Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
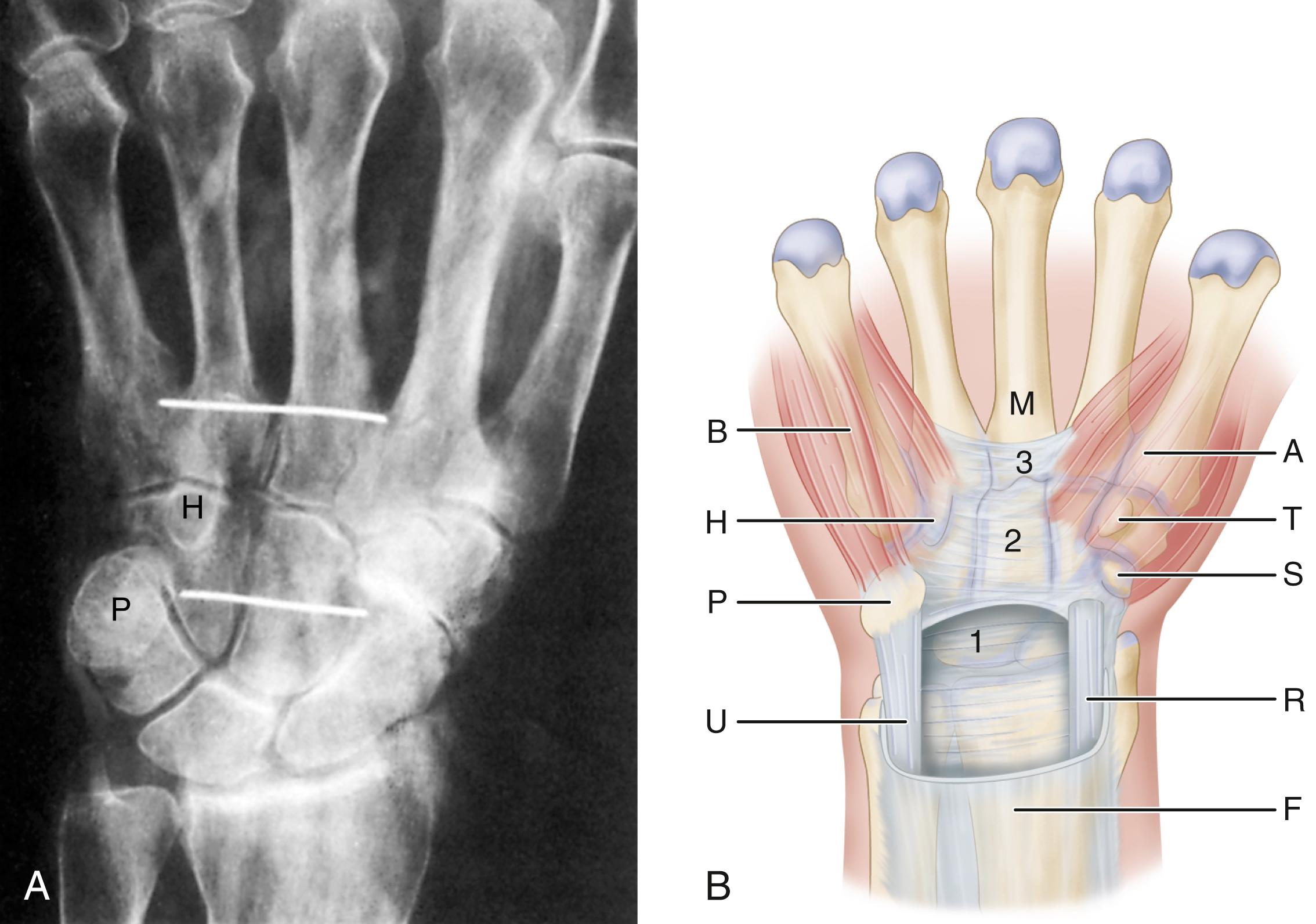
Carpal tunnel syndrome, described by Paget in 1854, is the most common upper extremity compression neuropathy and results from median nerve compression within the carpal tunnel. The carpal tunnel is bound by the carpal bones arching dorsally; the hook of the hamate and the pisiform medially; and the scaphoid tubercle and trapezial ridge laterally. The palmar aspect, or “roof,” of the carpal tunnel is formed by the flexor retinaculum, consisting of the deep forearm fascia proximally, the transverse carpal ligament (TCL) over the wrist, and the aponeurosis between the thenar and hypothenar muscles distally. The most palmar structure in the carpal tunnel is the median nerve. Lying dorsal (deep) to the median nerve in the carpal tunnel are the nine long finger and thumb flexor tendons.
Carpal tunnel syndrome is primarily a clinical diagnosis, with symptoms of tingling and numbness in the typical median nerve distribution (thumb, index, long, and radial side of ring fingers). Pain, described as deep, aching, or throbbing, occurs diffusely in the hand and may radiate up the forearm. Thenar muscle atrophy usually is seen in late-stage nerve compression. It occurs most often in patients 30 to 60 years old and is two to three times more common in women than in men. Carpal tunnel syndrome may affect 1% to 10% of the U.S. population. Older, overweight, and physically inactive individuals are more likely to develop carpal tunnel syndrome, and female sex, obesity, cigarette smoking, and vibrations associated with job tasks have been identified as carpal tunnel risk factors in industrial workers.
Elevation of carpal tunnel pressures of more than 20 to 30 mm Hg impedes epineurial blood flow, and nerve function is impaired. Reduction in cross-sectional area along the length of the carpal tunnel may result from various conditions such as malaligned Colles fractures, edema from infection or trauma, tumors or tumorous conditions, and other space-occupying lesions. Distal radial fracture immobilization with the wrist in marked flexion and ulnar deviation can cause acute median nerve compression immediately after reduction. Systemic conditions, such as obesity, diabetes mellitus, thyroid dysfunction, amyloidosis, rheumatoid arthritis, and Raynaud disease, sometimes are associated with the syndrome. Occasionally, a patient has carpal tunnel syndrome symptoms caused by a habitual sleeping posture in which the wrist is kept acutely flexed. Trauma caused by repetitive hand motions has been identified as a possible aggravating factor, especially in patients whose work requires repeated forceful finger and wrist flexion and extension. Laborers using vibrating machinery also are at risk. The causative effect of light, repetitive activities experienced by office workers is controversial and unresolved. Many factors are implicated in the causation and aggravation of carpal tunnel syndrome ( Box 77.1 ).
Bony abnormalities of the carpal bones
Acromegaly
Flexion or extension of wrist
Forearm and wrist fractures (Colles fracture, scaphoid fracture)
Dislocations and subluxations (scaphoid rotary subluxation, lunate volar dislocation)
Posttraumatic arthritis (osteophytes)
Musculotendinous variants
Aberrant muscles (lumbrical, palmaris longus, palmaris profundus)
Local tumors (neuroma, lipoma, multiple myeloma, ganglion cysts)
Persistent medial artery (thrombosed or patent)
Hypertrophic synovium
Hematoma (hemophilia, anticoagulation therapy, trauma)
Diabetes mellitus
Alcoholism
Double-crush syndrome
Exposure to industrial solvents
Rheumatoid arthritis
Gout
Nonspecific tenosynovitis
Infection
Pregnancy
Menopause
Eclampsia
Thyroid disorders (especially hypothyroidism)
Renal failure
Long-term hemodialysis
Raynaud disease
Obesity
Lupus erythematosus
Scleroderma
Amyloidosis
Paget disease
Vibration
Direct pressure
When carpal tunnel syndrome occurs in pregnant women, the symptoms usually resolve after delivery. Aberrant muscles of the forearm and thrombosis of the median artery also may contribute to median nerve compression. The cause, however, in most patients is idiopathic, its direct association with work is difficult to substantiate, and involvement of the nondominant hand is frequent.
