Physical Address
304 North Cardinal St.
Dorchester Center, MA 02124
Every clinical and public health decision has ethical components that are at times difficult to recognize and process. As John Glaser noted, there are “no ethics free zones.” , In the context of clinical care, ethical awareness is as essential as pathophysiology to bringing about a successful patient outcome. For the physician assistant (PA), the stage is set for complex ethical dilemmas, given their role in decision making and leadership. Also, being a dependent practitioner embedded within a complex health care team is apt for ethical dilemmas or uncertainty. Given the ubiquitous nature of ethical issues, all clinicians must familiarize themselves with ethical analysis and decision making. The study and application of ethics are not reserved for ethics consultation services: Significant and complex ethical conundrums emerge in all clinical settings with great frequency, and thus each provider will need to have a foundation in moral reasoning to assist in achieving excellent patient care. This chapter provides that foundation and is germane for PAs at all levels of their career.
This chapter constructs a foundation in ethics by introducing relevant historical and contemporary ethics cases alongside the methods of clinical ethics analysis. The organization of this chapter is unique in that we deliver the majority of ethics learning objectives through case-based reasoning. This case-based methodology relies less on formal theories, although we will discuss them intermittently throughout. Our goal is to provide practicing clinicians with a general understanding of some common ethical issues that one might see in a variety of practice settings. Through this broad survey, we hope to provide a foundational understanding of certain ethical terms, principles, and theories that are generalizable to other situations. Given this textbook’s diverse readership, the case studies presented highlight issues for the PA as a student, educator, clinician, and leader.
Clinical ethics is the practiced discipline that offers an organized system of recognition, evaluation, and methods to resolve ethical issues that arise in the practice of medicine. Those practicing ethics specialize in areas such as business, policy, and bioethics. The specialty of bioethics focuses on moral dilemmas as they intersect with biology and the policies and practices of medicine. Encompassed within this broad field are queries within the areas of clinical ethics, public health, and research ethics. This text is predominately focused on clinical ethics but introduces public health and research ethics. Ethical uncertainties and dilemmas are ubiquitous regardless of clinical setting or specialty. When a clinician is asked to identify bioethical cases, she or he might turn to visible and often deliberated end-of-life issues. This period in the life course engenders ethical issues such as futility, the right to refuse, surrogate decision making, and physician-assisted suicide. Additional oft-mentioned ethical dilemmas, such as conflict of interest, decision-making capacity, and informed consent, are equally visible. Nevertheless, many of the more prevalent ethical issues are less palpable. These include ethical questions such as: How much time will you spend with a patient? Should you prescribe a less effective treatment because it does not require insurance company prior authorization or costs less? Will you penalize patients who are late, unvaccinated, or nonadherent with a treatment plan?
The literature on theories of clinical ethics is vast, ranging from applications of standard ethical theories, such as consequentialism, to virtue and newer interpretations of narrative ethics. Although these established theories are useful and valuable to ethical decision making, our preference is to demonstrate how patient care often requires PAs to take a more comprehensive approach that also considers the values, principles, and concepts specific to a particular patient. This is different from the theory-based approach that most authors use that focuses on a central value or values. Many of our readers are well aware of the “four principles” of health care ethics : autonomy, nonmaleficence, beneficence, and justice. Nevertheless, we believe that focusing on only principles is too narrow for clinical ethics. Thus, throughout our case, presentations, discussion, and analysis, we invoke a larger array of principles, theories, concepts, and values. This method provides readers with a broader depth of knowledge of ethics and practical application in addressing ethical issues.
To assist with decision making and the resolution of complex ethics cases that arise during the daily care of patients, many individuals have developed frameworks and case analysis methodologies. Our preference in methodology is a hybrid that includes three methods. , , The core of our method is the approach developed by Kladjian et al., which views ethics cases with a reasoning process similar to all clinical encounters ( Fig. 36.1 ). This methodology provides a systematic process that readers can use to address ethical conflicts or uncertainties they face in their daily practice.

We will use the following case to illustrate our preferred methodology. Mrs. Roberts is a 68-year-old woman with metastatic colon cancer that has spread to her liver and lungs. She is bedridden and is currently residing in her daughter’s home. She recently was hospitalized for a severe case of pneumonia. The patient is unable to speak for herself because she also has end-stage dementia. The critical care PA approaches the patient’s next of kin, her daughter Regina, about resuscitation status. Regina states resoundingly, “I want her to be resuscitated no matter what.” Mrs. Roberts never completed an advance directive and has no other living family members. The providers are concerned with resuscitating Mrs. Roberts and they state, “CPR [cardiopulmonary resuscitation] cannot bring Mrs. Roberts any clinical benefit.” The clinical staff believe that resuscitation is futile and do not want to perform it. The surrogate wants CPR performed.
