Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
All radiologic contrast media available for intravascular use depend on iodine for their radiopacity. Ideally, contrast media should be inert in every respect. But unlike other therapeutic medications, contrast media are used in larger quantities and participate in numerous physiologic and pharmacokinetic interactions after intravenous administration. Their interactions not only affect tissue characteristics but also may have significant effects on patient health.
The principle of contrast enhancement is based on photoelectric interaction of the iodine atom with x-rays. The binding energy of the inner K shell electron of the iodine atom is called its K-edge and is equal to 33.2 keV. The mean energy of diagnostic radiographs is close to this value. When diagnostic radiographs interact with iodine atoms in the body there is increased absorption of the x-rays by the iodine atoms compared with the surrounding soft tissues. The attenuation of the x-ray beam increases with the concentration of iodine in the tissue because of more K shell interactions. This is the fundamental basis of contrast enhancement. There is a linear relation between iodine concentration and attenuation. Every milligram of iodine in a milliliter of blood or cubic centimeter of tissue elevates the attenuation by 25 Hounsfield units (HU).
All currently used computed tomography (CT) contrast agents are based on the triiodinated benzene ring. Whereas the iodine atom is responsible for the radiopacity of contrast media, the organic carrier is responsible for its other properties, such as osmolality, tonicity, hydrophilicity, and viscosity. The organic carrier is responsible for most of the adverse effects and has received a lot of attention from researchers. Some patients react to small amounts of contrast media, but most of the adverse effects are mediated by the large osmotic load. Thus, over the past few decades researchers have focused on developing contrast media that minimize the osmotic load after contrast agent administration.
Contrast media are classified as ionic or nonionic and as monomers or dimers. Ionic contrast media dissolve in water to dissociate into an iodinated benzene ring containing an iodized carboxyl group and a cation. Nonionic contrast media are water soluble (hydrophilic) but do not dissociate in solution. Monomers have a single triiodinated benzene ring, whereas dimers have a double benzene ring containing six iodine atoms. Figure 8-1 shows the chemical structure of contrast media molecules.
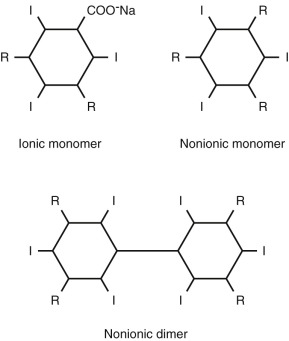
The most commonly used classification of contrast media is by their osmolality, which is defined as the number of osmotically active particles per kilogram of solvent. This is determined by the size of the contrast media molecule and whether it dissociates in solution. Table 8-1 lists commonly used contrast media.
| Classification | Iodine per Particle Ratio | Osmolality (mOsm/kg) |
|---|---|---|
| High osmolar contrast media | 3 : 2 | 1500-1800 |
| Diatrizoate (Hypaque) | ||
| Iothalamate (Conray) | ||
| Nonionic monomer low-osmolar contrast media | 3 : 1 | 600-700 |
| Iohexol (Omnipaque) | ||
| Iomeprol (Iomeron) | ||
| Iopamidol (Niopam) | ||
| Iopromide (Ultravist) | ||
| Ioversol (Optiray) | ||
| Ioxilan (Oxilan) | ||
| Iobitridol (Xenetix) | ||
| Ionic dimer low-osmolar contrast media | 3 : 1 | 560 |
| Ioxaglate (Hexabrix) | ||
| Nonionic dimer isosmolar contrast media | 6 : 1 | 300 |
| Iodixanol (Visipaque) | ||
| Iotrolan (Isovist) |
Ionic monomers consisting of a cation (e.g., sodium, meglumine) and an anion (iodine-containing benzoic acid derivative) were developed in the 1950s. These molecules dissociate in solution to give three atoms of iodine for every two particles in solution (iodine atoms to particle in solution ratio of 3 : 2). The osmolality of these agents ranges from 1500 to 1800 mOsm/kg, whereas that of human plasma is 290 mOsm/kg; thus, they are named high osmolar contrast media . Because of the higher incidence of adverse reactions, these agents are infrequently used today.
Nonionic monomers are widely used today and have better solubility and lower toxicity compared with ionic monomers. Nonionic monomers consist of a benzoic acid derivative containing three iodine atoms per molecule but do not contain an ionizing carboxyl group (iodine atoms to particle in solution ratio of 3 : 1). These particles do not dissociate in solution. Their osmolality is 600 to 700 mOsm/kg (double that of human plasma). Other than eliminating ionicity and reducing osmolality, newer low osmolar contrast media also have an increased number of, and more evenly distributed, hydroxyl groups. This improves their hydrophilicity by restricting access to the lipophilic areas of the molecule, decreasing affinity for cell membrane and plasma proteins and improving tolerance.
