Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Congenital vascular malformations (CVMs) are rare developmental abnormalities of the vascular system that include a broad spectrum of arterial, venous, lymphatic, and capillary lesions. The overall incidence is estimated at 1.2% to 1.5%. Congenital arterial malformations (AMs) without arteriovenous (AV) shunting are lesions that include abnormally formed arteries or anomalous anatomy due to persistence of the fetal arterial remnants. These pure AMs are extremely rare and represent only approximately 8% of all CVMs. Arteriovenous malformations (AVMs) and combined lesions that involve venous, lymphatic, and high-flow (HF) arterial components, comprise the majority of congenital AMs. The prevalence of both intracranial and extracranial AVMs ranges from 5 to 613 per 1000,000 population. Despite their relative rarity (10% to 20% of all CVMs), AVMs are the most challenging diagnostic and therapeutic pathology among all malformations ; therefore, the majority of this chapter is dedicated to discussing them.
The classification, clinical presentation, treatment, and outcomes of CVMs are dependent on the timing of developmental errors in the vascular system. Extratruncular CVMs arise before the formation of mature vascular trunks in the 4th to 6th weeks of gestation. They possess mesenchymal cell characteristics that lead to progression and aggressive recurrence. Truncular CVMs originate in the final stage of angiogenesis in the 6th to 7th week of development and manifest as abnormal major axial vessels or persistence of embryonic vasculature. They have lost embryonic characteristics and therefore, unlike extratruncular lesions, do not possess the potential to proliferate.
Blood vessels and blood elements arise from the common mesenchymal precursor cells (hemangioblasts), which differentiate into blood cell and vascular precursors (angioblasts). During vasculogenesis, angioblasts differentiate into endothelial cells that aggregate into small vessel cords and eventually form vessels. After endothelial cells are established as vascular elements, under the influence of vascular endothelial growth factor (VEGF), they begin to sprout and form the primitive capillary retiform plexus. As the vasculature matures, changes in hemodynamic requirements, growing metabolic demands, and growth factor secretion induce pruning and vessel remodeling.
Extratruncular CVMs are thought to arise due to failure of the fetal capillary networks to undergo appropriate regression. Interestingly, AVMs are 20 times more common in the central nervous system, where apoptosis is rare. These fetal nonfunctional AV channels later recanalize to form small AV connections that dilate, cause local ischemia, and subsequently enter a cycle of collateralization and recruitment. As such, AVMs are characterized by direct flow from an artery to a vein in the absence of a normal capillary bed. A central core or nidus forms as a network of densely packed, tortuous, poorly differentiated blood vessels with low resistance and vascular tone. The nidus with enlarged feeding arteries and draining veins is found in the majority of extratruncular AVMs.
Truncular CVMs arise during later stages of vascular development and originate from fetal vessel trunks. During early embryonic development the precursors of the heart, bulbus cordis and truncus arteriosus, are connected to the pair of dorsal aortas via a series of six pairs of pharyngeal arch arteries that develop in conjunction with the branchial pouches. In humans the five arch arteries are numbered 1, 2, 3, 4, and 6. The fifth arch eventually fuses with the fourth arch and carries no significance in later development. The paired dorsal aortas fuse in the midline to form a single dorsal aorta. The third and fourth dorsal arches, the seventh intersegmental artery, and the left dorsal aorta are the primary contributors to the normal aortic arch and its major thoracic branches. The segments of the bilateral aortic arch system that normally regress include the distal portion of the right sixth arch and the right-sided dorsal aorta. The distal part of the left sixth arch artery becomes ductus arteriosus or ligamentum arteriosum after birth. The right and left pulmonary arteries are formed from respective portions of sixth proximal arch segments. The left fourth arch becomes the aortic arch; the right fourth arch contributes to the innominate and proximal portion of the right subclavian artery. The third arches become the common carotid arteries and first segments of the internal carotid arteries. The seventh intersegmental arteries bilaterally become the subclavian and vertebral arteries. The left dorsal aorta becomes the descending thoracic aorta, as the entirety of the right dorsal aorta is lost. Abnormal regression of aortic arches results in truncular anomalies associated with abnormal site and course of adult arterial anatomy.