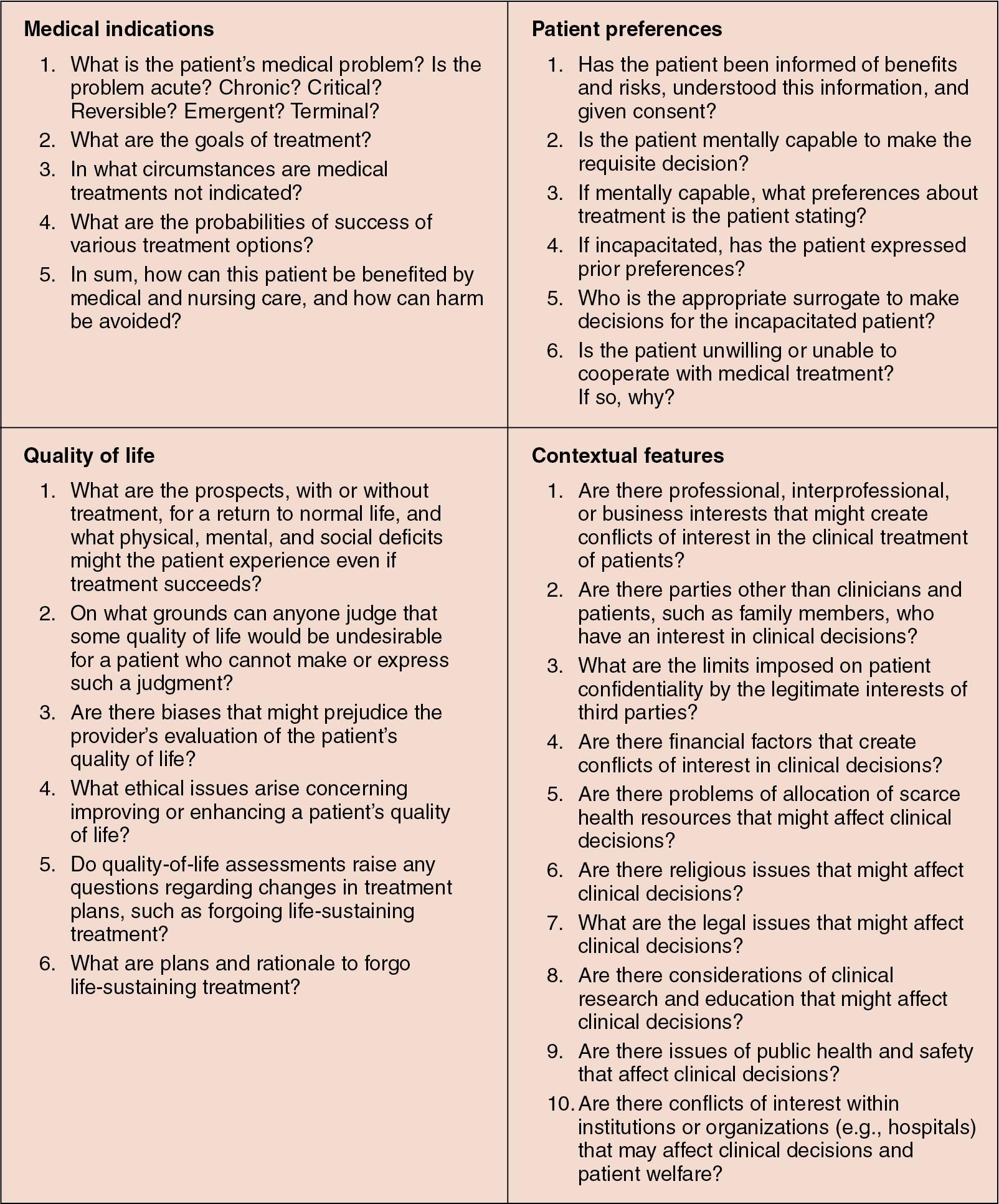
The first step in the process as outlined in Fig. 36.1 is to clearly identify the ethics problem or concern. As others have pointed out, getting clear on the presenting ethical issue is often part of the battle. In the case of Mrs. Roberts, the ethics problem is that the clinical team believes that CPR should not be initiated, and the family disagrees. Step two in the process requires the individual to gather and organize data. In this step, we rely on another methodology to look at the broad array of facts that one needs to consider. The method is from Jonsen, Siegler, and Winslade and looks at gathering data ( Fig. 36.2 ). Each section of “facts” looks at a different aspect of the patient’s care and how it might impact the ethical decision-making process. Medical indications ask the reader to look at diagnosis, prognosis, treatment options, and goals of care of any encounter. Patient preferences ask the reader to look at the clinical encounter from the patient’s viewpoint, considering whether the patient can make decisions or, if unable, whether he or she previously stated any preferences or whether he or she has a surrogate to make the decision. Step three in the process asks whether the issue is really an ethics problem or concern and, if so, what the ethics question is. Mrs. Roberts’ case is a classic ethical issue surrounding futility and the appropriateness of initiating CPR. The ethics question is likely, given that the providers’ obligation to not cause unnecessary harm and to provide interventions that will benefit the patient is in conflict with the surrogate decision maker’s right to decide on behalf of his or her loved one. Step four asks the reader to consider whether more dialogue is needed or whether more information should be sought. In our case, perhaps the clinicians should seek outside input on the success rates of CPR in patients in Mrs. Roberts’ condition. Or perhaps more dialogue with the patient’s daughter is needed to determine her level of understanding.