In children, carpal tunnel syndrome is unusual. Macrodactyly, lysosomal storage diseases, and a strong family history of carpal tunnel syndrome may be predisposing factors in children. Symptoms in children may be confusing and include decreased dexterity and diffuse pain. Findings such as thenar muscle atrophy and weakness suggest that the condition is severe by the time of presentation. The Phalen test and Tinel sign may be absent if the nerve compression has been present for a long time. Carpal tunnel syndrome frequently is associated with nonspecific tenosynovial edema and rheumatoid tenosynovitis, as are trigger finger and de Quervain disease. Flexor tendon synovium biopsy specimens from patients with idiopathic carpal tunnel syndrome show benign fibrous tissue without inflammatory changes. The tenosynovium in patients with carpal tunnel disease shows increased fibroblast density, collagen fiber size, vascular proliferation, and more type III collagen fibers than controls.
Patients with carpal tunnel syndrome usually have symptoms of numbness, pain, or paresthesia in the median nerve distribution.
Paresthesia in the median nerve sensory distribution is the most frequent symptom, often awakening patients with burning and numbness of the hand that is relieved by exercise. The Tinel sign also may be shown in most patients by percussing the median nerve at the wrist. Atrophy to some degree of the median-innervated thenar muscles has been reported in about half of patients treated by operation. Acute flexion of the wrist for 60 seconds (Phalen test) or strenuous use of the hand increases the paresthesia in some but not all patients.
Evaluation of the clinical usefulness of several commonly used provocative tests, including wrist flexion, nerve percussion, and the tourniquet test, found the most sensitive test to be the wrist flexion test, whereas nerve percussion was the most specific and the least sensitive. Gellman et al. found that with the wrist in neutral position, the mean pressure within the carpal tunnel in patients with carpal tunnel syndrome was 32 mm Hg. This pressure increased to 99 mm Hg with 90 degrees of wrist flexion and to 110 mm Hg with the wrist at 90 degrees of extension. The pressures in the control subjects were 25 mm Hg with the wrist in neutral position, 31 mm Hg with the wrist in flexion, and 30 mm Hg with the wrist in extension.
A carpal compression test (Durkan test), in which direct compression is applied to the median nerve for 30 seconds with the thumbs or an atomizer bulb attached to a manometer, was found to be more specific (90%) and more sensitive (87%) than either the Tinel or Phalen test. Szabo et al. evaluated the validity of tests for carpal tunnel syndrome, including Phalen wrist flexion, Tinel nerve percussion, Durkan compression, and Semmes-Weinstein monofilaments. Grip and pinch strength, a hand diagram, and patient symptoms were assessed. Durkan nerve compression, the hand diagram score, night pain, and Semmes-Weinstein testing after a Phalen test had the highest sensitivity. The most specific tests were the hand diagram and Tinel sign. These authors concluded that a patient with an abnormal hand diagram, a positive Durkan test, abnormal Semmes-Weinstein sensibility testing, and night pain had a very high probability of having carpal tunnel syndrome. Conversely, they found that if all four of the just-mentioned examinations were normal, the probability of the patient having carpal tunnel syndrome was very low.
Sensibility testing in peripheral nerve compression syndromes found that threshold tests of sensibility correlated accurately with symptoms of nerve compression and electrodiagnostic studies. Semmes-Weinstein monofilament pressure testing was the most accurate in determining early nerve compression. A combination of the Semmes-Weinstein monofilament test with the wrist flexion test for a “quantitative provocational” diagnostic test was reported to have 82% sensitivity and 86% specificity.
According to some authors, electrodiagnostic studies including nerve conduction velocities and electromyography (EMG) are reliable confirmatory tests. A distal motor latency of more than 4.5 milliseconds (ms) and a sensory latency of more than 3.5 ms are considered abnormal. EMG may show increased insertional activity, positive sharp waves, fibrillations at rest, decreased motor recruitment, and complex repetitive discharges indicative of nerve damage. These studies are occasionally normal, however, even in patients with classic clinical signs and symptoms of carpal tunnel syndrome are present. Similarly, electrodiagnostic tests may be abnormal in asymptomatic patients. Nerve conduction studies are reported to be 90% sensitive and 60% specific for the diagnosis of carpal tunnel syndrome. They also are helpful in evaluating the upper extremity for nerve compression at the elbow, axilla, and cervical spine and for showing changes of peripheral neuropathy. Studies have shown, however, that electrodiagnostic testing provides no significant data for prediction of functional recovery or reemployment after carpal tunnel release, nor does it increase the diagnostic value of the four commonly used tests (i.e., abnormal hand diagram, abnormal Semmes-Weinstein testing, positive Durkan compression, and night pain). These findings, combined with reported false-negative rates of 10%, limit the usefulness of this type of testing to determine treatment. Postoperative electrodiagnostic testing may be helpful in assessing recurrent symptoms. The various tests for nerve compression in the carpal tunnel are summarized in Table 77.1 .