Clinically, truncular lesions are defects in fully formed and often named vessels. A majority of the truncular lesions affecting arterial circulation are pure AMs. They are divided into obstructive (aplasia, hypoplasia, or stenosis) and dilated (localized and diffuse) forms. Such anomalies present with hemodynamic and structural abnormalities that are treated with standard vascular or endovascular techniques. Selected examples of truncular AMs are discussed at the end of the chapter. The truncular AMVs are extremely rare and will not be discussed in greater detail. They present with a fistulous connection between the artery and vein with no nidus. Examples include a patent ductus arteriosus, pulmonary arteriovenous fistula (AVF), direct communication between pelvic vessels, or femoral artery/vein.
The history of CVMs has been plagued by incorrect descriptive terminology, eponymous nomenclature, and classification systems that are not based on clinical, anatomic, or flow characteristics. This historical lack of adequate classification has been an important factor obscuring the optimal approaches to diagnosis, treatment, and prognosis of these complex lesions. The term “hemangioma” was used to describe a variety of vascular lesions with different etiologies and natural histories. Terms such as “capillary or cavernous hemangioma” were used to describe both infantile hemangioma and CVMs.
The seminal study by Mulliken and Glowacki in 1982, which later formed the basis of International Society for the Study of Vascular Anomalies (ISSVA) classification system, provided the first distinction between hemangiomas and CVMs. Hemangiomas (vascular tumors) were classified as neoplastic disorders that histopathologically demonstrate rapid neonatal growth and increased endothelial cell turnover during their proliferative phase; during their involution phase, they then undergo slow fibrosis and exhibit diminished cellularity. Vascular malformations were classified as embryologically developed, inborn errors of vascular morphogenesis, leading to structural anomalies present at birth and associated with normal endothelial proliferation.
The Hamburg classification system, first proposed by Dr. S. Belov in 1988, placed the emphasis on embryogenesis of CVMs and their anatomic relationship to the major vascular trunks. Initially, four major groups were identified: arterial, venous, arteriovenous (AV), and combined defects. Later, capillary and lymphatic malformations were added. Truncular and extratruncular categories subdivided lesions based on the predominant vessels present in the malformations—either peripheral vascular branches/tributaries or major axial trunks. Truncular and extratruncular lesions were separated based on the stage at which developmental defect occurred. Truncular CVMs were divided into those with obstruction or stenosis (aplasia, hypoplasia, coarctation) and those with dilations (localized aneurysm, diffuse ectasia). Extratruncular malformations were localized or they were diffuse and infiltrating. Occasionally both forms were combined and include capillary, arterial, venous, or lymphatic components. The distinction between truncular and extratruncular lesions is critical for successful treatment. Truncular lesions are treated to improve hemodynamics or eliminate physiologic burden with no risk of provocation. Extratruncular lesions are more challenging to manage due to their mesenchymal origin and proliferative potential that can be stimulated by interventions (“recruitment”).
In 1992 the ISSVA was founded and in 1996 proposed an updated classification system based on histopathologic and flow characteristics, in addition to the clinical appearance of the lesions. Vascular anomalies were divided into two categories: vascular tumors and vascular malformations ( Table 10.1 ). This classification emphasized that CVMs, in contrast to vascular tumors, histologically and physiologically were not a proliferative neoplastic lesions. Vascular malformations were further subdivided into low-flow (LF) and HF groups, as well as mixed syndromes with various histopathologic features and flow components. The latest version of ISSVA classification system was expanded in 2014 to include a section on malformations of individually named vessels (“truncular” malformations), as well as newly named anomalies and gene mutations involved in various subtypes of CVMs. This classification system was meant to reflect the state of the art in vascular anomalies classification and reflect recent advances in knowledge and clinical associations.
| VASCULAR ANOMALIES | ||||
|---|---|---|---|---|
| Vascular Tumors | VASCULAR MALFORMATIONS | |||
| Simple | Combined b | Of Major Named Vessels | Overgrowth Syndromes/Other Anomalies | |
Benign:
Locally aggressive:
Malignant:
|
Arteriovenous Malformations (AVM) a :
Arteriovenous Fistula (AV) a (congenital):
Capillary Malformations (CM):
Lymphatic Malformations (LM):
Venous Malformations (VM):
|
Truncular, aka channel type: Affect:
Anomalies of:
|
||
a Lesions with high-flow arterial component.
b Defined as two or more types of vascular malformations found in one lesion.
