Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Most congenital nasal lesions occur secondary to developmental errors in one of three embryologic zones: the anterior neuropore, the central midface, or the nasobuccal membrane.
Encephaloceles, gliomas, and dermoids share a common embryologic origin and frequently manifest as midline nasal masses. These lesions require surgical management.
Appropriate preoperative imaging with computed tomography and/or magnetic resonance imaging and careful intraoperative dissection are required to determine whether a dermoid extends intracranially. Lesions with intracranial extension are optimally managed by a combined approach involving otolaryngology and neurosurgery.
A cleft lip typically results in a flattened, retracted nostril and a broad nasal tip. Other congenital nasal disorders of the central midface are less common.
Congenital stenosis of the nasal piriform aperture is associated with holoprosencephaly and a central maxillary megaincisor in a majority of cases. Pituitary abnormalities are also common.
Up to 30% of infants are born with nasolacrimal duct obstruction but most do not require surgical intervention. Nasolacrimal duct cysts may cause nasal obstruction and feeding difficulties and require marsupialization.
Choanal atresia is a relatively common cause of congenital nasal obstruction and is more frequently unilateral in presentation.
Choanal atresia may be bony or combined bony and membranous in nature. Transnasal approaches are the most popular. Mucosa-sparing techniques are associated with the best outcomes.
Acquired malformations of the nose may arise from surgical disruption of the growth centers of the nose and chronic obstruction of the nasal airway.
Congenital malformations of the nose and paranasal sinuses are rare manifestations of disordered development, ranging from subtle cosmetic deformities to feeding difficulties and even life-threatening acute upper airway obstruction in neonates. Congenital lesions of the nose and paranasal sinuses result from developmental errors in specific anatomic zones, including (1) the anterior neuropore, (2) the central midface, and (3) the nasobuccal membrane. Mesodermal and germline malformations may also affect the nose and paranasal sinuses but are not unique to these sites. In contrast, acquired malformations of the nose are thought to result from disruption of the growth centers of the nose and/or the application of chronic forces on the facial skeleton from mouth breathing.