| TEST | HOW PERFORMED | CONDITION TESTED | POSITIVE RESULT | INTERPRETATION OF POSITIVE RESULT |
|---|---|---|---|---|
| Phalen test | Elbows on table, forearms vertical, wrists flexed | Paresthesia in response to position | Numbness or tingling on radial digits within 60 s | Probable CTS (sensitivity 0.75, specificity 0.47) |
| Percussion test (Tinel sign) | Lightly tap along median nerve from proximal to distal | Site of nerve lesion | “Electric” tingling response in fingers | Probable CTS if positive at the wrist (sensitivity 0.60, specificity 0.67) |
| Carpal tunnel compression test (Durkan) | Direct compression of median nerve at carpal tunnel | Paresthesia in response to compression | Paresthesia within 30 s | Probable CTS (sensitivity 0.87, specificity 0.90) |
| Hand diagram | Patient marks site of pain or altered sensation on outlined hand diagram | Patient’s perception of symptoms | Markings on palmar side of radial digits, without markings in palm | Probable CTS (sensitivity 0.96, specificity 0.73, negative predictive value 0.91) |
| Hand volume stress test | Hand volume measured by displacement, repeat after 7-min stress test and 10-min rest | Hand volume | Hand volume increased by ≥10 mL | Probable dynamic CTS |
| Direct measurement of carpal tunnel pressure | Wick or infusion catheter placed in carpal tunnel | Hydrostatic pressure in resting and provocative positioning | Resting pressure ≥25 mm Hg (variable and technique related) | Hydrostatic compression is probable cause of CTS |
| Static two-point discrimination | Determine minimal separation of two distinct points when applied to palmar fingertip | Innervation density of slow-adapting fibers | Failure to determine separation of at least 5 mm | Advanced nerve dysfunction |
| Moving two-point discrimination | As above, with movement of the points | Innervation density of fast-adapting fibers | Failure to determine separation at least 4 mm | Advanced nerve dysfunction |
| Vibrometry | Vibrometer placed on palmar side of digit, amplitude at 120 Hz, increased to threshold of perception; compare median and ulnar bilaterally | Threshold of fast-adapting fibers | Asymmetry compared with contralateral hand or median to ulnar in ipsilateral hand | Probable CTS (sensitivity 0.87) |
| Semmes-Weinstein monofilaments | Monofilaments of increasing diameter touched to palmar side of digit until patient can determine which digit is touched | Threshold of slowly adapting fibers | Value >2.83 | Median nerve impairment (sensitivity 0.83) |
| Distal sensory latency and conduction velocity | Orthodromic stimulus and recording across wrist | Latency, conduction velocity of sensory fibers | Latency >3.5 ms or asymmetry of conduction velocity >0.5 m/s vs. opposite hand | Probable CTS |
| Distal motor latency and conduction velocity | Orthodromic stimulus and recording across wrist | Latency, conduction velocity of motor fibers of median nerve | Latency >4.5 ms or asymmetry of conduction velocity >1 m/s | Probable CTS |
| Electromyography | Needle electrodes placed in muscle | Denervation of thenar muscles | Fibrillation potentials, sharp waves, increased insertional activity | Advanced motor median nerve compression |
Reports of MRI in carpal tunnel syndrome are promising, especially with newer techniques such as diffusion tensor imaging, but MRI is not routinely used for diagnosis. A major advantage of MRI is its high soft-tissue contrast, which gives detailed images of bones and soft tissues. Ultrasound sensitivity for carpal tunnel syndrome has been reported to be over 97% when the median nerve diameter is greater than 10 mm 2 at the level of the pisiform and is suggested to be a useful diagnostic technique by some authors. In patients with negative electrodiagnostic studies but a clinical diagnosis of carpal tunnel syndrome, high-resolution ultrasonography has been used to diagnose carpal tunnel, with a sensitivity of 73% if the cutoff of 9.4 mm 2 at the inlet of the carpal tunnel is used. Nonetheless, the diagnosis of carpal tunnel syndrome should be based on clinical acumen and physical examination in the vast majority of patients, and ancillary tests should be reserved for patients without clear presentations.
If mild symptoms have been present and there is no thenar muscle atrophy, the use of night splints and injection of cortisone preparations into the carpal tunnel may provide temporary relief, but long-term benefit is obtained in only about 10% of patients treated with corticosteroid injection and splinting. The response to injection treatment has been reported to be faster in men and in patients older than 40 years old. Care should be taken not to inject directly into the nerve. Injection also can be used as a diagnostic tool in patients without osteophytes or tumors in the canal. Most of these cases are probably caused by a nonspecific synovial edema, and these seem to respond more favorably to injection. Injection also helps to eliminate the possibility of other syndromes, especially cervical disc or thoracic outlet syndrome. Some patients prefer to receive injections two or three times before a surgical procedure is done. If the symptoms and physical findings improve, and there is no muscle atrophy, conservative treatment with splinting and injection is reasonable.
In a study of 331 patients with carpal tunnel syndrome, Kaplan, Glickel, and Eaton identified five important factors in determining the success of nonoperative treatment: (1) age older than 50 years, (2) duration longer than 10 months, (3) constant paresthesia, (4) stenosing flexor tenosynovitis, and (5) a positive Phalen test result in less than 30 seconds. Two thirds of patients were cured by medical treatment when none of these factors was present; 59.6% were cured when one factor was present; and 83.3% when two factors were noted. Of patients with three factors, 93.2% did not experience any improvement. No patient with four or five factors was cured by medical management.
Patients with intermediate and advanced (chronic) syndromes probably are better treated with early carpal tunnel release. Extensive neurolysis has not been shown to have any significant effect. Internal neurolysis does not improve the motor or sensory outcome of carpal tunnel release. Moreover, epineurotomy offers no clinical benefit to carpal tunnel release. Treatment of acute carpal tunnel syndrome should be individualized, depending on its cause. For carpal tunnel syndrome caused by an acute increase in carpal tunnel pressure (e.g., after a Colles fracture treated with flexed wrist immobilization), relief may be obtained by a change in wrist position without surgical release of the tunnel.
Patients with florid tenosynovitis caused by rheumatoid arthritis or other inflammatory conditions are managed by tenosynovectomy at the time of carpal tunnel release. The palmaris longus opponensplasty (Camitz) may be beneficial, particularly in an elderly patient with thenar muscle wasting, weakness, and poor opposition. Trapeziometacarpal arthroplasty and carpal tunnel release can be done safely through two incisions.
Idiopathic carpal tunnel syndrome in the pediatric population is uncommon; however, night pain, hand clumsiness, and thenar atrophy may be the initial findings as these patients rarely present with complaints of sensory disturbance. Congenital bone abnormalities, hypothyroidism, lysosomal storage disease, and myopathic contractures account for some of the predisposing etiologies in this age group.
If signs and symptoms are persistent and progressive, especially if they include thenar atrophy, then a carpal tunnel release should be performed. The results of surgery are good in most instances, and benefits seem to last in most patients. Maximal improvement is seen in the first 6 months after carpal tunnel release. After 6 months, there is no significant improvement in the Tinel and Phalen tests, pinch strength, motor latency, symptom severity, or functional scoring. Although thenar atrophy may disappear, it resolves slowly, if at all. Surgical release might not achieve complete relief of all symptoms for patients older than 70 years or those with advanced nerve compression. When symptoms of median nerve compression develop during treatment of an acute distal radial fracture, the constricting bandages and cast should be loosened, and the wrist should be extended to neutral position. When median nerve symptoms persist after a distal radial fracture and have not improved after positional change, surgery usually is indicated.
Despite variable recovery periods, the results of carpal tunnel release in patients with idiopathic carpal tunnel syndrome with intermittent symptoms usually are uniformly successful; however, carpal tunnel release in diabetic and nondiabetic patients was shown to be similarly beneficial in a prospective study with a follow-up period of 6 months. When the same patients were followed for long-term (10 years), however, patients with diabetes had worse surgical outcomes compared with patients with idiopathic carpal tunnel syndrome when using the self-administered Boston Questionnaire to assess symptom severity and functional status. According to Roh et al., patients with a metabolic syndrome were found to have only a delay in the recovery process. Metabolic syndrome was defined by the presence of at least three of the five criteria: a clinical diagnosis of diabetes or hypertension or use of antihypertensive medication; elevated plasma triglyceride level (150 mg/dL or higher); decreased high-density lipoprotein cholesterol levels (less than 50 mg/dL for females or less than 40 mg/dL for males); increased waist size (greater than 80 cm for females or 90 cm for males); and body mass index of more than 30. These authors found that metabolic syndrome was related to a more severe grade of carpal tunnel syndrome and was a risk factor for delayed functional recovery at up to 6 months’ follow-up, yet significant improvements in symptom severity and hand function were similar in both groups after 12 months, except for pinch strength being higher in the control group. Similar findings were noted by Kronlage and Menendez when the results of carpal tunnel release were compared among moderate and severe electrophysiologic carpal tunnel syndrome patients. Patients with moderate disease (prolonged sensory and motor latencies) had nearly complete resolution of pain and paresthesias at 3 months after surgery, whereas those with severe disease (prolonged sensory or motor latencies plus either absent sensory or mixed nerve action potential or low amplitude or absent compound motor action potential), despite considerable improvement, had prolonged and incomplete symptom reduction at 1 year after carpal tunnel release.
