ablation of the ZPA induces a loss of Fgf signaling. Wnt7a and Lmx1b also contribute to the maintenance of Shh secretion from the ZPA linking the dorsoventral and anteroposterior axes. Removal of dorsal ectoderm reduces Shh expression and disrupts posterior (ulnar) patterning. Thus, Shh plays a pivotal role during limb development, linking dorsoventral, proximodistal, and anterioposterior axes during outgrowth.
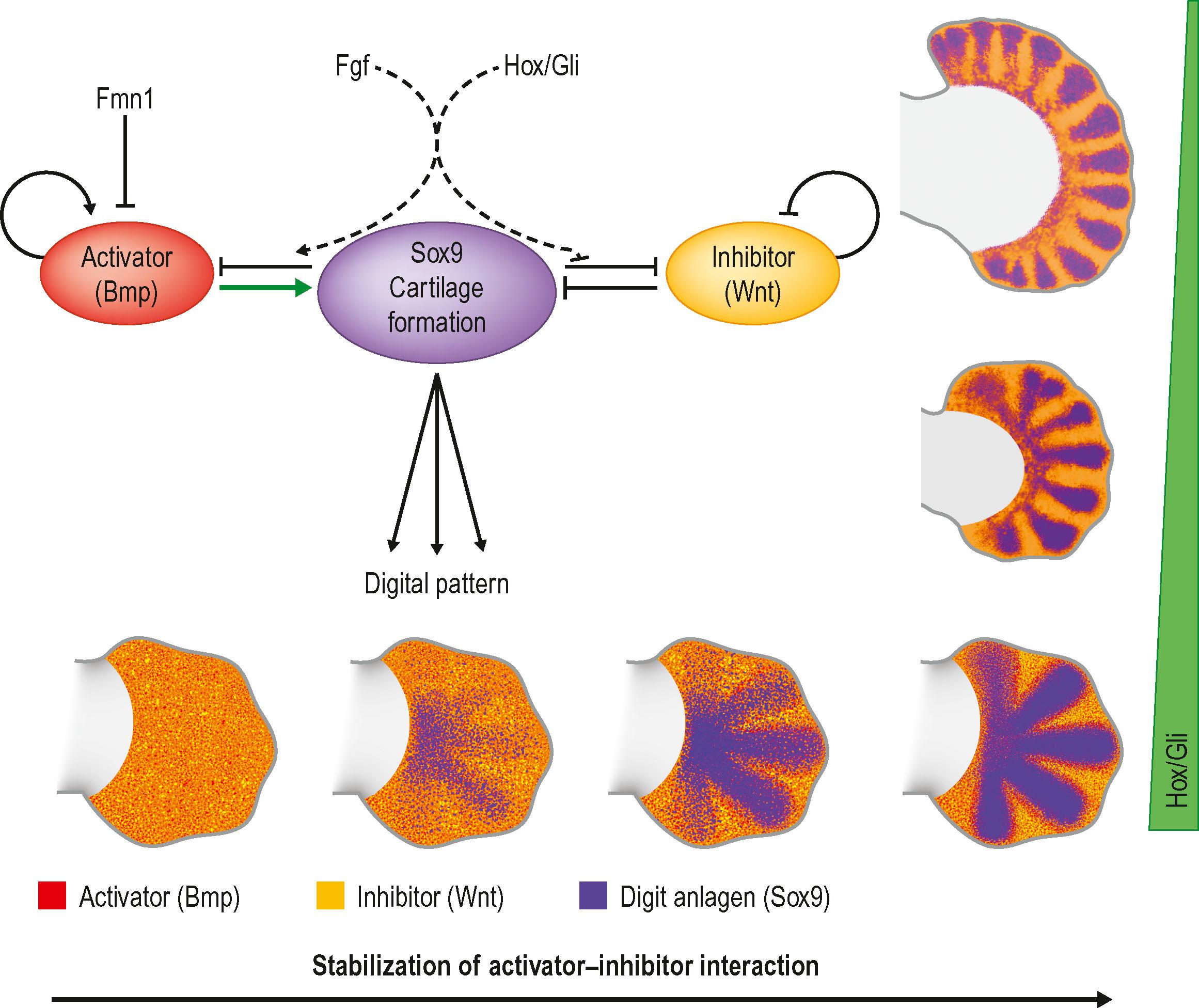
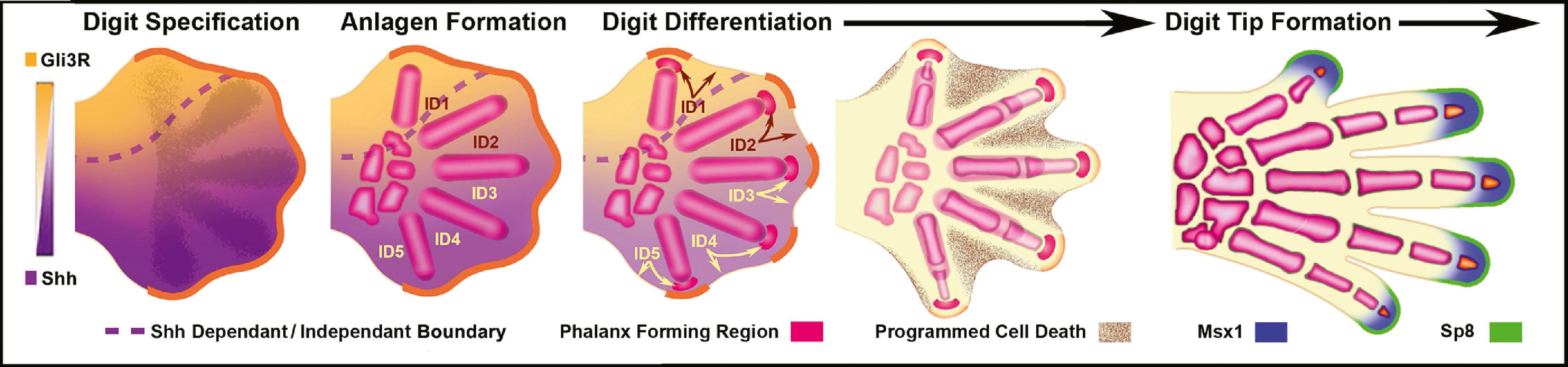
Around the end of the fifth week of development the hand plate becomes visible. Current data suggest that digit number is established by an intrinsic Turing-type pattern generator within the handplate using reciprocal activator (Bmps) and inhibitor (Wnt) interactions to define the alternating digit and interdigit pattern ( Fig. 32.4 ). Interplay between Hox transcription factors (particularly Hoxd9-13 and Hoxa9-13) and the Shh/Gli3 pathway subsequently establishes digit morphology ( Fig. 32.5 ). Shh also induces an ulnar to radial (posterior to anterior) gradient that appears to involve Bmps in at least two roles in the formation and differentiation of digits. First, Bmps induce programmed cell death, or apoptosis, via discrete interdigital signaling centers, in part by repressing Fgf expression in the overlying AER. In addition, Bmps play a role in completing digital identity via the phalanx-forming region, a region overlying the distal digital anlagen that regulates Sox9 expression and chondrogenesis. Applying a Bmp-laden bead or transplanting Bmp expressing interdigital tissue from the third interdigital space to the second transforms the second digit into a third digit. The phalanx-forming region also maintains digit-associated Fgf expression in the overlying AER for continued digital outgrowth. With regression of the phalanx-forming region and loss of overlying Fgf, the terminal phalanges form at the distal tip of each digit, invoking a unique mechanism of ossification that includes membranous ossification in addition to anlagen formation. The terminal phalanx is also demarcated by persistent expression of Msx1 in the mesoderm, and Sp8 in the ectoderm.
Limb bud formation and the progressive morphologic changes evident externally are accompanied by differentiation of various tissues internally that are regulated by the coordinate signaling centers described above. Differentiation of these tissues is coincident, but will be discussed separately to add clarity and to relate timing of formation to tissue-specific malformations.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here