The anterior neuropore persists medial to the optic recesses in the third week of life, and the skull base develops around the neuropore as the frontal, ethmoidal, and nasal bones. Behind the nasal bones but in front of the nasal and septal cartilages lies a potential space, the prenasal space. The foramen cecum forms a defect in the anterior skull base at the apex of the prenasal space, where the cribriform plate ultimately condenses. This structure is closed by its fusion with the fonticulus frontalis, a fontanelle between the inferior aspect of the frontal bones and the developing nasal bones. During the third through the eighth weeks of development, a projection of dura extends through the foramen cecum, traverses the prenasal space, and apposes ectoderm at the tip of the nasal bones (the future rhinion). As the foramen closes, the dural diverticulum detaches from the overlying ectoderm and retracts into the cranium. Irrevocably apposed ectoderm may be pulled posterosuperiorly toward and even through the foramen cecum, giving rise to a dermoid fistula, cyst, or sinus. Faulty or premature closure of the foramen may enable persistence of neural tissue in the nasal cavity as isolated heterotopic glial tissue (glioma) or as a patent central nervous system (CNS) communication (meningocele or encephalocele) ( Fig. 5.1 ). The latter can occur in a paramedian position, as a nasoethmoidal encephalocele, or, more laterally through a medial orbital wall defect, as a naso-orbital encephalocele. Similar errors may occur above the developing nasal bones at the fonticulus frontalis. Intracranial contents may herniate through the patent fonticulus frontalis until the eighth week. If the fonticulus also closes abnormally, an extranasal path may persist, leading to nasofrontal meningoceles, encephaloceles, or gliomas ( Fig. 5.2 ).
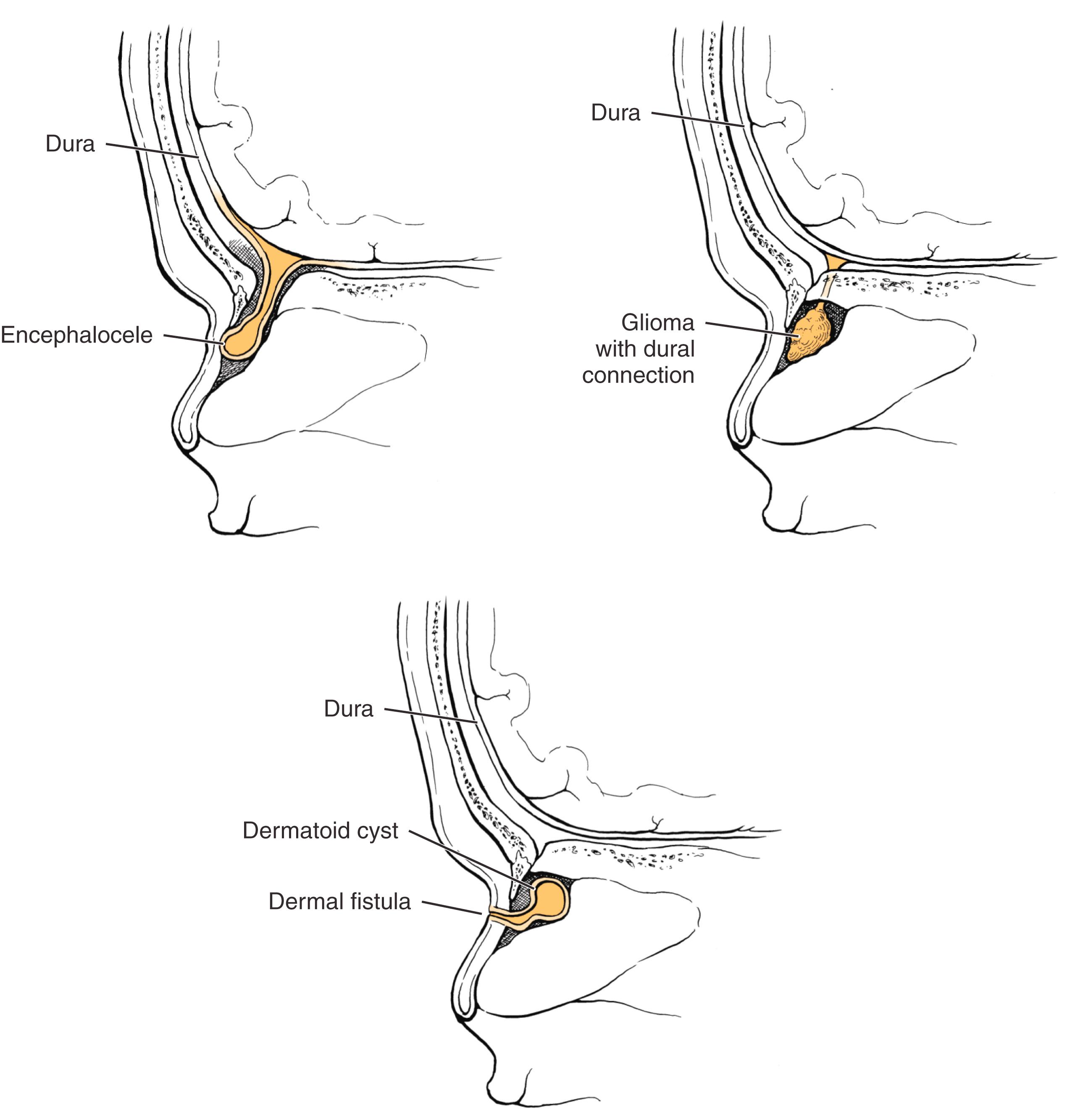
An encephalocele is an extracranial herniation of cranial contents through a defect in the skull. When the encephalocele includes meninges only, it is termed a meningocele; if it includes both brain and meninges, it is termed a meningoencephalocele. Estimates of the incidence of these lesions vary considerably, ranging from 1 in 3000 to 1 in 30,000 live births in North America and Europe. The incidence in Asian populations is higher, with a reported incidence of 1 in 6000 live births. Encephaloceles have no familial tendency or gender predilection. Approximately 40% of affected patients have other associated anomalies.
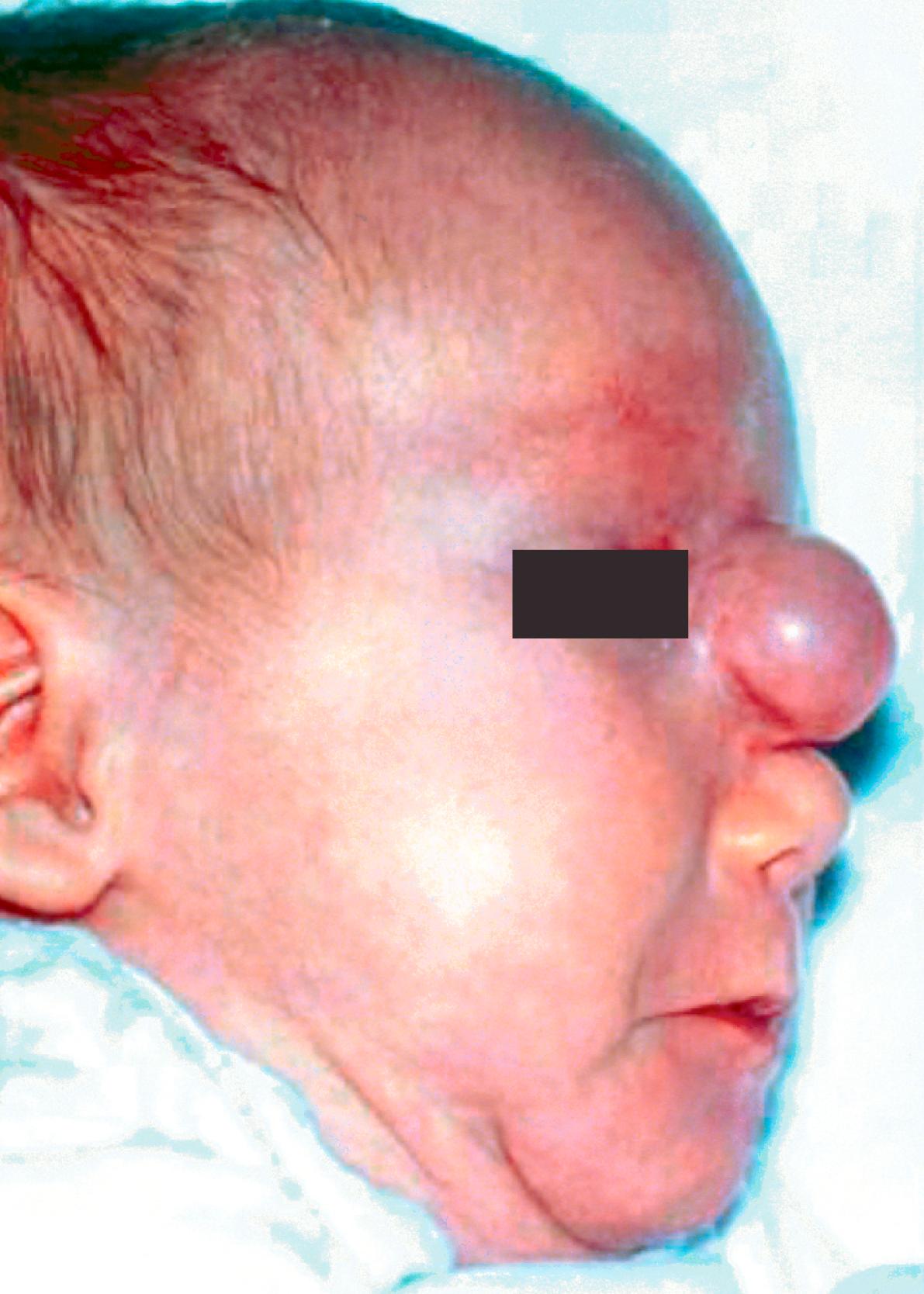
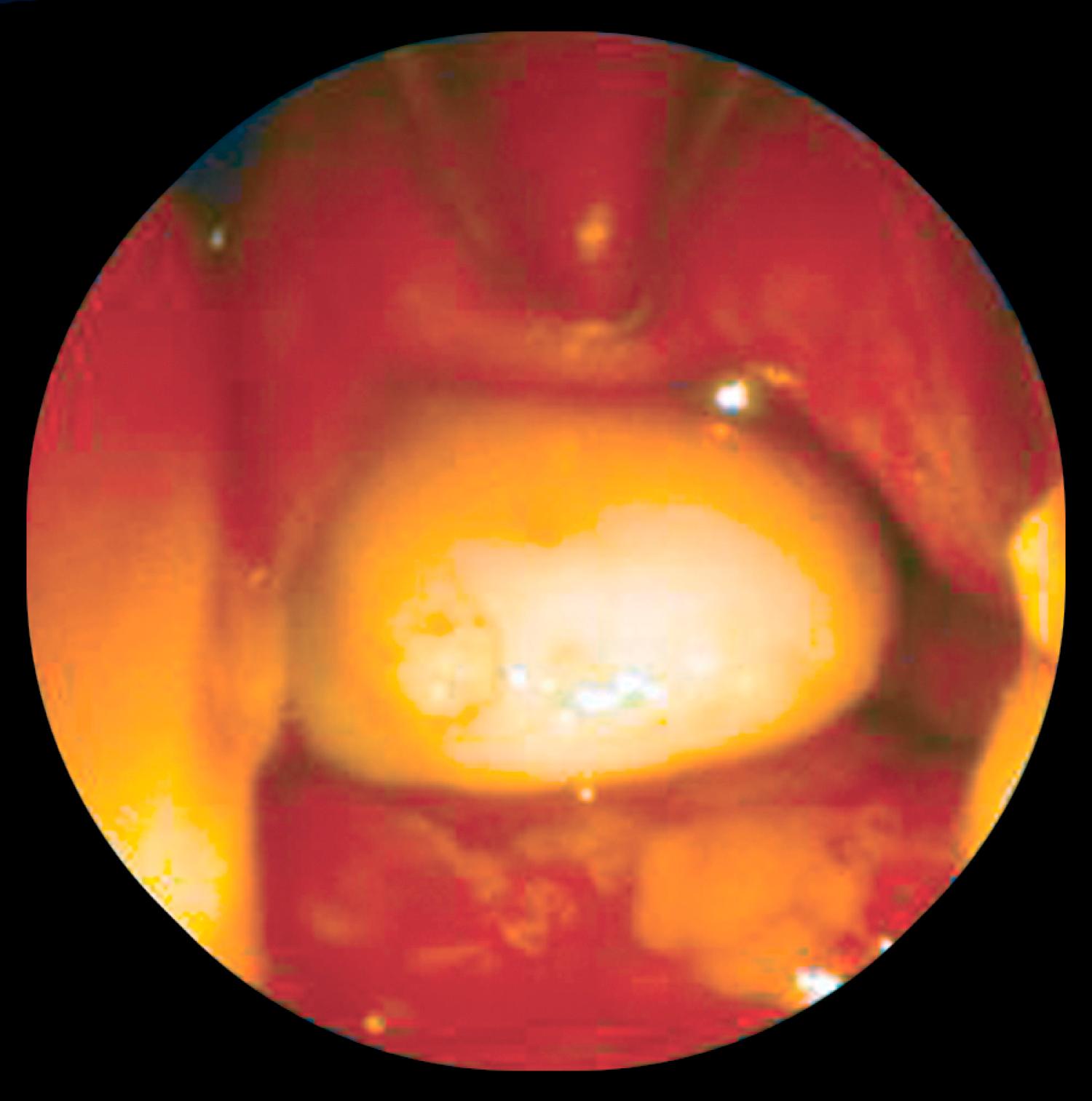