Worker’s compensation patients do well with carpal tunnel release overall; however, surgeon expectations for improvement similar to that in non–worker’s compensation patients should be cautious. In addition, patients should be counseled that outcomes may be delayed or inferior compared with non–worker’s compensation patient. Pallis et al. found that worker’s compensation patients had three times the number of complications and nearly twice the rate of persistent pain. They also took 5 weeks longer to return to work and were 16% more likely to not return to preinjury vocation.
The surgical technique chosen for median nerve decompression should be tailored by the surgeon’s expertise. Although minimally invasive techniques purport earlier return to work and less postoperative discomfort, the results suggest that outcomes are similar at 6 months. In a meta-analysis of high-level evidence (randomized controlled trials), Sayegh and Strauch found that endoscopic release allows earlier return to work and improved strength during the early postoperative period. Results at 6 months or later were similar according to current data except that patients with endoscopic release are at greater risk of nerve injury and lower risk of scar tenderness compared with open release. The authors concluded that, although endoscopic release may appeal to patients who require an early return to work and activities, surgeons should be cognizant of its elevated incidence of transient nerve injury amid its similar overall efficacy to open carpal tunnel release. Seiler et al. showed that since the introduction of endoscopic carpal tunnel release the frequency of vascular injuries has decreased; however, nerve injuries have not declined, and this should be taken into consideration when determining treatment options.
Publications have described ultra-minimally invasive techniques, such as ultrasound- guided carpal tunnel release and looped-thread carpal tunnel release. We have limited experience with these treatment options. A single study by Lytie et al. of 159 hands treated with a modified looped-thread technique showed improved short- and long-term Boston Carpal Tunnel Syndrome Questionnaire responses compared with the available data for open or endoscopic carpal tunnel release. Apard and Candelier reviewed ultrasound-guided carpal tunnel release and made several recommendations: the practitioner attempting these techniques (1) must be able to identify all structures clearly, (2) must be aware of all the possible intraoperative and postoperative complications, and (3) must be able to treat the complications appropriately.
Limited approaches, such as the “double incision” of Wilson ( Fig. 77.1A ) and the “minimal incision” of Bromley ( Fig. 77.1B ), may offer rapid recovery as ascribed to the endoscopic techniques. Similarly, the use of the “carpal tunnel tome” through a small palmar incision is a technical modification that may minimize the soft-tissue trauma of the traditional open technique and provides adequate exposure in most cases. Regardless of the technique selected, all structures to be incised should be seen and identified and safety of the median nerve verified before carpal tunnel release ( Fig. 77.2 ).
Mark the planned surgical incision with a skin pen so that the longitudinal incision begins just distal to the distal wrist flexion crease and slightly ulnar to the midline of the wrist (center dot reference point) and extends distally approximately 2.0 to 3.0 cm in line with the third web space ( Fig. 77.3A ). (Note: only rarely is it necessary to extend the incision into the distal forearm.)
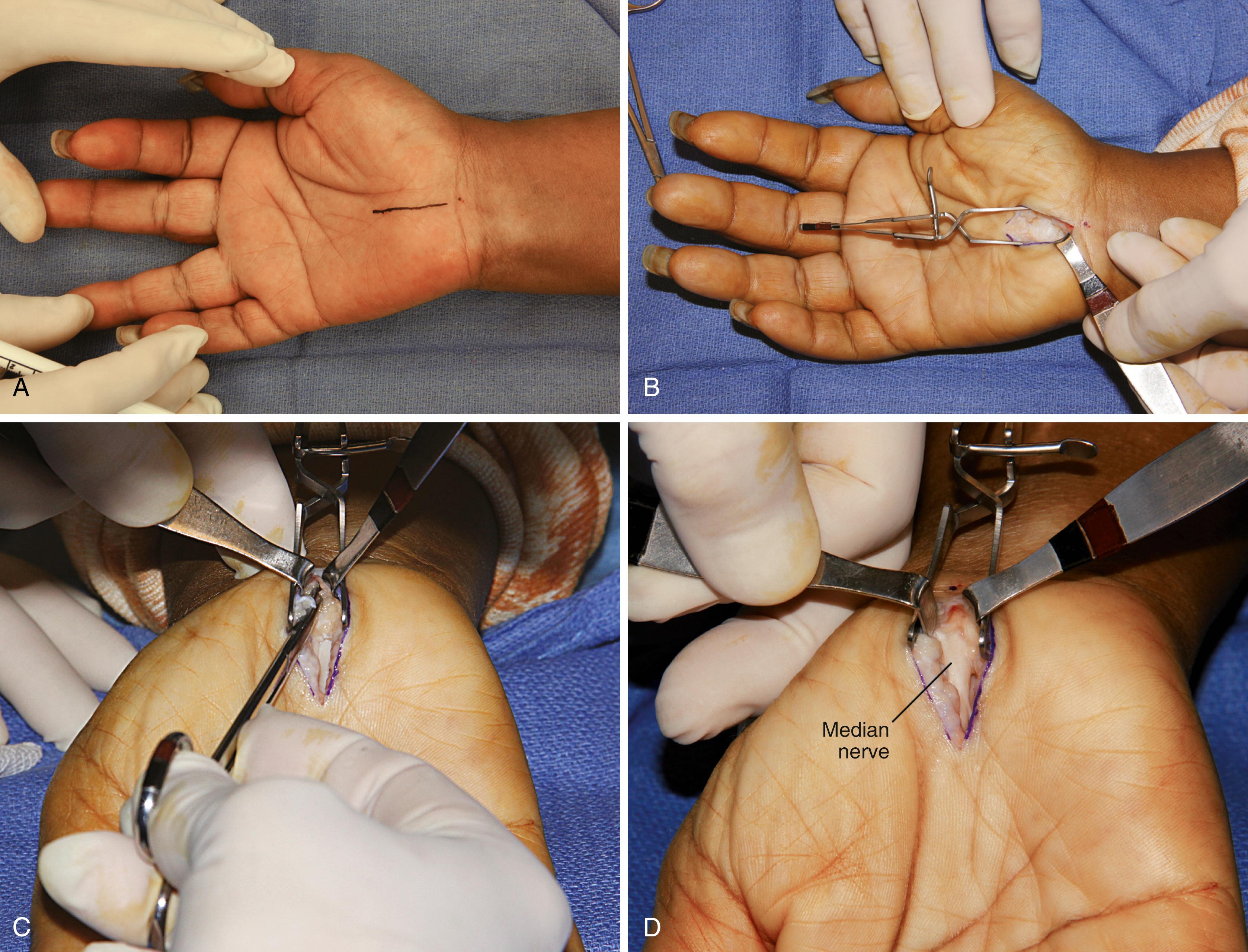
Exposure of the transverse carpal ligament (TCL) requires splitting of the parallel palmar fascia fibers and ulnar retraction of the hypothenar fat ( Fig. 77.3B ). Frequently, intrinsic muscles obscure the midline of the TCL and can be released from their origin and reflected away from the underlying TCL.
Carefully open the carpel tunnel by division of the TCL with a no. 15 blade. The TCL division should be such that 3 to 4 mm of it is left attached to the hamate hook to avoid flexor tendon ulnar subluxation. Make sure the contents of the carpal tunnel are not adherent to the undersurface of the TCL by gently spreading with a blunt instrument such as a mosquito hemostat the remaining portions of the distal and proximal portions of the undi vided TCL and antebrachial fascia. The distal 2.0 cm of the antebrachial fascia can then be safely divided with blunt-tipped Metzenbaum or Mayo scissors ( Fig. 77.3C ).