At the crux of any ethics case, a decision will have to be made, and that decision should be based on ethics values, concepts, principles, and so on. Thus, after gathering sufficient information through chart review and meeting with the clinical team and family, an ethics recommendation should be made based on values. In the current case, one could recommend that CPR should not be performed based on the principles of nonmaleficence, claiming that starting CPR would only cause the patient unnecessary harm.
This section analyzes several noteworthy moments in recent United States history that informed clinical ethics. These historical accounts are particularly useful because several illustrate ethics beyond the PA and patient encounter. This extension includes ethical dilemmas in the research and public health space. It is of little surprise that many of our bioethical historical accounts took place in the 1960s and 1970s, when we find a frustrated community reaction to a dramatic technological change in the hands of physicians who practiced medicine in a paternalistic fashion. The United States is a fascinating place to study bioethics, given the country’s unique health economy, history of social inequality, and biotechnology growth over the past several decades.
In 1961, a committee was formed in Seattle to determine which patients would be hooked up to a new machine designed to filter blood for those with end-stage renal disease. This committee was charged with the difficult task of deciding who would receive this early and expensive form of hemodialysis. Given economic and dialysis equipment constraints, the committee would ask themselves who should be chosen and on what basis. This charge was undertaken by a committee of seven non–bioethics-trained citizens—a lawyer, minister, banker, housewife, state government official, labor leader, and surgeon—selected by the King County Medical Society. They decided on factors such as gender, number of dependents, marital status, education, income, and emotional stability, alongside clinical factors. Many criticized the committee for using subjective criteria and allowing for “values” to creep into what some may have claimed was a clinical decision. It was famously said, “The Pacific Northwest is no place for a Henry David Thoreau with bad kidneys,” given his lack of employment, children, and religion. These concerns around the use of what some deemed inappropriate criteria led to the group being called the “God squad.”
The Tuskegee study was implemented in 1932 and remains one of the more sobering ethical violations of human experimentation in U.S. history. Tuskegee was an observational study of 399 subjects infected with syphilis matched with 200 similar but noninfected control subjects. A variety of morbidity outcomes were measured with the plan to follow study participants until death. As the history of Tuskegee unfolded, it is important to understand that those enrolled in the study possessed little capacity to avoid the undue influence the researchers had over them. Most of the research participants were illiterate, poor, and African American.
By 1947, penicillin was recognized as a highly successful treatment for syphilis. Despite this accessible information, the Tuskegee research team did not discontinue the study or transition to a study design that provided scientific value given the new treatment paradigm. The ability of these study participants to make autonomous decisions was further impacted by coercive practices from the research team. There are reports stating researchers made false claims of therapeutic benefit enticing participants to follow up. These follow-up visits even included lumbar punctures. The study continued until 1972 when popular media and public outcry influenced public health officials. In addition to the men who died of untreated syphilis, 40 wives and 19 children contracted syphilis. The Tuskegee study illustrates how vulnerable communities are at risk for unethical human subject research. These communities are also at risk for unethical practice in busy clinical settings where these often-complicated patients may receive less comprehensive medicine. Many of the contemporary cases presented in this chapter highlight examples of this concern. Common ethical dilemmas in human-subject research include unacceptable risk-to-benefit ratios, a lack of independent review and informed consent, and invalid research with low scientific value. The Tuskegee study failed in each of these areas. For example, given the state of knowledge surrounding untreated syphilis and the known benefits of penicillin, the participants should have received updated informed consent. Moreover, the risk-to-benefit ratio and scientific value should have called for the cessation of the study. Second, the study design lacked oversight, accurate data collection, and overall low validity, which all contributed to low scientific value.
In 1966, Dr. Henry K. Beecher published a landmark article titled “Ethics and Clinical Research” in the New England Journal of Medicine. In his paper, he reviewed 22 human-subject studies and found that many of the studies had a number of the aforementioned common ethical research concerns. Frequent ethical concerns included a lack of informed consent, a lack of proper study oversight, and study methods without validity or scientific utility.
Through Dr. Beecher’s work, we realize that the Tuskegee study was not unique and that ethical dilemmas were prevalent in a variety of clinical questions, research settings, and study populations. These findings are germane to the clinical setting as well. One theme throughout Beecher’s paper is how researcher bias can create unethical and invalid outcomes. Clinicians are also subject to bias, which influences the lens through which they view patient history, develop treatment plans, and deliver informed consent.
Become a Clinical Tree membership for Full access and enjoy Unlimited articles
If you are a member. Log in here