Encephaloceles are divided into occipital, sincipital, and basal types ( Tables 5.1 and 5.2 ). Occipital encephaloceles are not discussed in this chapter because they occur outside the nose. Sincipital encephaloceles account for 25% of all encephaloceles and are further classified according to their location (see Table 5.1 ). Nasofrontal encephaloceles manifest as glabellar masses, causing telecanthus and inferior displacement of the nasal bones. Nasoethmoidal lesions manifest as dorsal nasal masses, causing superior displacement of the nasal bones and inferior displacement of the alar cartilages. Naso-orbital lesions manifest as orbital masses, causing proptosis and visual changes. Basal encephaloceles are less common, arising between the cribriform plate and the superior orbital fissure or posterior clinoid fissure. These manifest as intranasal masses ( Fig. 5.3 ) and may not present until later in childhood, when nasal obstruction and drainage ensues. Sincipital and basal encephaloceles appear as pulsatile bluish compressible lesions that transilluminate. Classically, these lesions expand with crying, straining, or compression of the jugular veins.
| Type | Course | Clinical Features |
|---|---|---|
| Nasofrontal | Through bone defect between orbits and forward between nasal and frontal bones to the area superficial to the nasal bones | Glabellar mass Telecanthus Inferior displacement of nasal bones |
| Nasoethmoidal | Through foramen cecum deep to the nasal bones, turning superficially at the cephalic end of the upper lateral cartilage to expand superficial to the upper lateral cartilage | Mass on the nasal dorsum Superior displacement of nasal bones Inferior displacement of the alar cartilages |
| Naso-orbital | Through the foramen cecum deep to the nasal and frontal bones through a lateral defect in the medial orbital wall | Orbital mass Proptosis Visual changes |
| Type | Course | Clinical Features |
|---|---|---|
| Transethmoidal | Through the cribriform plate into the superior meatus medial to the middle turbinate | Most common type Nasal obstruction Hypertelorism Broad nasal vault Unilateral nasal mass |
| Sphenoethmoidal | Passes through a bony defect between the posterior ethmoid cells and sphenoid | Nasal obstruction Hypertelorism Broad nasal vault Unilateral nasal mass |
| Transsphenoidal | Through a patent craniopharyngeal canal into the nasopharynx | Nasopharyngeal mass Nasal obstruction Associated with cleft palate |
| Spheno-orbital | Through the superior orbital fissure and out the inferior orbital fissure into the sphenopalatine fossa | Unilateral exophthalmos Visual changes Diplopia |
On histopathologic analysis, sincipital and basal encephaloceles have a glial component, with astrocytes surrounded by collagen, submucosal glands, and sometimes nasal septal cartilage or calcification. Although it may be difficult to differentiate between gliomas and encephaloceles, the presence of ependymal tissue is diagnostic of an encephalocele.