If the median nerve is adherent to the divided radial TCL leaf ( Fig. 77.3D ), external neurolysis may be needed.
Close the incision in routine fashion ( Fig. 77.3E ) and apply a compressive dressing ( Fig. 77.3Fto H ).
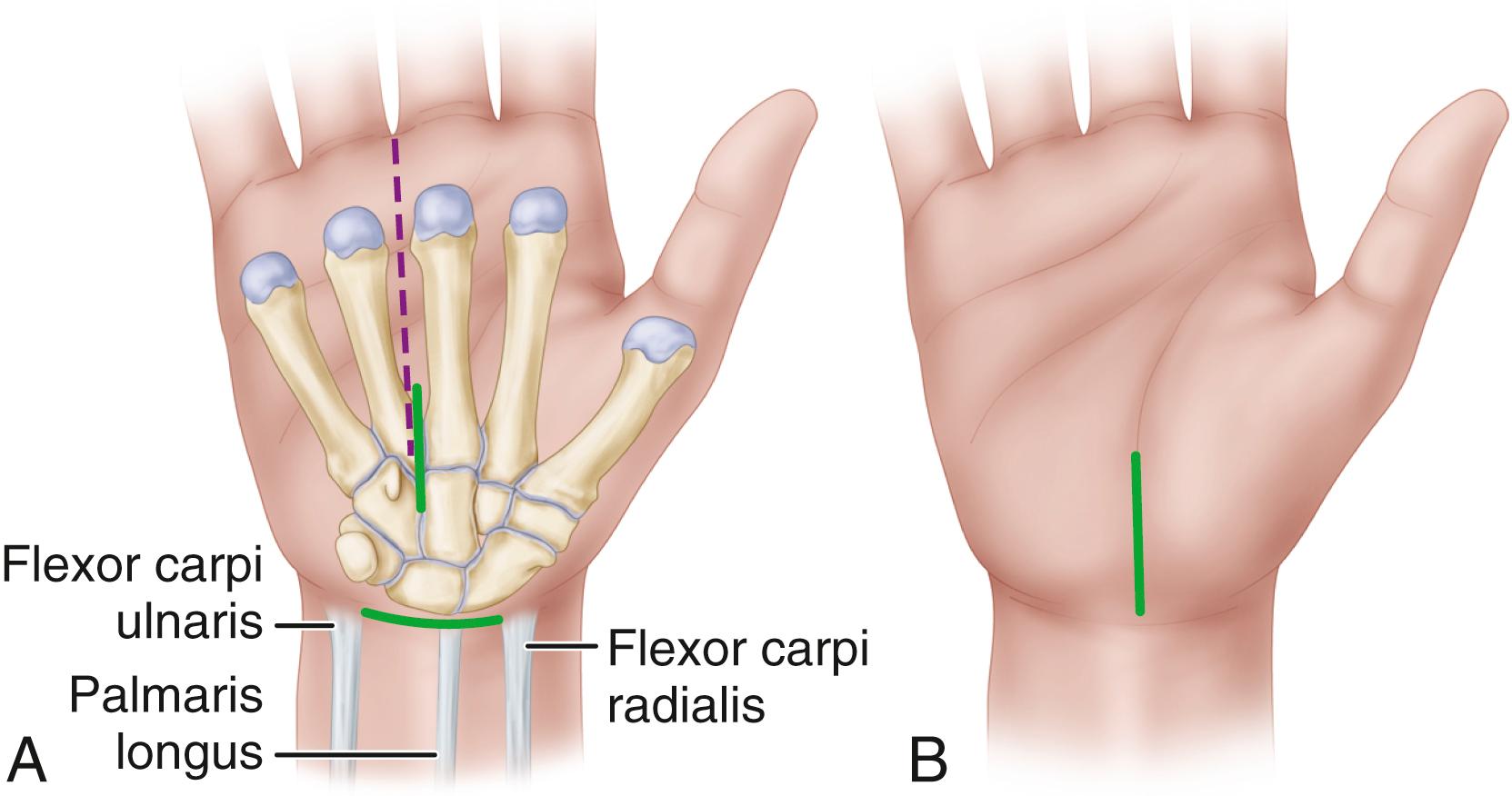
The thenar crease takes a variable course, and palmar incisions should be well ulnar to it to avoid the median nerve palmar cutaneous branch. A curved incision ulnar and parallel to the thenar crease is not advisable because the palmar cutaneous branch of the median nerve proximally may be more at risk of injury. We prefer to use the incision described for the mini-palm technique (see Technique 77.1).
Extend the incision proximally to the flexor crease of the wrist, where it can be continued farther proximally if necessary. Angle the incision toward the ulnar side of the wrist to avoid crossing the flexor creases at a right angle, but especially to avoid cutting the palmar cutaneous sensory branch, which lies in the interval between the palmaris longus and the flexor carpi radialis tendons ( Fig. 77.4 ). Maintain longitudinal orientation so that the incision is generally to the ulnar side of the long finger axis or radial border of the ring fourth ray. When severed, the palmar sensory branch frequently causes a painful neuroma that may later require excision from the scar. Should this nerve be severed, we do not attempt to repair it but section it more proximally to be covered by the middle finger sublimis muscle.
Incise and reflect the skin and subcutaneous tissue.