In 2013 the International Union of Angiology (IUA) created a comprehensive classification system informed by both the ISSVA and Hamburg classification systems. The new Integrated Classification System for the management of CVMs ( Table 10.2 ) includes flow and vessel characteristics, as well as embryologic derivation (truncular vs. extratruncular). In the future this classification system should facilitate effective diagnosis and treatment of CVMs.
| CONGENITAL VASCULAR MALFORMATIONS | |||||
|---|---|---|---|---|---|
| Low Flow | High Flow | ||||
| Venous (VM) | Truncular |
|
Arterial (AM) | Truncular |
|
| Extratruncular |
|
Extratruncular |
|
||
| Lymphatic (LM) | Truncular |
|
Arteriovenous (AVM) | Truncular |
|
| Extratruncular |
|
Extratruncular |
|
||
| Capillary/microvascular | |||||
| Combined | |||||
| Syndrome associated | Syndrome associated | ||||
Extratruncular lesions result from defective development during the earlier stages of embryogenesis, when the vascular structures are still in the state of plexiform primordial vessels. As such, they possess mesenchymal cell characteristics to grow when stimulated externally (e.g., trauma, surgery, intervention) or internally (e.g., menarche, pregnancy, hormones). Clinically, these lesions are masses of abnormal small/mid-sized vessels diffusely infiltrating various tissues and organ systems. The embryologic characteristics, combined with inappropriate treatment strategies, can provoke dormant, asymptomatic lesions to grow rapidly. Therefore, in addition to the hemodynamic impact on the affected vascular system, all extratruncular lesions, regardless of the type and location, carry the risk of progression and recurrence.
Most AVMs are present at birth but may not become evident until later in childhood or adolescence, after the lesion has enlarged or become symptomatic. AVMs are most common in the central nervous system ; however, intracranial lesions will not be discussed in this chapter. Extracranial AVMs are most commonly observed in the head and neck (50% to 70%), extremity (20% to 30%), trunk (5% to 10%), and visceral locations (1% to 5%). Unlike vascular tumors, AVMs do not regress and their growth occurs simultaneously with the patient's physical development. Lesions may progress more rapidly due to trauma, puberty, pregnancy, embolization, or resection-mediated ischemic injury. The mechanisms by which AVMs progress and recur following treatment include: collateralization and arterialization due to AV shunt, opening of latent AV shunts, aneurysm formation in arteries and veins, and angiogenesis and vasculogenesis. Hormonal changes during adolescence and pregnancy also stimulate progression. Estrogen, testosterone, and progesterone have been found to stimulate VEGF production, endothelial proliferation, and angiogenesis. During puberty, patients are at least a twofold risk of AVM progression. Although pregnancy is not contraindicated in women with AVMs, patients should be warned of the potential of an aggressive lesion behavior during pregnancy.
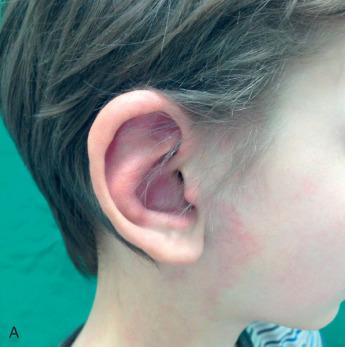
Extratruncular AVMs are classified according to the Schobinger severity scoring system ( Table 10.3 ). Stage I is quiescence: the AVM resembles a capillary malformation or involuting hemangioma ( Fig. 10.1A ). Stage II is expansion: the lesion becomes warmer and bigger and develops a throbbing sensation with a thrill and a bruit (see Fig. 10.1B ). Stage III is that of tissue destruction: the aforementioned symptoms are accompanied by ulcers, hemorrhage, pain, and bony lytic lesions (see Fig. 10.1C ). Stage IV is rare and signifies progression of all the previously mentioned symptoms to congestive failure with increased cardiac output, volume-pressure overload, and left ventricular hypertrophy. A high-output cardiac state occurs in 1% to 2% of peripheral AVMs.
| Stage | Clinical Description |
|---|---|
| I (quiescence) | Warm, pink-bluish stain, arteriovenous shunting on Doppler, resembles capillary malformation or involuting hemangioma |
| II (expansion) | Same as stage I, plus enlargement, pulsatility, thrill/bruit and tortuous/tense veins |
| III (destruction) | Same as stage II, plus ulceration, bleeding, pain, steal, soft tissue necrosis. Osteolytic lesions may develop |
| IV (decompensation) | Same as stage III, plus progression to high-output congestive heart failure and left ventricular hypertrophy. |
Most extratruncular AVMs are sporadic in nature and are caused by somatic (post)zygotic mutations with various penetrance patterns. The precise genetic basis is unknown, but molecules that participate in AV differentiation and the angiogenesis pathway, such as Notch signaling proteins, are likely involved. The genetic basis of several CVMs that occur as part of complex syndromes have been recently identified, and further studies will contribute to a better understanding of defective vascular morphogenesis. Newly discovered biomolecular pathways will serve as potential therapeutic targets for treatment of sporadic and syndromic CVMs.