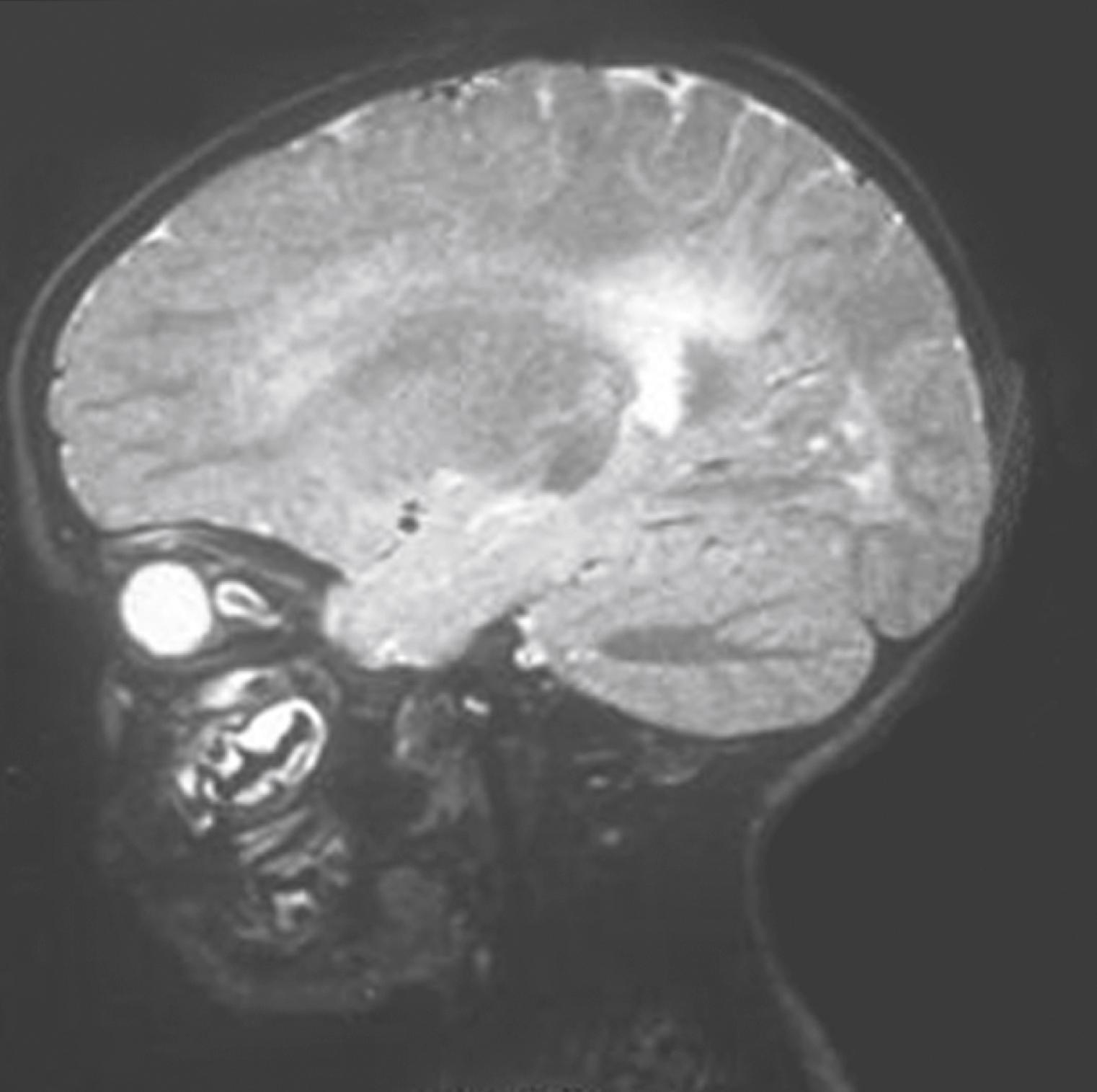
High-resolution computed tomography (CT) or magnetic resonance imaging (MRI) delineates encephaloceles and additionally helps to exclude associated anomalies such as agenesis of the corpus callosum and hydrocephalus. CT outlines the bony cartilaginous defect, whereas MRI provides complementary information regarding the soft tissue characteristics of the mass ( Fig. 5.4 ). MRI also differentiates meningoceles from meningoencephaloceles. Sagittal reconstructions and contrast enhancement are particularly advantageous for identifying an intracranial connection.
Once the diagnosis has been made, the management of encephalocele is surgical. Most authorities advocate intervention in the first few months of life to minimize the risk of meningitis and cosmetic deformity. As the intracranial connection is easier to identify in young patients, complete repair of the dural defect is easier to achieve. Prophylactic antibiotics are not necessary to prevent meningitis for patients awaiting surgery, as they are ineffective. Small lesions with minimal skull base defects may be managed endoscopically. Larger lesions require a combined approach with a craniotomy to resect condemned dura and cerebral tissue with concomitant endoscopic removal of the residual nasal tumor. The skull base defect can then be reconstructed using a pericranial flap or split-thickness calvarial bone graft. The most commonly encountered postoperative complications are cerebrospinal fluid (CSF) leak, meningitis, and hydrocephalus. Postoperative neurologic function should reflect the patient’s preoperative performance. Encephalocele recurrence rates of 4% to 10% have been reported.