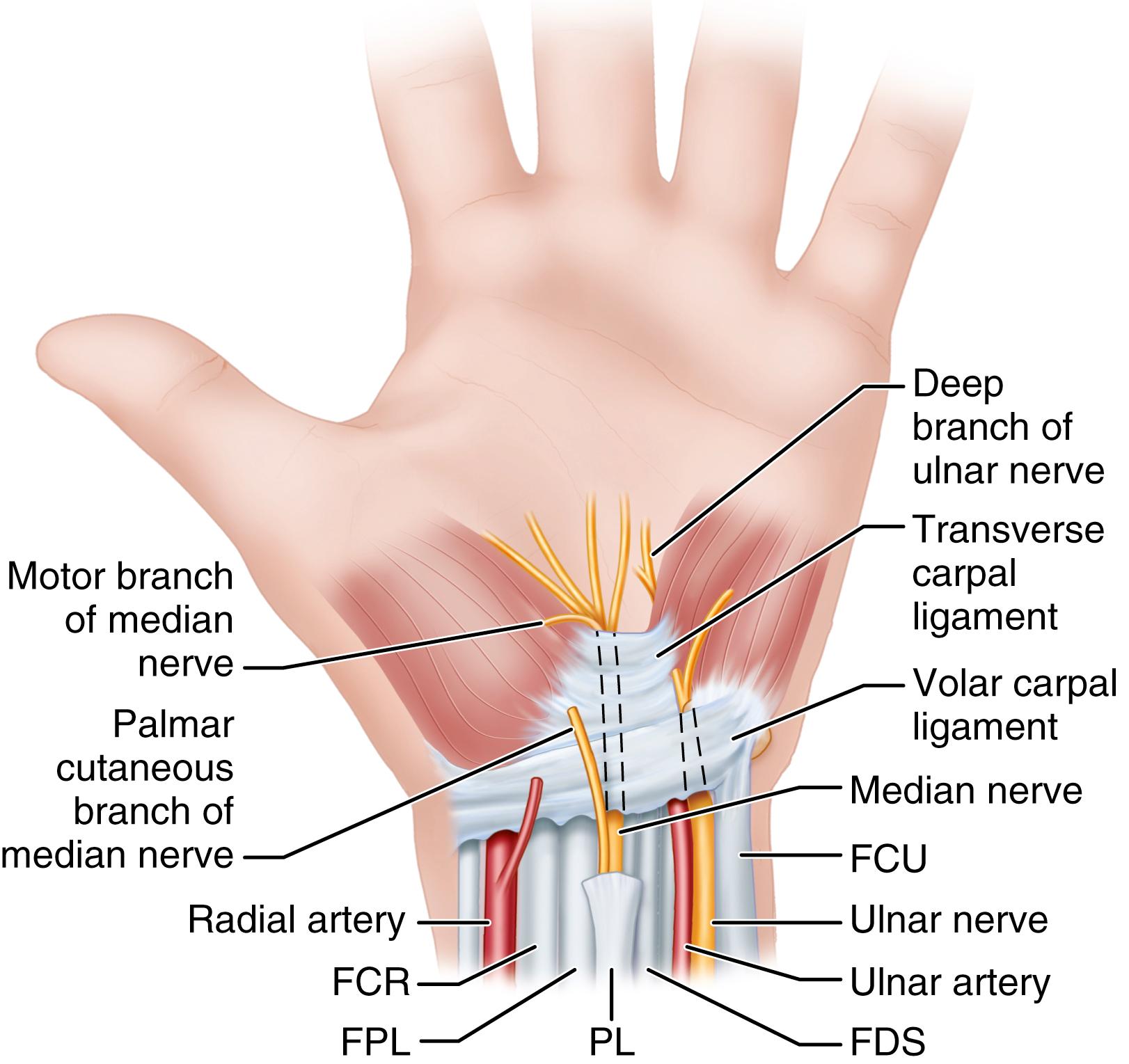
Identify the palmar fascia from the wrist flexion crease distally and the distal forearm antebrachial fascia proximally by subcutaneous blunt dissection. Split the palmar fascia, and expose the underlying transverse carpal ligament (TCL), avoiding the median nerve beneath it.
Identify the TCL, and carefully divide it and avoid damage to the median nerve and its recurrent branch, which may perforate the ligament and leave the median nerve on the volar side ( Fig. 77.5 ). Fibers of the TCL can extend distally farther than expected ( Fig. 77.6 ).
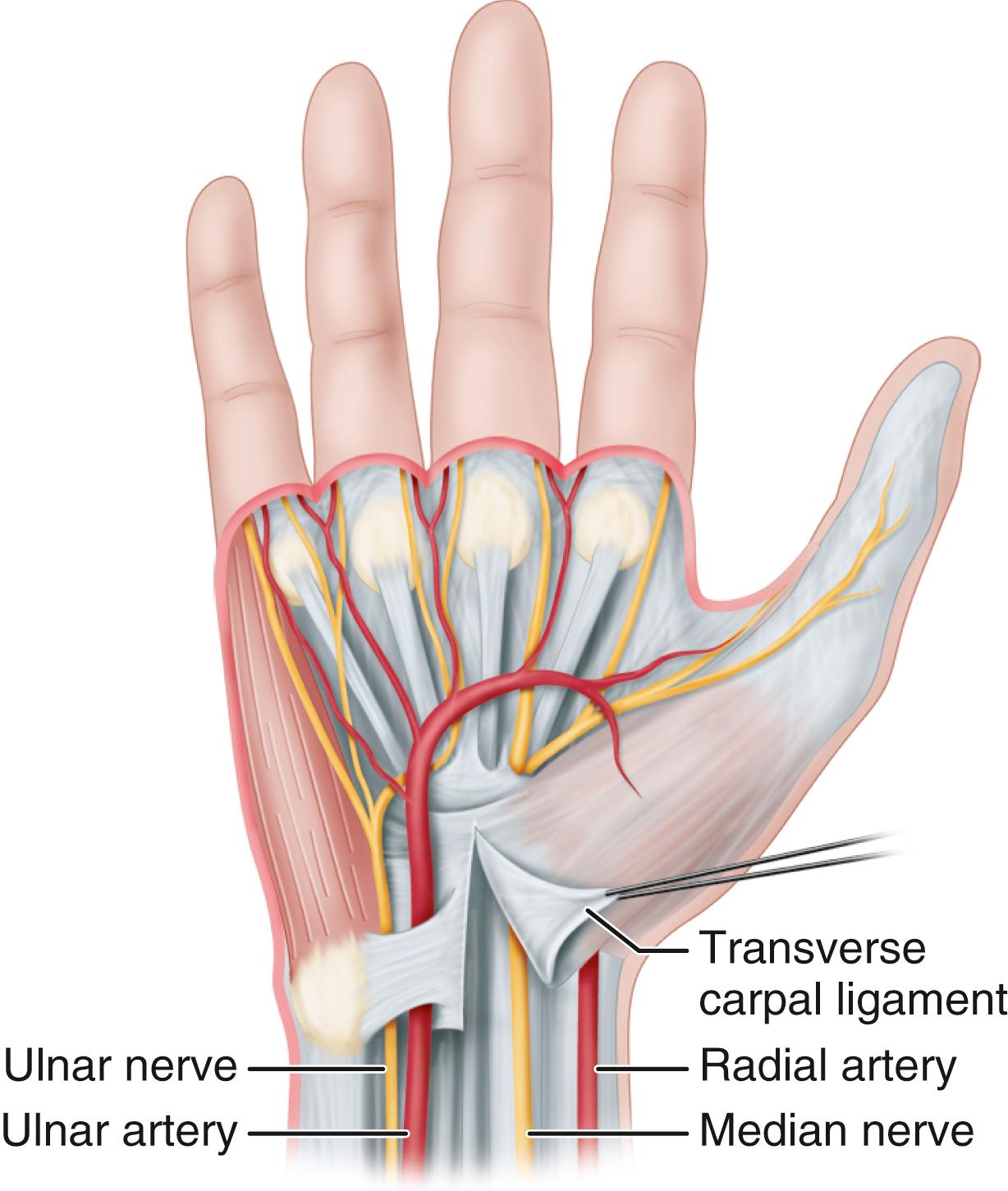
The flexor retinaculum includes the distal deep fascia of the forearm proximally, the TCL, and the aponeurosis between the thenar and hypothenar muscles. A successful carpal tunnel release usually requires division of all these components.
Be aware of potential anomalies: connections between the flexor pollicis longus and the index flexor digitorum profundus tendons; anomalous flexor digitorum superficialis; palmaris longus, hypothenar, lumbrical muscle bellies; and median and ulnar nerve branches and interconnections.
Avoid injury to the superficial palmar arterial arch, which is 5 to 8 mm distal to the distal margin of the TCL.
Inspect the flexor tenosynovium. Tenosynovectomy occasionally may be indicated, especially in patients with rheumatoid arthritis.
Close only the skin and drain the wound as needed.
A light compression dressing and a volar splint may be applied. The hand is actively used as soon as possible after surgery, but the dependent position is avoided. Usually the dressing can be removed by the patient at home 2 or 3 days after the surgery, and then gentle washing and showering of the hand is permitted. Gradual resumption of normal hand use is encouraged. The sutures are removed after 10 to 14 days. A splint may be continued for comfort as needed for 14 to 21 days.