Hereditary hemorrhagic telangiectasia (HHT, also known as Rendu-Osler-Weber disease) is an autosomal dominant disorder characterized by multiple telangiectasias of the skin and gastrointestinal tract, as well as AVMs in the liver, lungs, and brain. Spontaneous recurrent nosebleeds from telangiectasias of the nasal mucosa are the presenting sign in more than 90% of HHT patients. The two major types of disease, HHT1 and HHT2, are caused by mutations in the endoglin (ENG) and activin receptor kinase (ACVRL1) genes, respectively. Both genes belong to the transforming growth factor-β (TGF-β) signaling pathway that regulates cell proliferation, migration, and differentiation. TGF-β is also responsible for maintaining extracellular vascular matrix. HHT1 has earlier onset and is more likely to have cerebral and pulmonary AVMs, whereas HHT2 has a later onset, lower penetrance, and more common hepatic AVMs. Bevacizumab, a monoclonal antibody against VEGF, has been found to directly inhibit the VEGF proteins that can be elevated as a result of the HHT mutations and reduce epistaxis, telangiectasias, and iron-deficiency anemia.
RASA1 is another autosomal dominant mutation responsible for capillary arteriovenous malformation (CAVM) syndrome. Affected family members manifest small, multifocal capillary malformations, often with a pale halo. Approximately a third of the patients have AVMs, AVFs, or Parkes-Weber syndrome (PWS), which is characterized by soft tissue and skeletal overgrowth with or without lymphatic lesions, large cutaneous stains, and AV-microfistulas. Individuals with PWS who do not have multiple capillary lesions have a sporadic form of this syndrome without the RASA1 mutation. The RASA1 mutation leads to loss of inhibitory function on the Ras protein that takes part in endothelial cell proliferation, survival, and function, likely resulting in CAVM phenotype.
Phosphatase and tensin homolog gene (PTEN) is an autosomal dominant mutation of a tumor suppressor gene involved in Cowden and Bannayan-Riley-Ruvalcaba syndromes. PTEN hamartoma refers to the focal tissue overgrowth frequently containing AVMs. These syndromes share common features of macrocephaly, penile freckling, hamartomatous intestinal polyposis, asymmetrical overgrowth, and ectopic fat deposits (lipomas). Patients usually have multiple intramuscular, paraspinal, or intracranial AVMs that are aggressive and difficult to control with embolization.
Distinguishing vascular tumors from vascular malformations and HF from LF lesions are essential steps in proper evaluation and treatment selection. The presence of arterial flow is the hemodynamic characteristic used to differentiate between HF and LF CVMs. Failure to identify arterial flow can have detrimental consequences in treatment outcomes if the patient with HF lesion is erroneously treated with techniques appropriate for LF CVM and vice versa.
History and physical examination alone can differentiate 90% of AVMs. It is important to note the timing of lesion appearance, progression, and any treatment attempts. The most important clinical characteristic used to differentiate between vascular tumors and CVMs is the absence of clinical regression among true malformations. Infantile hemangiomas are neoplastic lesions that have an increased rate of cellular proliferation during the first year of life (proliferative phase) followed by slow, spontaneous regression (involution phase). Therefore, by definition an adult presenting with a vascular lesion does not have a hemangioma, despite the frequent use of this term by nonexperts.
Bedside Doppler examination with typical flow characteristics can help to distinguish HF from LF (such as capillary, lymphatic or venous) lesions. Further imaging should be used to confirm the type of malformation, define anatomic extent and involvement of adjacent structures, and facilitate treatment planning.