Gliomas consist of heterotopic glial tissue that lacks a patent CSF communication to the subarachnoid space; however, 5% to 20% of gliomas maintain a fibrous affiliation (see Fig. 5.1 ). Nasal gliomas are rare benign masses that may be extranasal (60%), intranasal (30%), or combined (10%). They occur more commonly in males than females in a ratio of 3:2. Extranasal gliomas are smooth, firm, noncompressible masses that occur most commonly at the glabella, although they may arise along the side of the nose or the nasomaxillary suture line. Intranasal gliomas are pale polypoid masses that may protrude from the nostril. The nasal fossa on the involved side may be obstructed. Intranasal gliomas most often arise from the lateral nasal wall near the middle turbinate and occasionally from the nasal septum. Nasal gliomas may rarely extend into the orbit, frontal sinus, oral cavity, or nasopharynx. Because they lack a patent CSF connection, gliomas do not change in size with crying or straining and do not transilluminate. On histopathology, gliomas demonstrate fibrovascular and dysplastic neuroglial tissue, similar to nasal glial heterotopias, nasal cerebral heterotopias, or congenital nasal neuroectodermal tumors. Gliomas do not contain ependymal tissue, which differentiates them from encephaloceles.
Diagnostic evaluation should include nasal endoscopy to assess the location, origin, and extent of the nasal mass. CT scan is useful to assess the bony anatomy of the skull base, and MRI serves to define soft tissue connections to the CNS. As with encephaloceles, sagittal reconstructions and contrast enhancement are useful imaging adjuncts.
Proper management of gliomas requires surgical extirpation using a multidisciplinary approach that involves both otolaryngology and neurosurgery. Delaying intervention may lead to infection or distortion of the septum or nasal bones. With cosmesis kept in mind, surgical access should provide exposure of the lesion and allow possible exploration of the skull base. For resection of extranasal gliomas, options include lateral rhinotomy, external rhinoplasty, and transglabellar, subcranial, bicoronal, and midline nasal approaches. Except in the case of large glabellar lesions, the external rhinoplasty approach provides adequate surgical exposure while minimizing the use of facial incisions. When a fibrous stalk is present that extends deep to the nasal bones toward the base of the skull, a nasal osteotomy is recommended to improve exposure. Following the stalk in its entirety is essential for assessing possible intracranial extension. As a result of advancements in surgical instrumentation, image guidance, and surgical techniques, most intranasal gliomas can be removed endoscopically. , Reported recurrence rates are between 4% and 10%.