Advocates of endoscopic carpal tunnel release cite less palmar scarring and ulnar “pillar” pain, rapid and complete return of strength, and return to work and activities at least 2 weeks sooner than for open release. Some studies comparing open and endoscopic carpal tunnel release found no significant differences in function. The advantages of the endoscopic technique in grip strength and pain relief are realized within the first 12 weeks and seem to benefit those patients not involved in compensable injuries. Anecdotal reports of intraoperative injury to flexor tendons; to median, ulnar, and digital nerves; and to the superficial palmar arterial arch emphasize the need to exercise great care and caution when performing the endoscopic procedure. Cadaver studies have shown the close proximity of the median and ulnar nerves, superficial palmar arterial arch, and flexor tendons to the endoscopic instruments. Problems related to endoscopic carpal tunnel release include (1) a technically demanding procedure; (2) a limited visual field that prevents inspection of other structures; (3) the vulnerability of the median nerve, flexor tendons, and superficial palmar arterial arch; (4) the inability to control bleeding easily; and (5) the limitations imposed by mechanical failure. Agee, McCarroll, and North developed the following 10 guidelines for the single-incision endoscopic technique to prevent injury to the carpal tunnel structures:
Know the anatomy.
Never overcommit to the procedure.
Ascertain that the equipment is working properly.
If scope insertion is obstructed, abort the procedure.
Ascertain that the blade assembly is in the carpal tunnel and not in Guyon’s canal.
If a clear view cannot be obtained, abort the procedure.
Do not explore the carpal canal with the scope.
If the view is not normal, abort the procedure.
Stay in line with the ring finger.
“When in doubt, get out.”
Although this technique has proved to be effective, it may not be applicable to every patient with carpal tunnel syndrome. If an endoscopic release cannot be accomplished safely, the procedure should be converted to an open technique.
There are various equipment manufacturers, but the two methods can be divided into single-portal (Chow) and two-portal (Agee) techniques. According to Chow, contraindications to endoscopic carpal tunnel release include the following: (1) the patient requires neurolysis, tenosynovectomy, Z-plasty of the TCL, or decompression of Guyon’s canal; (2) the surgeon suspects a space-occupying lesion or other severe abnormality of the muscles, tendons, or vessels in the carpal tunnel; and (3) the patient has localized infection or severe hand edema, or the vascular status of the upper extremities is tenuous. Fischer and Hastings added the following contraindications to the use of endoscopic technique: (1) revision surgery for unresolved or recurrent carpal tunnel syndrome; (2) anatomic variation in the median nerve, suggested by clinical findings of wasting in the abductor pollicis brevis without significant median sensory changes; and (3) previous tendon surgery or flexor injury that would cause scarring in the carpal tunnel, preventing the safe placement of the instruments for endoscopic carpal tunnel release. Additionally, limitation of wrist extension is another contraindication to an endoscopic procedure because the endoscopic instruments cannot be introduced into the carpal tunnel and remain juxtaposed to the dorsal surface of the TCL. The general scheme of the techniques is shown in Figures 77.7 and 77.8 . Before any surgeon attempts endoscopic carpal tunnel release, thorough familiarization with the technique through participation in “hands-on” laboratory practice sessions is recommended.
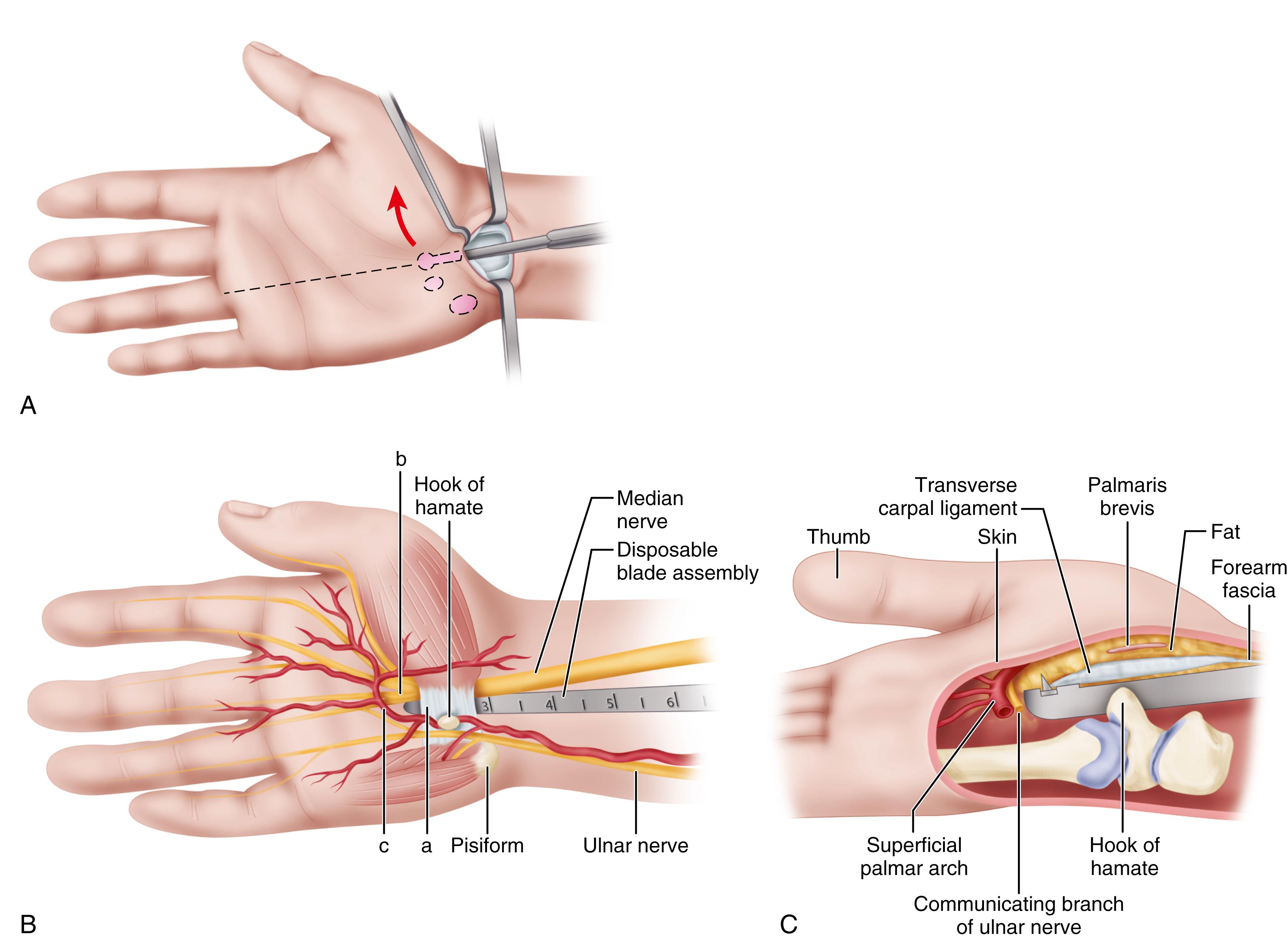
Endoscopic carpal tunnel release often has been performed with sedation or even general anesthesia. More recently, however, Tulipan et al. compared endoscopic carpal tunnel release using local anesthesia alone with the same procedure with local anesthesia and sedation. They found equal satisfaction and outcomes, indicating that the endoscopic technique can be done in a procedure room.