Duplex ultrasound (US) is the best initial diagnostic study, especially for more focal, superficial lesions, and indeed should be considered part of the bedside physical exam to confirm diagnosis. An HF AVM exhibits low-resistance high-velocity arterial flow with high diastolic flux and pulsatile venous outflow. Color Doppler shows a poorly defined hypervascular lesion with tortuous feeding arteries and dilated draining veins that can undergo aneurysmal degeneration as a result of long-standing arterialization. US can provide initial information about the lesion extent, proximity to the adjacent structures, relationship to normal anatomy, and patterns of drainage and reflux. On US the AVM can be differentiated from hemangioma because, in contrast to AVM, vascular tumors contain homogenous parenchymal tissue.
Magnetic resonance imaging (MRI) has become the most important contemporary diagnostic modality. This study allows the clinician to define the anatomic extent and lesion relationship to the surrounding structures. It also helps to distinguish among the HF and LF malformations. A typical CVM imaging protocol consists of spin echo (SE) or fast spin echo (FSE) T1-weighted sequences axial to the lesion with fat suppression in order to define the anatomy, tissue planes, and neurovascular structures. T2-weighted images in at least two planes (coronal, sagittal, or axial) have been found to be most sensitive and specific for detection of the extent and depth of the lesion because of a generally bright lesion signal intensity over a low signal intensity of surrounding fat, muscle, and bone. These sequences demonstrate the content of the malformation. SE sequences with gadolinium help to distinguish HF AVMs from LF VMs or LMs because the signal voids on T2 sequences represent arterial feeding vessels and early filling of draining veins. Dilated feeding arteries and draining veins with a paucity of venous lakes are also indicative of HF lesions. LF CVMs have increased intraluminal signal on T2-weighted images. AVMs also exhibit a characteristic lack of soft tissue mass.
Dynamic contrast-enhanced MRI (dceMRI) has been reported to have increased specificity and sensitivity in differentiating between HF and LF CVMs. Using multiple rapid bolus contrast injections over a period of time enables better demonstration of an enhanced lumen by eliminating background signal. In addition to providing robust hemodynamic data, it also delineates feeding vessels, nidus morphology, and surrounding structures. Overall, MRI is a noninvasive study that provides anatomic and hemodynamic information, but it can be lengthy and noisy and might require sedation in younger children. MRI is the preferred imaging modality for treatment follow-up.
Computed tomography (CT) is rarely used for the initial lesion evaluation because MRI is preferred; however, CT can complement MRI in demonstrating bony involvement, as well as provide some additional structural information. Intravenous contrast and appropriate timing protocol (contrast bolus tracking technique) is needed to better distinguish the type of CVM and associated structures. Asymptomatic AVMs on CT angiogram should have no soft tissue component. Soft tissue enhancement suggests either a tumor (sarcoma) or inflammation due to infection, ischemia, or bleeding. CT angiography is also reserved for patients who are unable to undergo MRI or for more rapid evaluation of acute symptoms, such as bleeding or airway compromise.
Catheter-based angiography can be obtained if the diagnosis and/or flow characteristics remain equivocal following noninvasive imaging techniques and is essential in defining the lesion type. Angiographic classification system has been proposed by Yakes and is used to evaluate the feasibility of embolization treatment ( Table 10.4 ). Similar to the appearance on MRI or CT angiography, on angiography the AVM lesion appears as tortuous vessels with enlarged feeding arteries that rapidly shunt into dilated draining veins via a nidus. Catheter-based angiography allows for real-time evaluation of flow dynamics, main feeding arteries, collateral vessels, nidus, and draining veins.
Conventional radiography is used to demonstrate erosive and intraosseous changes of adjacent bone and is recommended for evaluation of limb length discrepancy.
Whole body blood pool scintigraphy (WBBPS) and transarterial lung perfusion scintigraphy (TLPS) are nuclear imaging techniques that have been recently introduced to quantitatively evaluate AVMs. Using technetium 99m–tagged erythrocytes to detect abnormal blood pooling throughout the body, WBBPS allows localizing hidden lesions. Increased radiotracer pooling within the vascular lesions can be quantified before and after intervention to evaluate treatment efficacy. TLPS uses technetium 99m–labeled microsphere albumin. Measuring radiotracer activity reaching the lungs that has passed unfiltered though the AVM shunt provides quantitative information on blood volume shunting through the AVM and allows calculation of shunt percentage. It can identify hidden micro-AV shunting that is difficult to detect on other modalities. These two modalities are unique because they can document quantitative AV shut percentage through the nidus and can be used to objectively follow treatment response.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here