Nasal dermoids are frontonasal inclusion cysts or tracts related to embryologic errors localized to the anterior neuropore. Congenital midline nasal masses occur in 1 in 3000 to 1 in 40,000 live births, and dermoids are by far the most common. Nasal dermoids account for 1% to 3% of all dermoids and approximately 10% to 12% of head and neck dermoids. Most dermoid cysts occur sporadically, with a slight male preponderance, , although familial associations have been reported. Nasal dermoids are typically isolated lesions, but 5% to 41% have associated anomalies, including aural atresia, pinna deformities, developmental delay, hydrocephalus, branchial arch anomalies, cleft lip and palate, hypertelorism, and hemifacial microsomia.
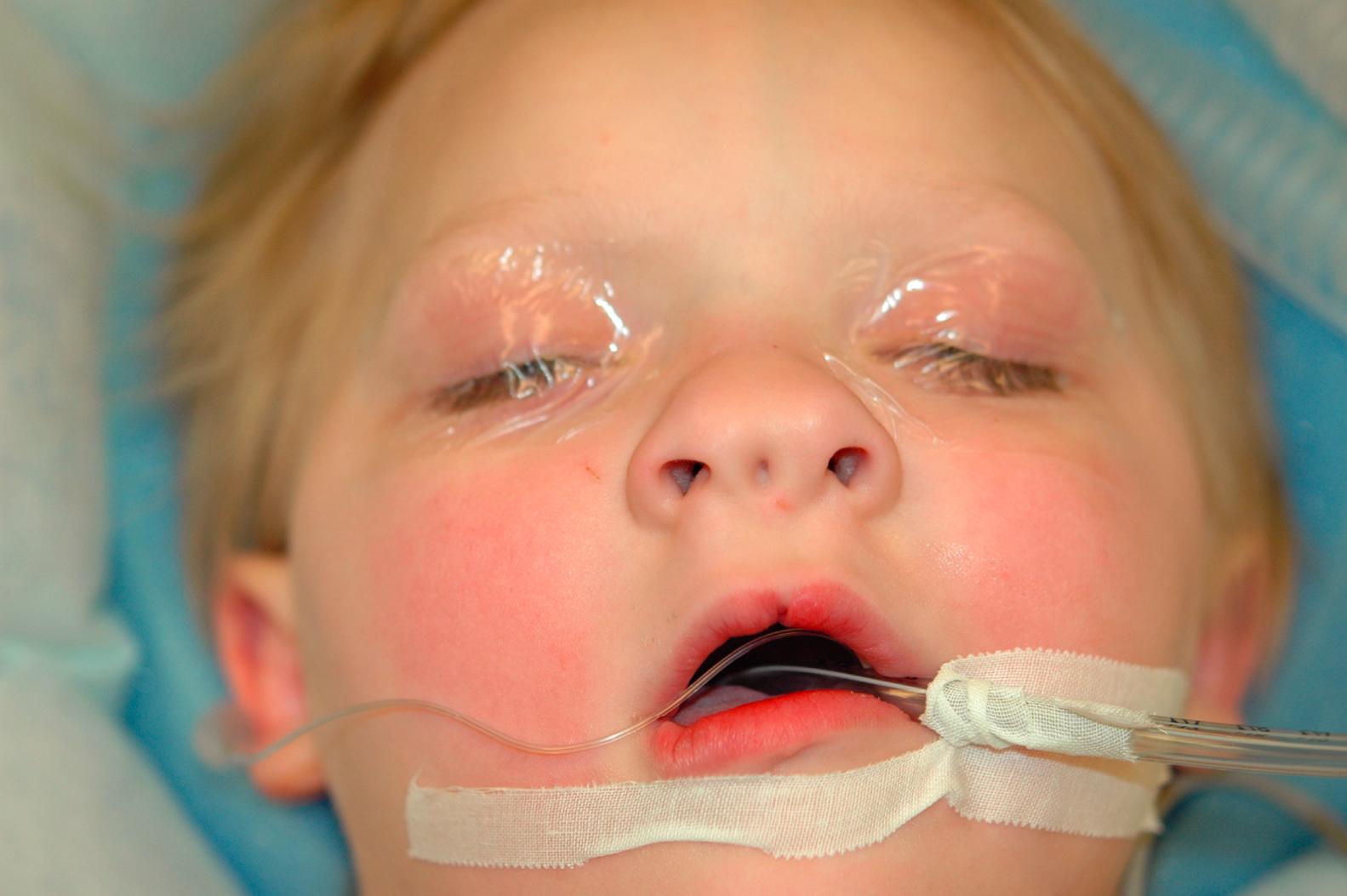
A nasal dermoid usually manifests as a midline pit or mass. In approximately 50% of cases, a dimple is present at or near the rhinion, along with a widened nasal bridge; however, the true spectrum of disease includes cysts, sinuses, or fistulas that may occur anywhere along the embryologic line from the nasal tip into the cranial space. Mass lesions are firm, lobulated, and noncompressible and may be associated with a sinus opening ( Fig. 5.5 ) with intermittent caseous discharge or infection. A protruding hair is pathognomic for a nasal dermoid, although it is seen in only a minority of patients. A lesion within the nasal septum may present as nasal obstruction. The incidence of intracranial extension ranges from 4% to 45%. Recurrent meningitis caused by organisms typical of skin flora may indicate an intracranial tract. Nasal dermoids do not enlarge with crying or straining and do not transilluminate.
Unlike teratomas, which contain all three embryonal germ layers, congenital dermoids contain only ectodermal and mesodermal embryonic elements such as hair follicles, sebaceous glands, and sweat glands. The presence of these elements in the wall of the cyst differentiates dermoids from simple epidermoid cysts. Keratin debris is another prominent feature on histologic examination, and dermoids lack the glial features of encephaloceles and gliomas.
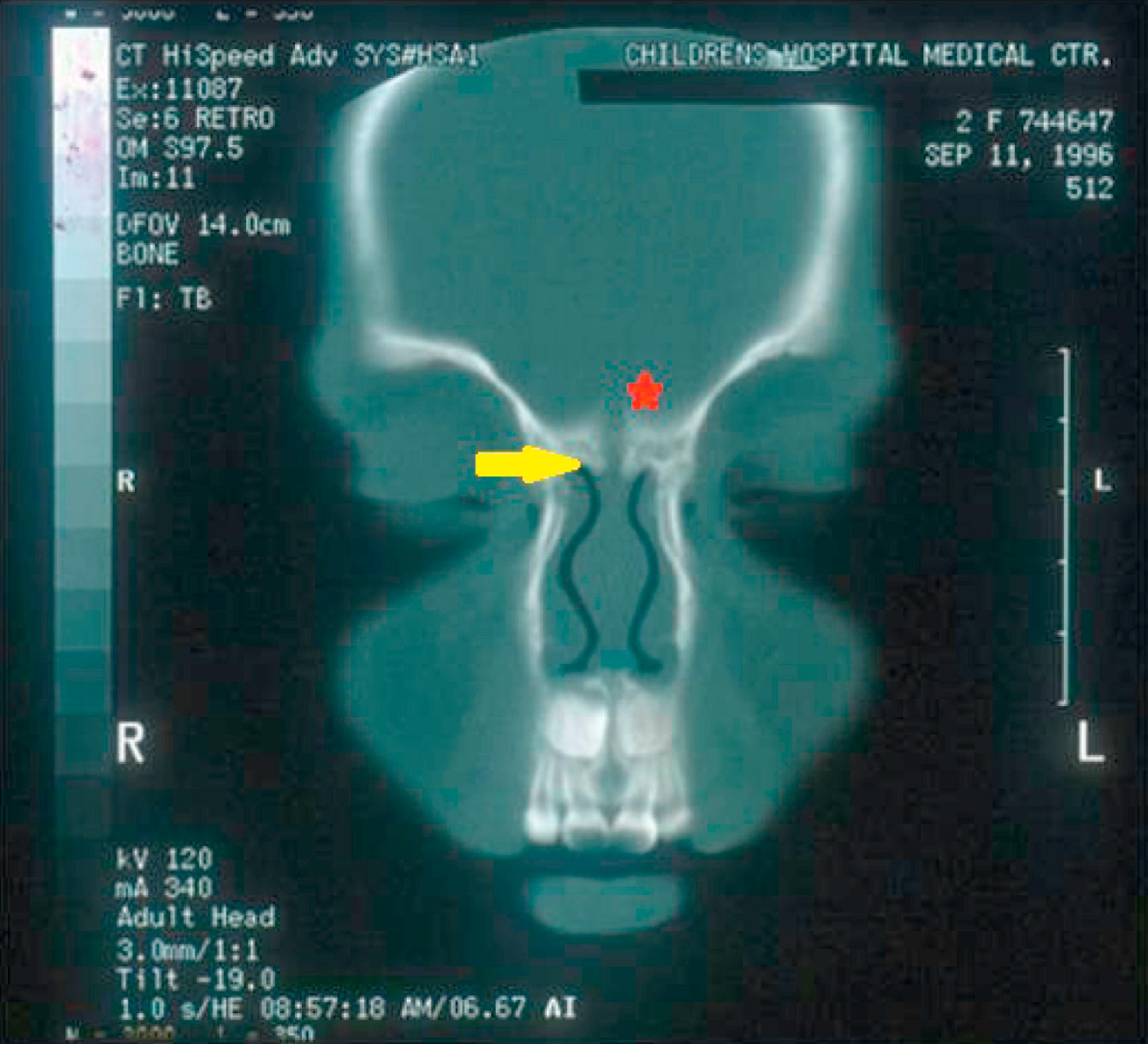
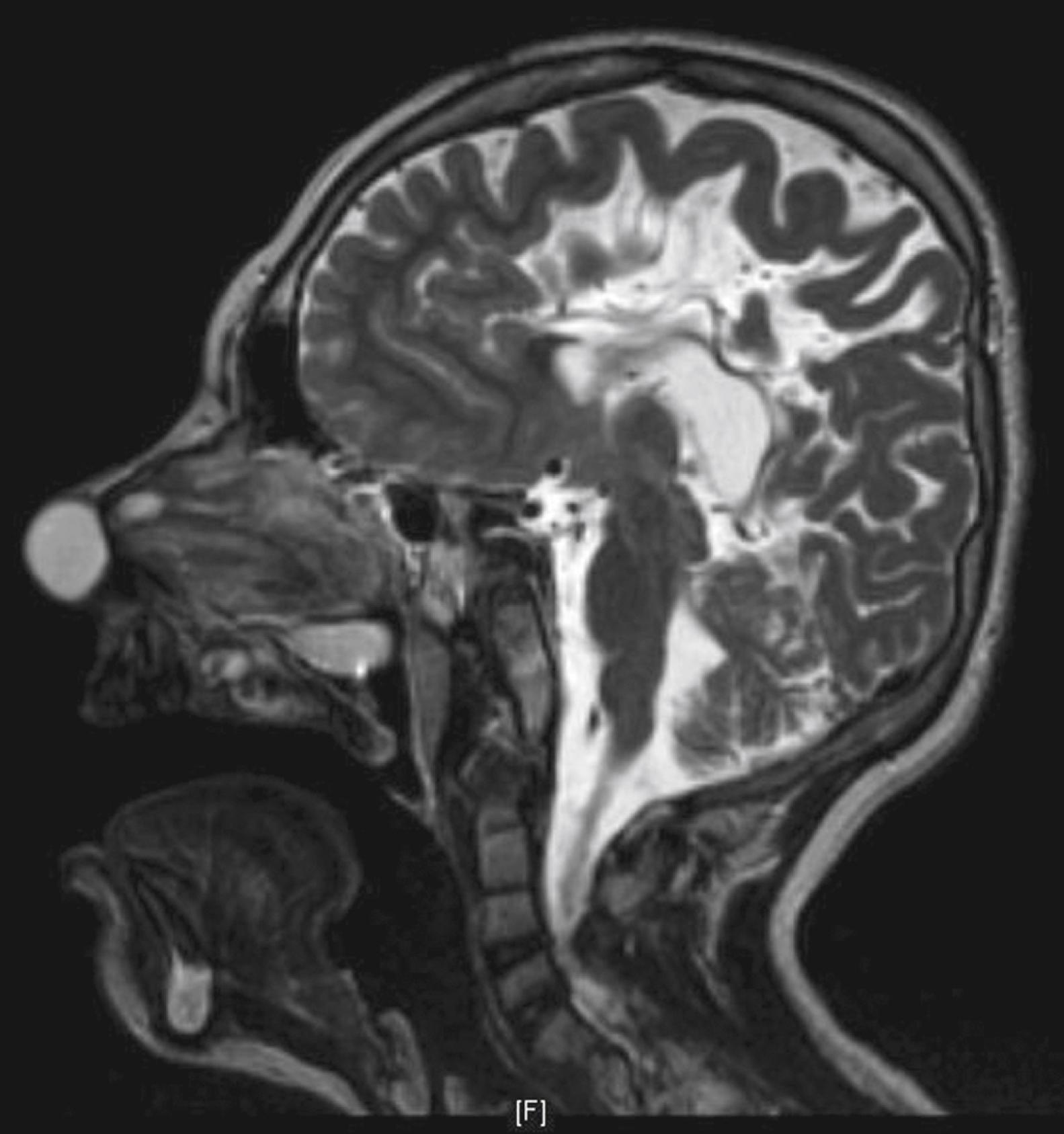