(Agee)
Ascertain that the operating room setup is satisfactory. Ensure there is an unobstructed view of the patient’s hand and the television monitor.
Use general or regional anesthesia. Although the procedure can be done safely using local anesthesia, the increase in tissue fluid can compromise endoscopic viewing.
Exsanguinate the limb with an elastic wrap and inflate a pneumatic tourniquet applied over adequate padding. Leave the arm exposed distal to the tourniquet.
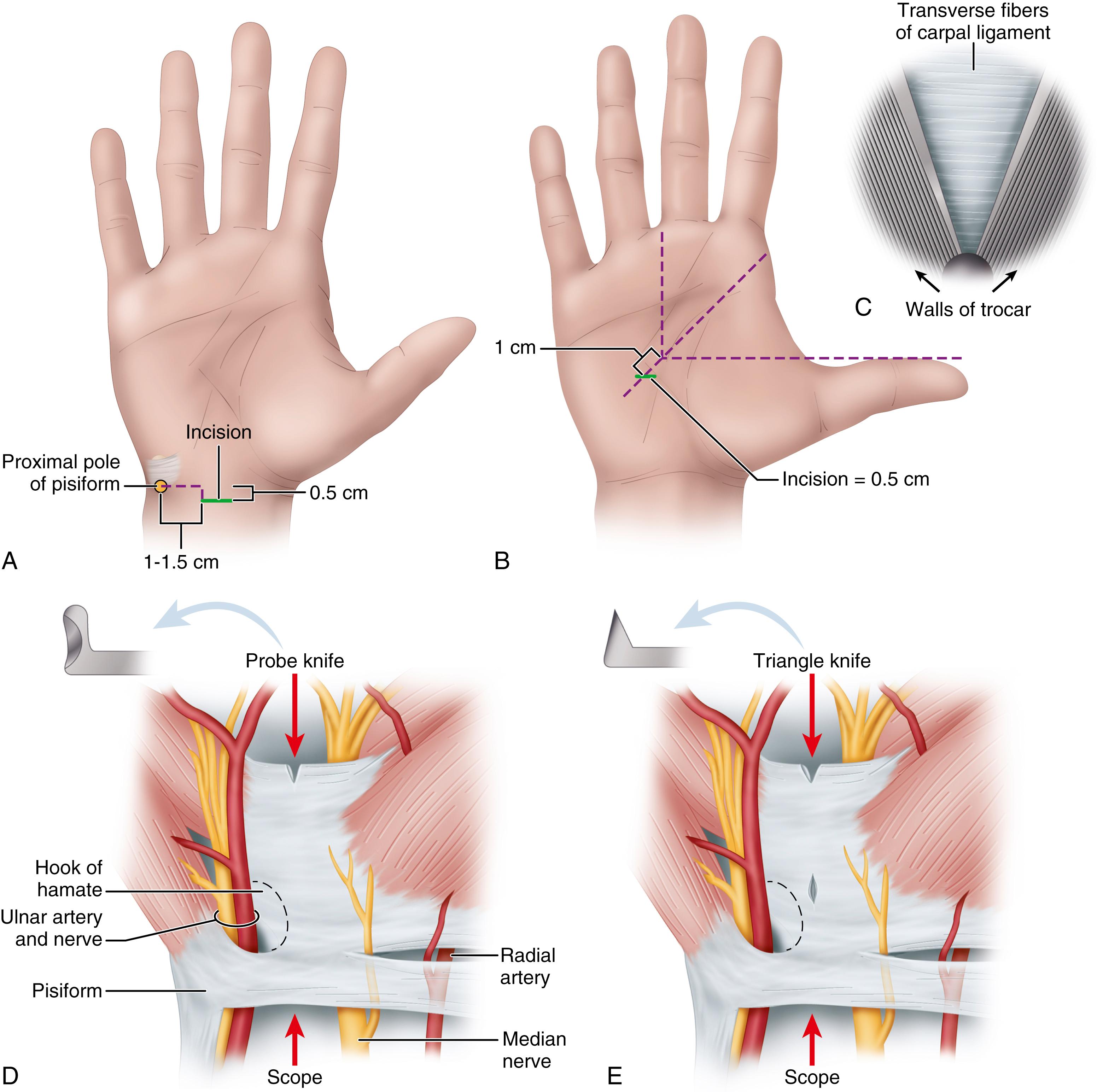
In a patient with two or more wrist flexion creases, make the incision in the more proximal crease between the tendons of the flexor carpi radialis and flexor carpi ulnaris ( Fig. 77.7A ).
Use longitudinal blunt dissection to protect the subcutaneous nerves and expose the forearm fascia.
Incise and elevate a U-shaped, distally based flap of forearm fascia ( Fig. 77.7B ), and retract it palmarward to facilitate dissection of the synovium from the deep surface of the ligament, creating a mouth-like opening at the proximal end of the carpal tunnel.
When using the tunneling tools and the endoscopic blade assembly, keep them aligned with the ring finger, hug the hook of the hamate, and keep the tools snugly apposed to the deep surface of the transverse carpal ligament (TCL), maintaining a path between the median and ulnar nerves for the instruments.
Use the synovium elevator to scrape the synovium from the deep surface of the TCL. Extend the wrist slightly; insert the blade assembly to the carpal tunnel, pressing the viewing window snugly against the deep surface of the TCL ( Fig. 77.7C ). While advancing the blade assembly distally, maintain alignment with the ring finger and hug the hook of the hamate, staying to the ulnar side. Make several proximal-to-distal passes to define the distal edge of the TCL with the fat overlying it.
Define the distal edge of the TCL by viewing the video picture, ballottement, and light transilluminated through the skin. Correctly position the blade assembly and touch the distal end of the ligament with the partially elevated blade to judge its entry point for ligament division. Elevate the blade and withdraw the device, incising the ligament.
Fat from the proximal palm may compromise endoscopic viewing by protruding through the divided proximal half of the ligament, leaving an oil layer on the lens. Avoid this by first releasing only the distal one half to two thirds of the ligament ( Fig. 77.7D ).
Using the unobstructed path for reinsertion of the instrument, accurately complete the distal ligament division with good viewing. Complete proximal ligament division with a final proximal pass of the elevated blade.
Assess the completeness of ligament division using the following endoscopic observations.
Through the endoscope, note that the partially divided ligament separates on the deep surface, creating a V-shaped defect ( Fig. 77.7E ).
Make subsequent cuts viewing the trapezoidal defect created by complete division as the two halves of the ligament spring apart. Through this defect, observe the longitudinal palmar fascia fibers intermingled with fat and muscle. Force these structures to protrude by pressing on the palmar skin.
Confirm complete division by rotating the blade assembly in radial and ulnar directions, noting that the edges of the ligament abruptly “flop” into the window, obstructing the view.
Palpate the palmar skin over the blade assembly window, observing motion between the divided TCL and the more superficial palmar fascia, fat, and muscle.
Ensure complete median nerve decompression by releasing the forearm fascia with tenotomy scissors ( Fig. 77.7F ).
Use small right-angle retractors to view the fascia directly, avoiding nerve and tendon injury ( Fig. 77.7G ).
Close the incision with subcuticular or simple stitches.
Apply a nonadhering dressing. Apply a well-padded volar splint, or, in selected patients, leave the wrist unsplinted.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here