Dimeric ionic contrast media contain six atoms of iodine per molecule. They dissociate in solution into an anion (six-iodine atom double-benzene ring) and a cation, yielding a ratio of iodine atoms to particle in solution of 6 : 2 or 3 : 1. The osmolality of ionic dimers is only slightly lower than that of nonionic monomers.
Nonionic dimers have been more recently developed. These molecules consist of a double-benzene ring with six iodine atoms. Because they do not dissociate in solution, they have a ratio of iodine atoms to particle in solution of 6 : 1 and are isosmolar to plasma. The larger molecule size in isosmolar contrast media results in higher viscosity. Nonionic dimers have generally been shown to be less nephrotoxic compared with other compounds, but this is not established.
All currently used iodinated contrast media have low lipid solubility. Contrast media molecules are not metabolized in the body but are excreted by the kidney via glomerular filtration without tubular reabsorption. They have a half-life of 2 hours in patients with normal renal function, and 75% of the dose is excreted within 4 hours of administration.
A multitude of factors determine the enhancement of a particular organ or tissue after contrast media administration. Broadly, these can be divided into organ-specific factors, patient-related factors, and injection-related factors.
The enhancement patterns of organs vary mainly because of differences in vascular supply. Factors that affect enhancement include vascular anatomy, vascular resistance, and percentage of cardiac output received.
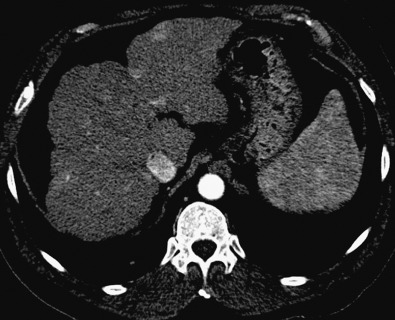
Seventy-five percent of the hepatic blood supply is from the portal venous system, and the remaining 25% is from the hepatic arterial circulation. This unique dual blood supply of the liver can be exploited to bring out the differences between normal parenchyma and pathologic lesions. The hepatic arterial phase occurs 20 to 30 seconds after the start of intravenous contrast injection (or 20- to 30-second delay). During this phase, hepatic tumors that primarily derive blood supply from the hepatic artery “hypervascular tumors” enhance ( Figure 8-2 ). The normal hepatic parenchyma shows only minimal enhancement during this phase. The hepatic parenchyma enhances only after the contrast media pass through the splanchnic or splenic circulation and reach the portal vein. This phase is known as the portal venous phase (60- to 90-second delay). Hypovascular lesions are relatively hypoattenuating compared with the parenchyma during this phase.
After intravenous injection, the contrast media rapidly diffuse along the concentration gradient into the extravascular (interstitial) space. This redistribution occurs by passive diffusion along the concentration gradient until equilibrium is reached in the equilibrium phase (1.5- to 3-minute delay). Contrast media accumulate in the interstitial spaces of the tumor, reducing the attenuation difference with the hepatic parenchyma. The onset of the equilibrium phase depends on injection duration. Because the attenuation difference between tumor and normal parenchyma is lost in the equilibrium phase, hepatic imaging must be completed before the equilibrium phase.
Although pancreas contrast dynamics are not as well defined as for the liver, the enhancement can be divided into arterial, parenchymal, and portal venous phases. The initial arterial phase (20- to 25-second delay) best opacifies the arterial tree and is followed by the pancreatic phase (40- to 45-second delay) when the parenchymal enhancement is maximum. The portal venous phase (60- to 70-second delay) opacifies the portal veins and is also useful for evaluating hepatic metastatic disease. Pancreatic neuroendocrine tumors are hypervascular and are best seen on arterial phase, whereas the peak tumor to parenchymal attenuation difference for hypovascular malignancies (e.g., adenocarcinoma) is best appreciated later during the parenchymal or portal venous phase. Most practices use a biphasic pancreatic protocol, with some variation in the exact timing.
The kidney has unique enhancement characteristics because of the greater vascularity of the renal cortex compared with the medulla, which results in greater enhancement of the renal cortex compared with the medulla during the corticomedullary phase (25- to 35-second delay). Small renal tumors can be obscured by this differential enhancement. A more homogeneous enhancement occurs during the nephrographic phase (90- to 110-second delay), which is primarily used to detect renal parenchymal tumors. A more delayed excretory phase (up to 8- to 10-minute delay) is used to opacify the collecting system and ureter and also in CT urography.
In a healthy patient, the only significant factor that determines the amount of iodine required to achieve desired enhancement is body weight. Patient body weight is inversely related to hepatic enhancement, and the total iodine dose needs to increase with increasing body weight to achieve optimal enhancement. Certain comorbid conditions, such as cardiac failure, can alter circulation time and increase the time required to achieve maximal aortic and hepatic enhancement.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here