CT and MRI provide complementary information in the radiologic evaluation of nasal dermoids. Thin-slice 1- to 3-mm CT scans with intravenous contrast are recommended to differentiate the dermoid from the surrounding nasal mucosa and to define the bony anatomy of the nose and anterior skull base. Findings unique to intracranial dermoids include a bifid crista galli ( Fig. 5.6 ) and an enlarged foramen cecum; when these structures are normal, intracranial extension is ruled out. Multiplanar thin-section contrast-enhanced MRI ( Fig. 5.7 ) is helpful to depict the soft tissue anatomy of the anterior skull base and to differentiate a dermoid (which does not enhance) from enhancing lesions such as hemangiomas and teratomas. High signal intensity on T1-weighted images is suggestive of an intracranial dermoid because the neonatal crista galli does not contain marrow fat.
As with other lesions of the congenital anterior neuropore, the management of nasal dermoids is surgical. Aspiration, incision and drainage, curettage, and subtotal excision are associated with high recurrence rates and are generally not advocated. It is critical to assess the degree of intracranial extension, and patients with suspected intracranial extension should be prepared for a combined intracranial-extracranial approach with a collaborating neurosurgeon. Extracranial access should fulfill four criteria: (1) excellent access to the midline, (2) access to the base of the skull, (3) adequate exposure for reconstruction of the nasal dorsum, and (4) an acceptable scar. Several different extracranial approaches have been described. The external rhinoplasty incision is the most widely used approach as it generally gives the best cosmetic result and allows access to the skull base and exposure of the nasal dorsum. However, the external rhinoplasty provides only limited access to lesions in the glabellar region; lateral rhinotomy and midline vertical incisions are preferred alternatives. Despite the possibility of a widened scar, glabellar lesions with a sinus opening require an elliptical incision to excise the ostium. A transglabellar subcranial approach, a paracanthal incision, or a bicoronal approach may be used for glabellar lesions without sinus tracts. For lesions that extend into the cranial cavity, frontal craniotomy is performed by a multidisciplinary team. A large frontal craniotomy is typically carried out through a coronal incision using a combined intracranial-extracranial approach; however, anterior small window craniotomies have been described. Some authors suggest that the tract may be adequately treated with suture ligation without craniotomy if frozen section analysis of the tract is negative for epidermal and adnexal structures at the skull base. , , However, others have suggested that epidermal elements may be staggered along the tract such that a single biopsy site is inadequate to rule out intracranial extension. The overall recurrence rate after adequate excision is low; however, long-term follow-up is essential to diagnose the occasional late recurrence.
The eruption of the nasal pits into the choanae, fusion of the palatal shelves, and growth of the nasal septum and soft palate coincide with the development of the lateral nasal wall and primitive sinuses. For normal nasal and paranasal growth to occur, all of these rapid changes must take place with complete precision.
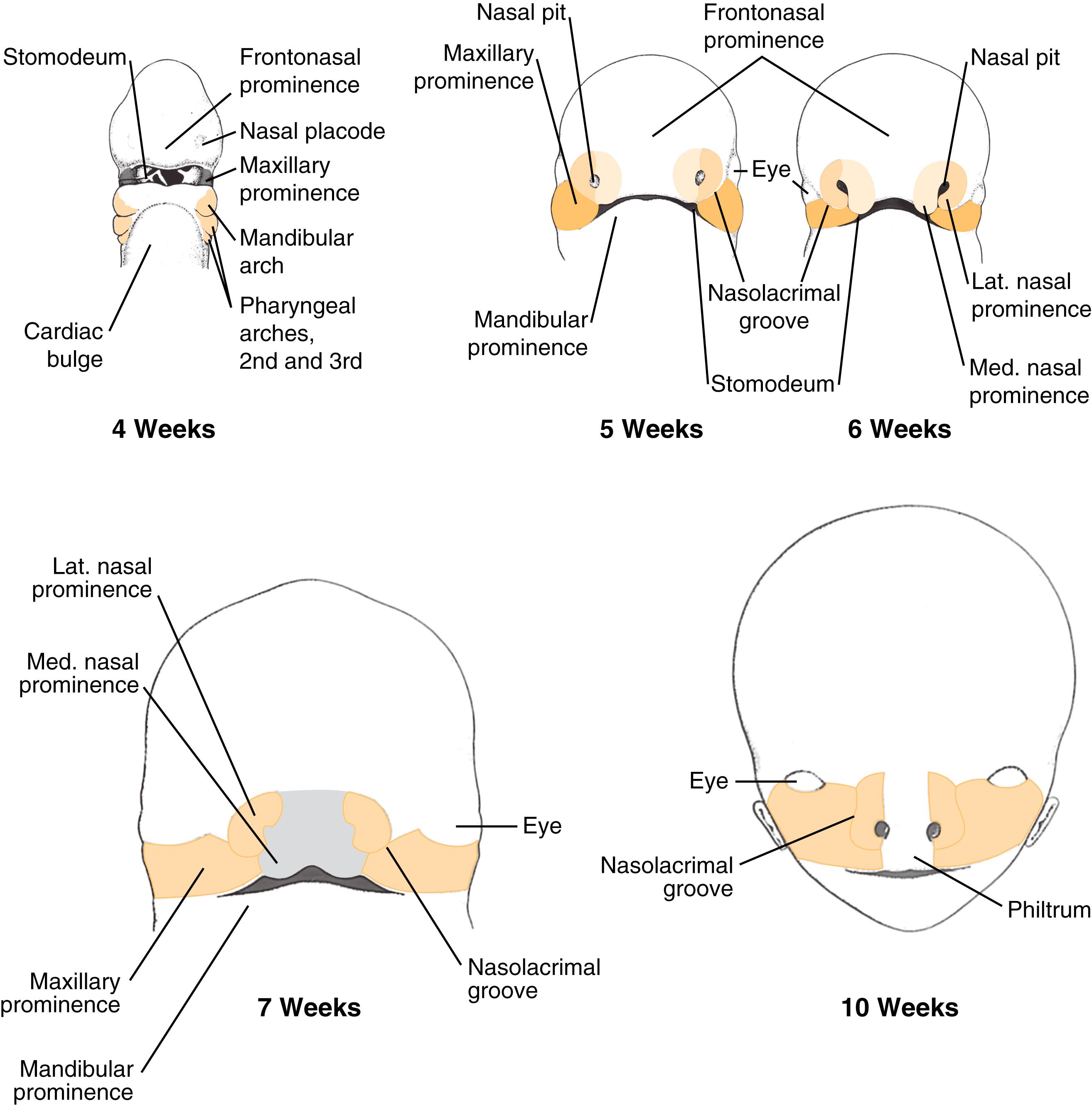
Normal nasal development occurs between weeks 4 and 12 of life. During this time, neural crest cells migrate from their origin in the dorsal neural folds around the eye to form the first and second branchial arch–derived facial prominences surrounding the stomodeum. The stomodeum is flanked by the frontonasal prominence superiorly, the maxillary processes laterally, and the mandibular processes inferiorly. Two small thickenings in the frontonasal prominence known as the nasal placodes begin to burrow, forming nasal pits. By the fifth week, continued invagination creates ridges of tissue around the pit; these are referred to as the lateral and medial nasal prominences. Nasolacrimal duct development begins as a thickening of the ectoderm that becomes buried in the mesoderm of the nasal pits between the lateral nasal prominence and the maxillary process. This buried ectoderm canalizes from superiorly to inferiorly postnatally. The lateral and medial prominences finalize interaction with the developing maxillary process, creating the philtrum and medial lip (from the fused medial nasal prominences) and the lateral upper lip (from the maxillary prominences). The lateral nasal prominences form the nasal alae ( Fig. 5.8 ).
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here